Abstract
Hematologic disorders can be caused by sporadic or inherited mutations. However, the molecular mechanisms that lead to pathogenicity are only partially understood. An accurate method to generate mouse models is conditional gene manipulation facilitated by the Cre-loxP recombination system. To enable identification and genomic manipulation of erythroid progenitor cells, we established a knock-in mouse model (ErGFPcre) that expresses an improved GFPcre fusion protein controlled by the endogenous erythropoietin receptor (EpoR) promoter. We show that ErGFPcre mice enable the identification of GFP-positive erythroid progenitor cells and the highly specific genomic manipulation of the erythroid lineage. Analysis of GFP-positive erythroid progenitor cells suggests a developmental switch in lineage progression from the hematopoietic stem cell compartment to early erythroid progenitor cells that are stem cell antigen-1–negative (Sca-1–) and c-kithigh. Within the hematopoietic system, Cre-mediated recombination is limited to erythroid progenitor cells and occurs in the adult bone marrow at a frequency of up to 80% and in the fetal liver with an efficiency close to 100%. Differential transcriptional activity of the wild-type and the knock-in locus was observed in nonhematopoietic tissues. Thus, our ErGFPcre mouse model could promote the identification of regulatory elements controlling nonhematopoietic EpoR expression and facilitates the characterization and genomic manipulation of erythroid progenitor cells.
Introduction
Cells of the hematopoietic system such as erythrocytes have a short half-life and are continuously renewed in a tightly controlled growth process. Dysregulation of erythropoiesis results in erythroleukemias or in anemias. The molecular cause for many of these diseases is still unknown. A key regulator of erythropoiesis is the hormone erythropoietin (Epo) and its cognate receptor, the erythropoietin receptor (EpoR). Besides some recent evidence for EpoR expression in the neuronal system, endothelial cells, and the heart,1-5 the EpoR is predominantly expressed in the erythroid lineage. The receptor is present from the erythroid burst-forming unit (BFU-E) stage, maximally expressed at the erythroid colonyforming unit (CFU-E) stage and/or proerythroblast stage, and declines thereafter.6-9 The essential and nonredundant role of the EpoR for the onset of erythropoiesis was confirmed by gene-targeting experiments.10 A reliable surface marker for the identification and enrichment of early erythroid progenitor cells in the murine system is missing, hence signal transduction through the EpoR has been primarily studied in hematopoietic cell lines. However, to determine the in vivo role of signaling molecules, the regulatory pathways have to be studied in the context of an organism.
To introduce precise genetic alterations and modifications in the mouse, gene targeting in murine embryonic stem (ES) cells is used.11 Although gene inactivation by classical gene knock-out has been highly informative, broad effects in multiple tissues or early embryonic lethality often obscure the role of genes in specific tissues or at later developmental stages. Several of the signaling molecules implicated in EpoR signaling or linked to hematologic disorders showed early embryonic lethality upon homozygous inactivation. Examples are the tyrosine phosphatase SHP-212 (Src homology 2 domain containing tyrosine phosphatase-2) and the signal transducer and activator of transcription 3 (STAT3),13 which is constitutively activated in polycythemia rubra vera, a disease characterized by hyperproliferation of the myeloid, erythroid, and megakaryocytic lineage.14
Conditional gene-targeting strategies such as the Cre-loxP system have the potential to circumvent these limitations by the induction of temporal and/or cell-type–restricted DNA rearrangements. Cre is a site-specific DNA recombinase of bacteriophage P1 that catalyzes reciprocal recombination between two 34–base pair (bp) DNA recognition sequences called loxP. In mice, conditional excision of a target gene is achieved by spatial and/or temporal expression of Cre in cells carrying the gene flanked (“floxed”) by 2 directly repeated loxP sites.15-17 This strategy has been successfully used for conditional gene manipulations in the neuronal system and other tissues, but only a few lines of mice have been generated expressing the Cre recombinase specifically in hematopoietic lineages. As yet, no Cre-transgenic mouse line is available to promote gene activation exclusively in the erythroid lineage. The majority of Cre recombinase–expressing mouse lines, including the lines expressing Cre in hematopoietic lineages, are transgenic animals generated by random integration of the Cre-encoding DNA. A disadvantage of this strategy is that insertion effects can result in founder strain–dependent variation of tissue specificity18 ; the use of upstream transcriptional regulative DNA fragments can result in mice that show a deletion pattern differing from the expression pattern of the endogenous gene.19,20 Cre-expressing mice using GATA-1 transcriptional regulatory elements to facilitate Cre expression in the erythroid and megakaryocytic lineage revealed conflicting results regarding tissue specificity.21,22 In contrast, the targeted insertion (knock-in) of the Cre recombinase gene into a defined genetic locus more often results in mice that maintain the full spatial and temporal activity of the endogenous promoter. Therefore, the key for conditional gene inactivation using the Cre-loxP system is the establishment of Cre-expressing mice with strictly defined tissue specificity and high recombination efficiency.
Here, we report a Cre knock-in mouse line (ErGFPcre) that simultaneously facilitates the conditional genomic manipulation and the identification of early erythroid progenitor cells. Within the hematopoietic system of this mouse line, Cre and the green fluorescence protein (GFP) are solely expressed by erythroid progenitors and recombination of floxed alleles reaches efficiencies close to 100%.
Material and methods
Generation of recombinant ES cells and ErGFPcre mice
The replacement-type targeting vector was constructed based on the plasmid pEPOR containing the murine EpoR gene (kindly provided by Dr Hong Wu, UCLA School of Medicine, Los Angeles, CA). The enhanced GFP with improved thermostability (tEGFP) has been described elsewhere.23 To eliminate a potential splice donor site in iCre, codon V343 was altered by site-directed mutagenesis from GTG to GTC. A DNA fragment of pEPOR encompassing parts of exon 2 as well as exons 3 to 8 of the EpoR gene was replaced by a neomycin selection cassette flanked by 2 FLP recombinase recognition (FRT) sites, thereby leaving the splice acceptor site of exon 2 upstream and the authentic poly-A signal downstream of the selection cassette (Figure 1A). The cDNA encoding GFPcre was introduced in exon 1 of the genomic EpoR locus. By polymerase chain reaction (PCR), the authentic 5′–untranslated region (UTR) of the EpoR was fused to the translational start ATG of the tEGFP cDNA, introducing a linker sequence encoding the dodeca-meric peptide (Gly-Glu-Ser-Ser-Gly-Ser-Ile-Ser-Ser-Leu-Ser-Thr) upstream of a short cloning site at the 3′ end. To complete the GFPcre cDNA, a fragment encoding iCreV343 was introduced in-frame downstream of the linker sequence, thereby connecting the splice donor site of exon 1 directly to the stop codon of GFPcre. The GFPcre cassette, transitions formed by molecular cloning, and parts amplified by PCR were confirmed by sequencing.
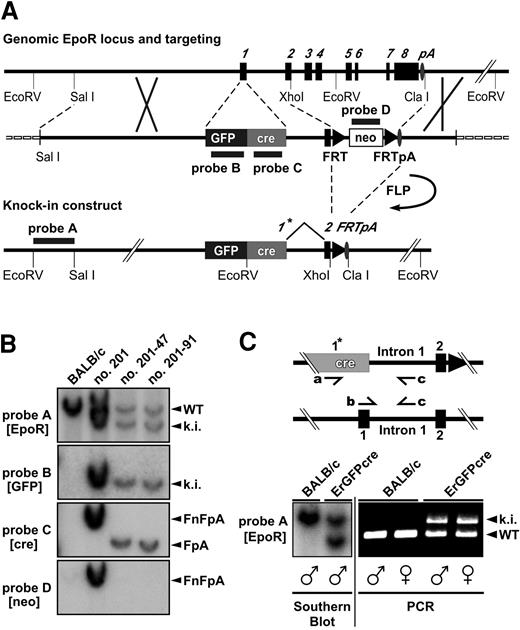
Targeted insertion of the GFPcre reading frame into the EpoR locus and screening strategy. (A) Structure of the genomic and the targeted locus of the EpoR. The targeting vector is shown in the middle of panel A. The exons of the EpoR gene are numbered and shown as black boxes. The oval indicates the polyadenylation signal (pA); triangles, FRT sites; a black/dark gray box, the GFPcre sequence inserted in exon 1 forming the new exon 1*; an open box, the neor selection marker; and a dashed line, the vector backbone of the targeting construct. Annealing sites of external probe A (EpoR) as well as internal probe B (GFP), C (cre), and D (neo) are indicated by black lines. (B) Southern blot analysis of EcoRV-digested genomic DNA derived from Balb/c and homologous recombinant ES cell clones. A blot probed by external probe A as well as internal probes B, C, and D is shown. The 5′-EcoRV fragment of the targeted knock-in allele (ki) is represented by a 7.4-kb band, and the wild-type EpoR 5′-EcoRV fragment (WT) is represented by a 9.0-kb band (probe A, B). The 3′-EcoRV fragment of the knock-in allele carrying the FRT-flanked neo-cassette (FnFpA; clone no. 201) is displayed by a 6.9-kb fragment (probe C and D) and the same allele having eliminated the selection marker (FpA; clone no. 201-47, no. 201-91) by a 5.6-kb band (probe C). (C) Multiplex PCR analysis of ErGFPcre mice. The wild-type allele (WT) displays a 431-bp PCR product created by the primers b and c, whereas a 679-bp band represents the GFPcre knock-in (ki) allele generated with the primers a and c. The PCR method was tested on Southern blot–confirmed (bottom left) genomic tail DNA derived from male and female wild-type Balb/c or hemizygous ErGFPcre mice.
Targeted insertion of the GFPcre reading frame into the EpoR locus and screening strategy. (A) Structure of the genomic and the targeted locus of the EpoR. The targeting vector is shown in the middle of panel A. The exons of the EpoR gene are numbered and shown as black boxes. The oval indicates the polyadenylation signal (pA); triangles, FRT sites; a black/dark gray box, the GFPcre sequence inserted in exon 1 forming the new exon 1*; an open box, the neor selection marker; and a dashed line, the vector backbone of the targeting construct. Annealing sites of external probe A (EpoR) as well as internal probe B (GFP), C (cre), and D (neo) are indicated by black lines. (B) Southern blot analysis of EcoRV-digested genomic DNA derived from Balb/c and homologous recombinant ES cell clones. A blot probed by external probe A as well as internal probes B, C, and D is shown. The 5′-EcoRV fragment of the targeted knock-in allele (ki) is represented by a 7.4-kb band, and the wild-type EpoR 5′-EcoRV fragment (WT) is represented by a 9.0-kb band (probe A, B). The 3′-EcoRV fragment of the knock-in allele carrying the FRT-flanked neo-cassette (FnFpA; clone no. 201) is displayed by a 6.9-kb fragment (probe C and D) and the same allele having eliminated the selection marker (FpA; clone no. 201-47, no. 201-91) by a 5.6-kb band (probe C). (C) Multiplex PCR analysis of ErGFPcre mice. The wild-type allele (WT) displays a 431-bp PCR product created by the primers b and c, whereas a 679-bp band represents the GFPcre knock-in (ki) allele generated with the primers a and c. The PCR method was tested on Southern blot–confirmed (bottom left) genomic tail DNA derived from male and female wild-type Balb/c or hemizygous ErGFPcre mice.
Thirty micrograms of the SalI linearized pErGFPcreFRT2neoPA targeting vector was introduced by electroporation into 1 × 107 BALB/c-I ES cells.24 The transfected cells were selected with G418 (320 μg/mL; Sigma, St Louis, MO) and resistant colonies were screened by Southern blot analysis of EcoRV-digested genomic DNA to discriminate between homologous and random recombinant ES cell clones. Two homologous recombinant hemizygous clones were chosen for FLP-recombinase–mediated neor gene deletion using pCAGGS-FLPe plasmid DNA.25
ES cells carrying the ErGFPcre-FRTpA–targeted allele were injected into C57BL/6-derived blastocysts, which were transplanted into the uteri of NMRI foster mothers. Chimeras were mated to BALB/c mice. Offspring generated through germ line transmission of the ES cell genome, as indicated by coat color, were analyzed by PCR on tail DNA, prepared as described.26 PCR amplification was performed for 36 cycles of 20 seconds at 94° C, 20 seconds at 60° C, and 45 seconds at 72° C using the primers a (5′-GTGTGGCTGCCCCTTCTGCCA-3′), b (5′-GGCAGCCTGGGCACCTTCAC-3′), and c (5′-CAGGAATTCAAGCTCAACCTCA-3′).
Cell preparation and cultivation
Fetal liver cells of 12.5–days after coitus (dpc), 13.5-dpc, or 14.5-dpc embryos were prepared by resuspending the isolated liver in 50 μL ice-cold phosphate-buffered saline (PBS) supplemented with 0.3% bovine serum albumin (BSA) to a single-cell suspension. Single-cell suspensions from bone marrow (2 femurs from each mouse) were prepared by flushing bones with ice-cold 0.3% BSA/PBS. Single-cell suspensions from spleen were prepared by gently teasing the cells from the splenic capsule in ice-cold 0.3% BSA/PBS. Fetal liver cells, bone marrow, and splenic cells were filtered trough a 40-μm cell-strainer, treated with Tris-buffered 0.155 M NH4Cl for 5 minutes on ice to remove erythrocytes, and washed by centrifugation through 0.3% BSA/PBS. Primary murine fetal liver cells were cultured in modified form as described.27
Purification of erythroid cells
Erythroid cells expressing the cellular surface marker Ter119 were purified from spleen, bone marrow, or fetal liver by magnetic activated cell sorting (MACS). Isolated spleen, bone marrow, or fetal liver cells were incubated for the enrichment with rat anti-Ter119 antibodies (gift from Dr Albrecht Müller, Julius-Maximilians-University, Würzburg, Germany) or for the sort with anti-Ter119–allophycocyanin (APC) (eBioscience, San Diego, CA) at a dilution of 1:200 for 30 minutes at 4° C. Cells were washed 3 times in 0.3% BSA/PBS and were incubated for 30 minutes at 4° C with anti–rat antibody–coupled magnetic beads according to the manufacturer's instructions (MACSbeads; Miltenyi Biotech, Bergisch-Gladbach, Germany). The cells were sorted to positive and negative fractions using the standard positive selection program of the AutoMACS machine (Miltenyi Biotech).
Flow cytometry
Flow cytometry analysis of fetal liver, bone marrow, thymic, or splenic cells was either performed with APC-conjugated anti-Ter119 (eBioscience) or by APC-conjugated anti–c-kit and phycoerythrin (PE)–conjugated anti–stem cell antigen-1 (anti–Sca-1; Pharmingen, San Diego, CA) antibodies. Alternatively, flow cytometry analysis was performed by a combination of Sca-1, granulocytic lineage (Gr-1), macrophage antigen-1 (Mac-1), CD14, CD41, B220 (all purchased from Pharmingen), and YBM/42 (gift from Dr S. Watt) specific monoclonal rat antibodies or by a single monoclonal Ter119 rat antibody (gift from Dr A. Müller) in combination with a cyanin 5 (Cy5) conjugated anti–rat secondary antibody (Dianova, Hamburg, Germany), respectively. Fetal liver, bone marrow, and spleen cells isolated by magnetic cell sorting were analyzed using APC-conjugated anti-Ter119 antibody (eBioscience). Gated cells were analyzed for GFP and surface marker expression by a FACScan (Becton Dickinson, Heidelberg, Germany).
Immunoprecipitation and immunoblotting
Epo-cultured fetal liver cells (1 × 107) of embryonic day 13.5 (E13.5) embryos were used to prepare detergent lysates. Cells were washed once with PBS, pelleted, and resuspended in 500 μL RIPA buffer (1% nonidet P-40 [NP40]; 150 mM NaCl; 50 mM Tris, pH 7.5; 0.5% sodium deoxycholate; 0.1% sodium dodecyl sulfate [SDS]), supplemented with 1 μg/mL aprotinin (Sigma) and 100 μg/mL AEBSF (4-(2-aminoethyl)-benzenesulfonyl fluoride; Sigma). After sonication, cells were lysed for 30 minutes at 4° C, and supernatants equivalent to 500 μg protein were subjected to immunoprecipitation using anti-GFP antiserum.28 The immunoprecipitates were eluted, resolved by 15% SDS–polyacrylamide gel electrophoresis (PAGE), and transferred to a nitrocellulose membrane. Detection by immunoblotting was performed with an anti-cre antibody (Novagen, Madison, WI) followed by enhanced chemiluminescence (Amersham Biosciences Europe, Freiburg, Germany). The blots were stripped and reprobed with anti-GFP antiserum.28
Southern blot analysis
DNA of adult tissues or embryonic yolk sac was prepared as previously described.26 For qualitative and quantitative determination of Cre activity in tissues and cell populations of R26R × ErGFPcre reporter mice, Southern blot analysis was performed on EcoRV-digested genomic DNA hybridized to the pCMV-β (Clontech, Palo Alto, CA) derived XmaI-EcoRV β-Gal fragment. Southern blot quantitation was carried out on a Fuji BAS 1300 phospho-imager (Fuji, Tokyo, Japan).
RT-PCR analysis
Reverse transcription–polymerase chain reaction (RT-PCR) analysis was performed using 1 μg total RNA isolated from each of the tissue samples as template for the oligo-dT–primed RT reaction with Moloney murine leukemia virus (MMLV) reverse transcriptase (Promega, Madison, WI). Equivalent dilutions of the cDNA samples were assessed by PCR for EpoR (primers are 5′-CTGTTGCTGACGGTTCTGG-3′ and 5′-GACAAGGCTGTTCTCATAGG-3′) and GFPcre (primers are 5′-ACCGATGGTGGGAGAATGC-3′ and 5′-CACACCAAGTCTTCCAAGCG-3′) messages, whereas exon-intron junctions were included to discriminate cDNA template from genomic DNA. Housekeeping gene expression was examined to yield a standard representing the mRNA level within the sample (beta-actin, 5′-GTCCACACCCGCCACCAGTTC-3′ and 5′-ACCGCTCGTTGCCAATAGTGATGA-3′).
Fluorescence microscopy
Fetal liver cells were grown in 0.8% methylcellulose (No. M3231; StemCell, Vancouver, BC, Canada) supplemented with 0.5 U/mL Epo (Janssen-Cilag, Bad Homburg, Germany) and 5 mM Hoechst 33258 on glass-bottom dishes (Nunc, Wiesbaden, Germany) for 24 to 48 hours prior to analysis. Fluorescence microscopy was performed on a Zeiss Axiovert S100 TV inverted microscope together with a Zeiss Plan-NEOFLUAR 100 × 1.30 oil (∞/0.17) objective (Thornwood, NY). Image acquisition was performed using Metamorph 3.51 imaging software (Universal Imaging, Downingtown, PA).
Results
Generation of erythroid-specific GFPcre knock-in mice
To generate mice that specifically express a fluorescent-tagged Cre recombinase in the erythroid lineage, we used a knock-in approach to insert the Cre gene into the erythropoietin receptor (EpoR) locus by homologous recombination. Thereby the endogenous EpoR promoter controls expression of a fusion protein consisting of the enhanced green fluorescent protein (EGFP) and the site-specific DNA recombinase Cre.
To establish a fluorescent-labeled Cre recombinase detectable in living cells, we fused a thermostable EGFP23 in-frame to the N-terminal coding sequence of a codon-improved Cre recombinase.29 As linker sequence between the 2 functional domains, a dodecameric peptide was introduced to allow flexibility. A potential splice donor predicted by in silico analysis (NetGene2; http://www.cbs.dtu.dk/services/NetGene2/) was eliminated by a silent mutation changing within the improved Cre codon V343 from GTG to GTC.
The GFPcre gene was inserted into the EpoR locus under the control of the EpoR promoter (Figure 1A). The coding sequence of GFPcre was inserted in exon 1 replacing the translational start codon ATG of the EpoR by the translational start site of the GFPcre cDNA, leaving the 5′-untranslated region intact. Downstream of the translational stop of the GFPcre gene, the last 10 bp of the splice-donor site of original exon 1 was preserved resulting in the GFPcre encoding artificial exon 1*. Exons 2 to 8 and introns 2 to 7 were replaced by a neomycin resistance (neor) cassette flanked by 2 FRT sites, leaving intron 1 and the authentic splice acceptor site of exon 2 upstream and the authentic EpoR poly-A signal downstream of the FRT-flanked selection marker.
The targeting construct was introduced into ES cells by homologous recombination. Homologous recombination could occur as desired by insertion of the complete GFPcre and FRT-neo-FRT sequences or between intron 1 and 3′-flanking region resulting in a “partial knock-in” of the neor gene only. To distinguish ES cell clones harboring the GFPcre reading frame correctly inserted from a partial knock-in and to identify additionally inserted copies, a Southern blot screening strategy using 4 different DNA probes was applied (Figure 1B). The external probe A facilitates the identification of clones harboring homologous recombination. The internal probes B, C, and D are specific for GFPcre and neor and confirm the correct insertion of GFPcre as well as the absence of additional random integration events. Of 372 screened ES cell clones, 19 (5.4%) were homologous recombinant. Of these clones, 2 (0.8% of total) contained a partial knock-in and 1 (0.3% of total) was a EpoR knock-out with both alleles targeted (data not shown). Of the 16 hemizygous homologous recombinant ES cell clones carrying the complete GFPcre sequence, 10 harbored, as desired, a single copy of the entire targeting construct (single knock-in), whereas 6 of them carried additional random integrations. After FLP-mediated neor removal, 2 single knock-in ES cell clones were injected into blastocysts. Both ES cell clones resulted in germ line transmission, and the corresponding mice were designated ErGFPcre.
Since the homozygous GFPcre allele results in a lethal EpoR knock-out manifested at embryonic stage E12.5, the ErGFPcre mouse line has to be propagated in the hemizygous state. The hemizygous state of male and female ErGFPcre mice can be confirmed as shown in Figure 1C by Southern blot and multiplex polymerase chain reaction (PCR) resulting in a 431-bp PCR product (primers b and c) for the wild-type and a 679-bp PCR product (primers a and c) for the GFPcre allele.
Analysis of GFPcre expression in ErGFPcre mice
To confirm expression of the GFPcre fusion protein in ErGFPcre mice, we examined the fetal liver, which is the primary organ of embryonic erythropoiesis. Fetal liver cells (FLCs) of hemizygous (GFPcre/+) and wild-type (+/+) embryos were analyzed by PCR in combination with immunoblotting, fluorescence activated cell sorting (FACS), and fluorescence microscopy (Figure 2).
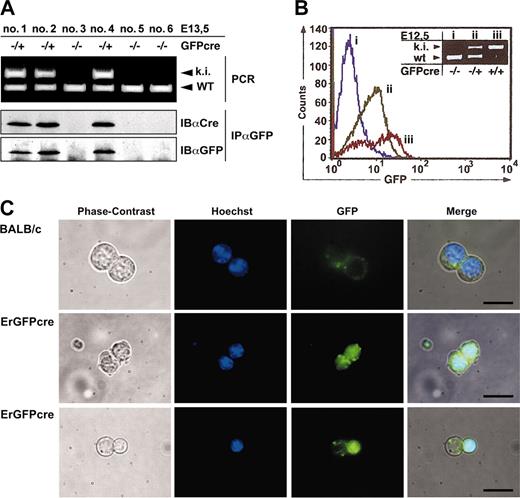
Expression of GFPcre in fetal liver cells of ErGFPcre mice. (A) Immunoblot analysis of FLCs. Littermates hemizygous GFPcre positive (–/+; ki) or GFPcre negative (–/–) and wild type at the EpoR (WT) were isolated at 13.5dpc and genotyping was performed by multiplex PCR on yolk sac DNA (top). Cell lysates of FLCs were immunoprecipitated with an anti-GFP antibody and analyzed by immunoblotting with an anti-Cre or anti-GFP antibody (bottom). (B) Analysis of FLCs by flow cytometry. FLCs derived from wild-type (i), hemizygous (ii), or homozygous (iii) ErGFPcre embryos at E12.5 were analyzed for GFP fluorescence. Data were plotted as relative fluorescence intensity versus the cell number. The genotype of the embryos used for FLC preparation was determined by multiplex PCR analysis on yolk sac DNA (inset). (C) Analysis of FLCs by fluorescence microscopy. FLCs derived from wild-type (Balb/c) or hemizygous ErGFPcre (ErGFPcre) littermates at embryonic stage E13.5 were cultivated in 0.8% methylcellulose supplemented with 0.5 U/mL Epo and 5 mM Hoechst 33258. After 24 hours, cells were analyzed for Hoechst and GFP fluorescence using fluorescent microscopy. The merged pictures were obtained by overlaying the corresponding phase contrast, Hoechst, and GFP images (scale bar, 10 μm).
Expression of GFPcre in fetal liver cells of ErGFPcre mice. (A) Immunoblot analysis of FLCs. Littermates hemizygous GFPcre positive (–/+; ki) or GFPcre negative (–/–) and wild type at the EpoR (WT) were isolated at 13.5dpc and genotyping was performed by multiplex PCR on yolk sac DNA (top). Cell lysates of FLCs were immunoprecipitated with an anti-GFP antibody and analyzed by immunoblotting with an anti-Cre or anti-GFP antibody (bottom). (B) Analysis of FLCs by flow cytometry. FLCs derived from wild-type (i), hemizygous (ii), or homozygous (iii) ErGFPcre embryos at E12.5 were analyzed for GFP fluorescence. Data were plotted as relative fluorescence intensity versus the cell number. The genotype of the embryos used for FLC preparation was determined by multiplex PCR analysis on yolk sac DNA (inset). (C) Analysis of FLCs by fluorescence microscopy. FLCs derived from wild-type (Balb/c) or hemizygous ErGFPcre (ErGFPcre) littermates at embryonic stage E13.5 were cultivated in 0.8% methylcellulose supplemented with 0.5 U/mL Epo and 5 mM Hoechst 33258. After 24 hours, cells were analyzed for Hoechst and GFP fluorescence using fluorescent microscopy. The merged pictures were obtained by overlaying the corresponding phase contrast, Hoechst, and GFP images (scale bar, 10 μm).
In lysates from FLCs of hemizygous ErGFPcre littermates at embryonic stage E13.5, a distinct 62-kDa band was detected by anti-GFP immunoprecipitation and immunoblotting with anti-Cre or anti-GFP antibodies that was absent in lysates from wild-type embryos (Figure 2A). This indicated that a full-length GFPcre fusion protein is expressed. Similar results were obtained by immunoblotting analysis of bone marrow preparations from hemizygous ErGFPcre mice in comparison to wild-type Balb/c mice (data not shown).
To examine GFP fluorescence, isolated FLCs of wild-type, hemizygous, or homozygous ErGFPcre embryos at E12.5 were analyzed by flow cytometry and the genotype was verified by yolk sac PCR (Figure 2B). Green fluorescence was detected in both hemizygous and homozygous mutant FLCs. In accordance with deficient erythropoiesis and embryonic lethality described for the EpoR knock-out, embryos homozygous for GFPcre showed a significant reduction in GFP-positive cells within the fetal liver but an approximately 2-fold increase in fluorescence intensity compared with hemizygous littermates. This increase is likely caused by a gene dosage effect due to the second EpoR promoter–driven GFPcre allele.
To visualize the subcellular localization of GFPcre in erythroid progenitor cells, FLCs of either hemizygous or wild-type embryos at the embryonic stage E13.5 were cultivated under conditions favoring proliferation as well as differentiation of erythroid progenitor cells and were analyzed by fluorescence microscopy (Figure 2C). As expected, FLCs from hemizygous littermates displayed a GFP signal confined to the nucleus that was absent in FLCs from wild-type control mice. Upon natural ejection of the nucleus in differentiated erythrocytes, the GFPcre fusion protein is eliminated together with the nucleus (Figure 2C bottom). These findings indicate that the GFPcre protein is present in erythroid progenitor cells up to the late normoblast stage.
GFPcre expression within the hematopoietic compartment
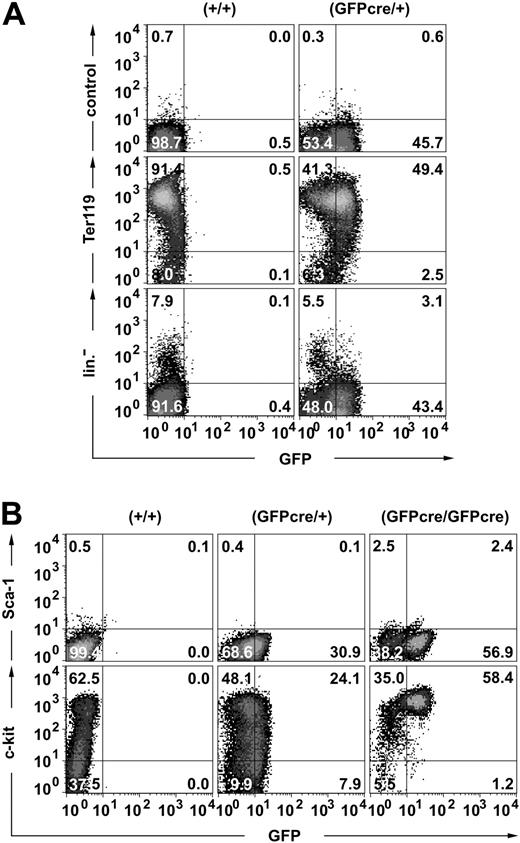
To confirm that GFPcre expression in ErGFPcre mice is restricted to the erythroid lineage, different hematopoietic subpopulations were analyzed by flow cytometry. Approximately 90% of the day-E13.5 FLCs stained positive for the erythroid surface marker Ter119, and 49% of these cells expressed both Ter119 and GFP (Figure 3A middle). A small population of cells (2.5%) was detected that was GFP-positive but Ter119-negative, potentially representing early erythroid progenitor cells. To verify that GFPcre is exclusively expressed in the erythroid lineage, E13.5 FLCs were stained with a combination of antibodies specific for different hematopoietic lineages (Figure 3A bottom). The GFP signal was only detected in cells that did not show contribution to megakaryocytes and platelets (CD41), macrophages, granulocytes and monocytes (Mac-1, Gr-1), leukocytes (CD41), or the lymphoid compartment (B220, YBM/4230 ). Thus, within the hematopoietic compartment, expression of GFPcre is restricted to the erythroid lineage.
Expression of the GFPcre fusion protein in the erythroid lineage. Flow cytometric analysis of FLCs derived from (A) E13.5 wild-type (+/+) and hemizygous (GFPcre/+) or (B) E12.5 wild-type (+/+), hemizygous (GFPcre/+), and homozygous (GFPcre/GFPcre) ErGFPcre littermates. (A) To determine the lineage specificity of GFPcre expression, cells were stained either with only the secondary antibody (control) or with a Ter119-specific antibody followed by the secondary antibody (middle). A combination of CD41-, Mac-1–, Gr-1–, CD14-, Sca-1–, B220-, and YBM/42-specific antibodies was used to identify the major pool of nonerythroid cells (lin–). (B) To determine surface marker expression of erythroid progenitor cells, cells were simultaneously stained with Sca-1– and c-kit–specific antibodies. The quadrant statistics are indicated.
Expression of the GFPcre fusion protein in the erythroid lineage. Flow cytometric analysis of FLCs derived from (A) E13.5 wild-type (+/+) and hemizygous (GFPcre/+) or (B) E12.5 wild-type (+/+), hemizygous (GFPcre/+), and homozygous (GFPcre/GFPcre) ErGFPcre littermates. (A) To determine the lineage specificity of GFPcre expression, cells were stained either with only the secondary antibody (control) or with a Ter119-specific antibody followed by the secondary antibody (middle). A combination of CD41-, Mac-1–, Gr-1–, CD14-, Sca-1–, B220-, and YBM/42-specific antibodies was used to identify the major pool of nonerythroid cells (lin–). (B) To determine surface marker expression of erythroid progenitor cells, cells were simultaneously stained with Sca-1– and c-kit–specific antibodies. The quadrant statistics are indicated.
To further characterize GFPcre-expressing erythroid progenitor cells, E12.5 FLCs from ErGFPcre embryos were monitored for Sca-1 and c-kit expression (Figure 3B). In contrast to hematopoietic stem cells (HSCs) that are Sca-1– and c-kit–positive,31 GFP-positive erythroid FLCs from hemizygous ErGFPcre mice are Sca-1– and range between c-kithigh and c-kitlow. However, erythroid FLCs from homozygous ErGFPcre embryos show enrichment of cells that are c-kithigh but Sca-1– and only a minor population of these cells might potentially be Sca-1low. Thus, our study in combination with the finding that lineage progression in EpoR knockout mice is blocked at the CFU-E stage10 suggests that CFU-E cells are c-kithigh and Sca-1–.
GFPcre activity in ErGFPcre mice
To determine whether expression level and activity of GFPcre is sufficient for tissue-specific recombination of loxP-flanked target sequences in vivo, ErGFPcre mice were crossed with Rosa26 (R26R) reporter mice.32 In these mice, expression of lacZ is mediated by the removal of a loxP-flanked DNA segment. We directly monitored GFPcre-mediated recombination by Cre-induced lacZ expression in embryonic mice hemizygous for GFPcre and R26R. Whole-mount analysis of E13.5 embryos hemizygous for GFPcre and R26R displayed strong X-Gal staining in the fetal liver (Figure 4A right) that was absent in control embryos lacking GFPcre but carrying the R26R reporter gene (Figure 4A left). No other organs than the fetal liver and single blood vessels showed Cre-mediated activation of lacZ expression. Similar results were obtained by lacZ stainings performed on cryosections (Figure 4B). This analysis confirms that in the embryo Cre activity is restricted to the vascular system and to the fetal liver, the major site of erythropoiesis in mice at embryonic stage E13.5.
Tissue specificity of GFPcre-mediated recombination and expression of the EpoR and GFPcre in ErGFPcre mice. (A) Whole-mount lacZ stainings of R26R-positive (left) and GFPcre/R26R–double-positive (right) embryos at developmental stage E13.5. (B) LacZ stainings performed on sagittal cryosections of embryos (E13.5) hemizygous for GFPcre and R26R. FL indicates for fetal liver; and BV, blood vessel. X-Gal stainings on whole-mount and tissue-sections were performed according to standard procedures.33 Images were acquired using a Zeiss Stemi SV11Apo binocular microscope in combination with a Zeiss AxioCam Color camera. (C) Southern blot analysis of ErGFPcre-R26R reporter mice hemizygous for GFPcre and R26R. EcoRV-digested genomic DNA from different tissues and Ter119-positive and -negative sorted bone marrow fractions were analyzed by Southern blot analysis (for details see Figure 5A). The fragments representing the excised (-◃-) and the nonrecombined (◃-◃) reporter allele are indicated. (D) RT-PCR analysis of different tissues derived from adult ErGFPcre mice hemizygous for GFPcre. Total RNA derived from tissue samples was reverse transcribed and cDNA samples were used for PCR analysis monitoring EpoR and GFPcre expression. PCR analysis monitoring beta-actin was used to confirm comparable total mRNA levels within the samples.
Tissue specificity of GFPcre-mediated recombination and expression of the EpoR and GFPcre in ErGFPcre mice. (A) Whole-mount lacZ stainings of R26R-positive (left) and GFPcre/R26R–double-positive (right) embryos at developmental stage E13.5. (B) LacZ stainings performed on sagittal cryosections of embryos (E13.5) hemizygous for GFPcre and R26R. FL indicates for fetal liver; and BV, blood vessel. X-Gal stainings on whole-mount and tissue-sections were performed according to standard procedures.33 Images were acquired using a Zeiss Stemi SV11Apo binocular microscope in combination with a Zeiss AxioCam Color camera. (C) Southern blot analysis of ErGFPcre-R26R reporter mice hemizygous for GFPcre and R26R. EcoRV-digested genomic DNA from different tissues and Ter119-positive and -negative sorted bone marrow fractions were analyzed by Southern blot analysis (for details see Figure 5A). The fragments representing the excised (-◃-) and the nonrecombined (◃-◃) reporter allele are indicated. (D) RT-PCR analysis of different tissues derived from adult ErGFPcre mice hemizygous for GFPcre. Total RNA derived from tissue samples was reverse transcribed and cDNA samples were used for PCR analysis monitoring EpoR and GFPcre expression. PCR analysis monitoring beta-actin was used to confirm comparable total mRNA levels within the samples.
We analyzed the specificity of GFPcre-mediated recombination in adult mice hemizygous for GFPcre and carrying the R26R reporter gene. DNA preparations of various organs were analyzed by Southern blot monitoring Cre-mediated recombination. Bone marrow preparations enriched in Ter119-expressing erythroid cells were used as positive control. Cre-mediated recombination was not detected in any tissue except of Ter119-positive enriched bone marrow cells (Figure 4C).
Recent studies provide evidence for EpoR promoter activity within nonhematopoietic tissues like brain, heart, and endothelial cells of the vascular system.1-5 To monitor EpoR and GFPcre expression in hematopoietic and nonhematopoietic tissues of adult ErGFPcre mice, total RNA preparations of different organs were subjected to RT-PCR analysis. In agreement with previous studies, EpoR expression was detected in the brain, the heart, and the vascular system, as well as in the erythroid organs, spleen, and bone marrow (Figure 4D). However, in nonhematopoietic tissues, differential expression of GFPcre was observed. Surprisingly GFPcre expression within the vascular system was significantly increased compared with the EpoR and no GFPcre expression was detected in the brain or the heart. Thus, in correlation with the results obtained by whole-mount analysis of embryos, GFPcre is expressed in the erythroid system and to some extent in vascular tissues and facilitates Cre-mediated recombination primarily in the erythroid lineage of adult ErGFPcre mice.
Cre-mediated recombination frequency in ErGFPcre mice
To determine the recombination frequency of GFPcre in adult and embryonic mice, hematopoietic cell fractions from MACS sortings were subjected to quantitative Southern blot analysis (Figure 5). The bone marrow and spleen cells from 3 adult GFPcre-expressing R26R mice were pooled and sorted into Ter119-positive and -negative fractions. By Southern blot analysis, a 1.7-kilobase (kb) fragment indicating the nonrecombined (“floxed”) allele and a 3.4-kb fragment representing the excised (“del”) reporter allele were detected. The Cre-mediated recombination efficiency in the Ter119-positive preparation was quantified to be 61.7% in the spleen and 76.3% in the bone marrow (Figure 5A). To determine the purity of the MACS-sorted Ter119-negative and Ter119-positive cell fractions, Ter119 expression in the sorted fractions in comparison to the unsorted preparations was analyzed by flow cytometry. As shown in Figure 5B, separation by MACS is highly efficient, since the Ter119-negative cell fraction of bone marrow and spleen lacked cells expressing the erythroid marker Ter119. In contrast, 94.4% (Figure 5B bone marrow) and 76.7% (Figure 5B spleen) purity of Ter119-positive cells was obtained in the Ter119 sorted fractions. The values quantified by Southern blot analysis in correlation with the results obtained by flow cytometry indicate that the loxP-flanked reporter cassette is efficiently eliminated in Ter119-positive cells from both spleen and bone marrow. Thus, adult ErGFPcre mice provide sufficient Cre activity for specific and efficient recombination of loxP-flanked target sequences within the erythroid lineage.
Determination of GFPcre-mediated recombination efficiency. (A-B) GFPcre-mediated recombination efficiency in adult mice. Bone marrow and spleen cells from 3 mice positive for the R26R reporter allele and hemizygous GFPcre positive (GFPcre/+) for the EpoR locus were sorted by MACS for Ter119-positive (+) and -negative (–) fractions. The EcoRV-digested DNA was analyzed by quantitative Southern blot analysis (A). The quantitation was performed by phospho-imager analysis and the values for the recombinant (del) as well as the nonrecombinant (flox) allele were calculated after background subtraction per lane in percent (table). The genomic organization of the reporter allele is depicted. The letters indicate the restriction site EcoRV (E) and the probe (P) used for hybridization. LoxP sites are shown as ◃. The size of the deleted (del) as well as the loxP-flanked (flox) reporter allele is indicated. Each cell fraction was analyzed by flow cytometry for Ter119 content (B). Data were plotted as relative fluorescence intensity versus the cell number. The Ter119-positive (Ter119+, gray line) and -negative sorted fractions (Ter119–, thick black line) of bone marrow (left) and spleen (right) were compared with the corresponding pool of unsorted cells (thin black line). The number in each panel represents the relative amount of Terr119-positive cells within the Ter119-positive sorted fraction as defined by the indicated gate. (C-D) GFPcre-mediated recombination efficiency in embryonic mice. FLCs derived from E14.5 embryos hemizygous for the R26R reporter allele and wild-type for the EpoR locus (+/+) or hemizygous positive for GFPcre (GFPcre/+) were sorted for Ter119-positive and -negative fractions. EcoRV-digested DNA derived from sorted FLC fractions were subjected to quantitative Southern blot analysis (C). The left lane displays the results obtained from Ter119-positive control FLCs, negative for GFPcre (+/+) and positive for the R26R reporter allele directly after sorting. The middle left, middle right, and right lanes in panel C show the results obtained from either Ter119-negative (–) or Ter119-positive (+) sorted FLCs hemizygous for GFPcre (GFPcre/+) and R26R directly after sorting (t = 0) or after 24-hour cultivation in Epo (t = 24). Sorted fractions were analyzed for Ter119 content by flow cytometry (D) either directly after sorting (0 h) or after 24-h cultivation in Epo (24 h).
Determination of GFPcre-mediated recombination efficiency. (A-B) GFPcre-mediated recombination efficiency in adult mice. Bone marrow and spleen cells from 3 mice positive for the R26R reporter allele and hemizygous GFPcre positive (GFPcre/+) for the EpoR locus were sorted by MACS for Ter119-positive (+) and -negative (–) fractions. The EcoRV-digested DNA was analyzed by quantitative Southern blot analysis (A). The quantitation was performed by phospho-imager analysis and the values for the recombinant (del) as well as the nonrecombinant (flox) allele were calculated after background subtraction per lane in percent (table). The genomic organization of the reporter allele is depicted. The letters indicate the restriction site EcoRV (E) and the probe (P) used for hybridization. LoxP sites are shown as ◃. The size of the deleted (del) as well as the loxP-flanked (flox) reporter allele is indicated. Each cell fraction was analyzed by flow cytometry for Ter119 content (B). Data were plotted as relative fluorescence intensity versus the cell number. The Ter119-positive (Ter119+, gray line) and -negative sorted fractions (Ter119–, thick black line) of bone marrow (left) and spleen (right) were compared with the corresponding pool of unsorted cells (thin black line). The number in each panel represents the relative amount of Terr119-positive cells within the Ter119-positive sorted fraction as defined by the indicated gate. (C-D) GFPcre-mediated recombination efficiency in embryonic mice. FLCs derived from E14.5 embryos hemizygous for the R26R reporter allele and wild-type for the EpoR locus (+/+) or hemizygous positive for GFPcre (GFPcre/+) were sorted for Ter119-positive and -negative fractions. EcoRV-digested DNA derived from sorted FLC fractions were subjected to quantitative Southern blot analysis (C). The left lane displays the results obtained from Ter119-positive control FLCs, negative for GFPcre (+/+) and positive for the R26R reporter allele directly after sorting. The middle left, middle right, and right lanes in panel C show the results obtained from either Ter119-negative (–) or Ter119-positive (+) sorted FLCs hemizygous for GFPcre (GFPcre/+) and R26R directly after sorting (t = 0) or after 24-hour cultivation in Epo (t = 24). Sorted fractions were analyzed for Ter119 content by flow cytometry (D) either directly after sorting (0 h) or after 24-h cultivation in Epo (24 h).
To determine the recombination frequency of GFPcre in the fetal erythroid lineage, embryos at E14.5 from 3 separate breedings of hemizygous ErGFPcre mice with homozygous R26R mice were analyzed independently (Figure 5C). Fetal liver cells from each embryo were prepared separately and genotyped according to GFP expression and by PCR analysis. Fetal liver cells either carrying the GFPcre allele or not were purified by MACS to isolate Ter119-positive and -negative fractions. To analyze the possibility that GFPcre expressing early erythroblasts within the Ter119-positive fraction required more time for successful recombination, half of each fraction was cultivated for 24 hours in Epo to facilitate further maturation. The isolated DNA of Ter119-positive and -negative fractions from R26R FLCs either carrying the GFPcre allele or not were analyzed, and the percentage of Cre-mediated recombination was quantified by Southern blot analysis directly after sorting (t = 0 h) and after cultivation in Epo (t = 24 h; Figure 5C). The mean of 3 independent Southern blot quantitations was calculated and revealed directly after sorting a recombination frequency of 93.7% ± 1.3% (n = 3). After 24 hours of cultivation in Epo, the same Ter119-positive cell fractions displayed an excision rate of 97.8% ± 1.3% (n = 3). In contrast, GFPcre-negative but R26R-positive control cells quantified directly after sorting revealed a recombination rate of 0.5% ± 3.2% (n = 3), whereas GFPcre- and R26R-positive but Ter119-negative sorted control fractions displayed recombination of the floxed reporter allele due to an impurity of Ter119-positive cells.
Figure 5D displays representative FACS analysis of the corresponding Ter119-positive and -negative sorted cell fractions. The positive sorted cell fraction obtained from embryos negative for GFPcre (Figure 5D left) included 75.9% Ter119-positive cells. The same fraction obtained from embryos hemizygous for GFPcre contained 83.5% of Ter119-positive cells (Figure 5D middle), whereas only 68.3% of Ter119-positive cells were measured in the same fraction after cultivation for 24 hours in Epo (Figure 5D right). This decrease of Ter119 content over time could be due to further maturation of erythroblasts to erythrocytes that are Ter119 negative in the embryonic state only. The mean content of Ter119-positive cells within the individually analyzed Ter119-positive fractions was calculated with 83.8% ± 4.4% (n = 5), whereas impurity of Ter119-positive cells in the corresponding negative sorted fractions was calculated to be 27.2% ± 13.7% (n = 6). These results indicate that Cre-mediated recombination in ErGFPcre embryos is specific for the erythroid lineage and highly efficient during early stages of erythropoiesis.
Discussion
Mice expressing a fluorescent marker or Cre recombinase under a tissue- and/or temporal-specific promoter are useful tools for either expression studies or conditional gene-targeting approaches elucidating the function of genes in vivo. The power of a Cre transgenic mouse line depends on the specificity and level of Cre-recombinase expression to achieve the desired genomic alteration.17 The majority of transgenic mouse lines have been generated by pronuclear DNA injection leading to random transgenesis and thereby to problems with penetrance and site of insertion effects.18 In contrast, gene-targeting–based gene knock-in has been shown to create a defined genomic mutation that allows transgene expression controlled by endogenous gene regulation.20,34 Previous attempts to generate a Cre mouse line facilitating Cre-mediated recombination in the erythroid lineage made use of GATA-1 transcriptional regulatory elements.21,22 Analysis of 2 transgenic mouse lines independently generated by random integration of a GATA-1–controlled Cre-transgene revealed conflicting results regarding the tissue specificity of Cre expression. In contrast, our ErGFPcre mouse line was generated by targeted integration of a fluorescent-tagged Cre-recombinase encoding gene into the endogenous EpoR locus. In accordance with the expectation that gene targeting increases the reliability and reproducibility of transgene expression, in our mouse model GFPcre expression was exclusively detected in the vascular system and in Ter119-positive fetal liver, adult spleen, and bone marrow cells that correspond to definitive erythroid cells. The EpoR is also expressed during Epo-independent primitive erythropoiesis,35 but the relative amount of EpoR expression within primitive erythroid progenitor cells remains to be elucidated. Assuming that EpoR expression in primitive erythroid progenitors is comparable to the expression level detected during definitive erythropoiesis, our mouse model could facilitate efficient conditional gene knock-out not only in definitive but also in primitive erythroid progenitor cells.
Besides tissue specificity, recombination efficiency is a critical parameter for the usefulness of a Cre mouse line. Most commonly, the excision rate in transgenic Cre mouse lines has been estimated by crossing the mouse line with floxed reporter strains and analyzing the offspring by Southern blot and/or visual counting of cells expressing the recombined reporter allele in stainings of whole-mount preparations as well as tissue sections. In most cases, cell morphology or separately analyzed samples were used to determine the tissue- or cell type–specificity of Cre-mediated recombination leading to estimated rates ranging from 60% to 100% recombination efficiency depending on the Cre mouse line analyzed.34,36,37 To quantify the recombination frequency in the ErGFPcre mouse line we developed an assay combining magnetic activated cell sorting with quantitative Southern blot analysis and flow cytometry to simultaneously determine the tissue specificity and efficiency of Cre-mediated recombination. Applying this assay, we determined a Cre-mediated recombination rate of 93.7% ± 1.3% in preparations of fetal liver Ter119-positive cells 83.8% ± 4.4% pure. Presumably the discrepancies regarding these values are due to the presence of low Ter119-expressing cells in the Ter119-positive sorted fraction that were scored negative by flow cytometry. Thus, up to 100% of all erythroid fetal liver cells are potentially affected by Cre-mediated recombination. A comparable analysis of adult spleen and bone marrow cells derived from ErGFPcre mice revealed a GFPcre-mediated excision rate of 80.8% (bone marrow) and 80.4% (spleen), assuming that the recombination occurred only in the Ter119-positive cell population defined by flow cytometry analysis. This reduced excision rate in the adulthood compared with embryonic stages is coherent with the lower fluorescent intensity of GFPcre observed in adult bone marrow and spleen cells and possibly could be enhanced by treatments leading to stress erythropoiesis such as phenylhydrazine administration.38
In a variety of tissue culture cells, high as well as long-term Cre expression was shown to be associated with aneuploidy and a high incident of chromosomal aberrations.39 In other experiments, Cre expression was linked to toxic effects when expressed in postmeiotic spermatids of transgenic mice,40 and growth suppressive effects of a retroviral-driven Cre recombinase have been reported in embryonic fibroblasts.41 Although the mean fluorescence intensity detected in hemizygous fetal liver, bone marrow, and spleen cells indicates a moderate expression of GFPcre, the fluorescence signal is sufficient to distinctly identify erythroid cells among other hematopoietic cell lineages by flow cytometry. Thus, in our mouse model, moderate and tissue-specific GFPcre expression presumably prevents detectable toxic side effects yet supporting maximal Cre-mediated recombination in erythroid cells.
Expression of the EpoR is crucial for proliferation and terminal differentiation of CFU-E erythroid progenitor cells but not essential for commitment to erythroid lineage.10 Expression of the gene encoding the hematopoietic cytokine receptor EpoR arises during definitive erythropoiesis at the BFU-E stage and reaches a maximum at the CFU-E/proerythroblast stage. Upon maturation to the late erythroblast stage, EpoR expression declines.6-9 In EpoR knockout mice, CFU-E erythroid progenitor cells develop but their inability to mature finally leads to apoptosis. Similarly, expression of GFPcre instead of the EpoR in homozygous ErGFPcre mice causes embryonic lethality. However, in these mice, erythroid progenitor cells arrested at the CFU-E stage can be detected by flow cytometry. Our data propose a phenotypic surface marker switch from hematopoietic stem cells that are Sca-1+/c-kit+31 to CFU-E, which can be described by Sca-1–/c-kithigh. In addition, heterozygous GFPcre-positive erythroid cells range between c-kithigh and c-kitlow. Assuming a linear regulation of c-kit expression, a gradual down-regulation of c-kit during lineage progression could be hypothesized.
Nonhematopoietic EpoR expression has been detected in tissues, such as brain, heart, and endothelial cells, and was shown to be crucial for normal brain development.5 Although it was reported that in the embryonic central nervous system (CNS) the EpoR is expressed at comparable levels as in the adult bone marrow and spleen,4,42 we were not able to detect Cre-mediated recombination in the CNS of ErGFPcre mice. By lacZ stainings as well as by Southern blot analysis of ErGFPcre-R26R reporter mice we show that Cre-mediated recombination is highly tissue specific and occurs selectively in cells of the erythroid lineage and the vascular system. Analysis of adult ErGFPcre mice revealed differentially expressed GFPcre and EpoR alleles in nonhematopoietic tissues. Previous results obtained from human EpoR transgenic mice suggest that nonhematopoietic EpoR expression in the brain is controlled by an additional promoter region upstream from the proximal EpoR promoter.43 Recent studies provide evidence for different cis-regulative elements in paired box gene 6 (PAX6) intron 7 that control tissue-specific gene expression.44 Because the promoter region in the GFPcre allele is unaffected by the genomic alteration, whereas intron 2 to 7 of the endogenous EpoR is removed, cis-regulative elements within these introns might be responsible for the differential expression of the EpoR and GFPcre.
In summary, our studies demonstrate that the ErGFPcre mouse model can successfully be used for the identification of erythroid cells as well as for highly specific and efficient manipulation of genes within the erythroid lineage. This mouse model represents a genetic tool that potentially facilitates the elucidation of mechanisms regulating nonhematopoietic expression of the EpoR and can be expected to improve our understanding of principal mechanisms regulating erythropoiesis in the context of a living animal.
Prepublished online as Blood First Edition Paper, April 15, 2004; DOI 10.1182/blood-2003-05-1442.
Supported by the Boehringer Ingelheim Fonds (A.C.H.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank A. Geist for excellent technical assistance, Dr C. Haas for technical advise as well as extensive discussion regarding the expression of the EpoR in neuronal tissue, and U. Stauffer for taking care of the mice. Dr B. Kanzler is acknowledged for ES cell injection. We are grateful to Drs B. G. Neel and L. C. Cantley for critically reading the manuscript. The anti-Ter119 antibody was generously provided by Dr A. Müller and the YBM/42 antibody by Dr S. M. Watt. We thank Dr H. Wu for providing the vector pEPOR, Dr R. Sprengel for supplying the iCre cDNA, and Dr F. Stewart for providing the pCAGGS-FLPe plasmid.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal