Abstract
Human hematopoietic stem cells (HSCs) are commonly purified by the expression of cell surface markers such as CD34. Because cell phenotype can be altered by cell cycle progression or ex vivo culture, purification on the basis of conserved stem cell function may represent a more reliable way to isolate various stem cell populations. We have purified primitive HSCs from human umbilical cord blood (UCB) by lineage depletion (Lin-) followed by selection of cells with high aldehyde dehydrogenase (ALDH) activity. ALDHhiLin- cells contained 22.6% ± 3.0% of the Lin- population and highly coexpressed primitive HSC phenotypes (CD34+ CD38- and CD34+CD133+). In vitro hematopoietic progenitor function was enriched in the ALDHhiLin- population, compared with ALDHloLin- cells. Multilineage human hematopoietic repopulation was observed exclusively after transplantation of ALDHhiLin- cells. Direct comparison of repopulation with use of the nonobese diabetic/severe combined immunodeficient (NOD/SCID) and NOD/SCID β2 microglobulin (β2M) null models demonstrated that 10-fold greater numbers of ALDHhi-Lin- cells were needed to engraft the NOD/SCID mouse as compared with the more permissive NOD/SCID β2M null mouse, suggesting that the ALDHhiLin- population contained committed progenitors as well as primitive repopulating cells. Cell fractionation according to lineage depletion and ALDH activity provides a viable and prospective purification of HSCs on the basis of cell function rather than cell surface phenotype. (Blood. 2004;104:1648-1655)
Introduction
Conventionally, human hematopoietic stem cells (HSCs) have been purified on the basis of the expression of cell surface molecules such as CD34 and CD133.1-5 Cells expressing CD34 are capable of long-term hematopoietic reconstitution in immune-deficient mice and fetal sheep,1-3,6,7 and most clinical protocols involving gene transfer, purified stem cell transplantation, and stem cell expansion have been designed with the use of CD34+ populations.8-10 However, findings in a variety of mammalian systems suggest that the human hematopoietic stem cell compartment is heterogeneous, including an array of cell phenotypes that exhibit repopulating function.11-14 In fact, human CD34+ and CD34- populations possess repopulating ability, and CD34 expression is reversible in mouse and human cells.15-19 Although CD34 selection has proven extremely useful for the isolation and characterization of human cells with enhanced repopulating function, there are several limitations to purifying hematopoietic cells based solely on cell surface phenotype. First, cell surface markers can vary between species and stem cell source. Murine long-term repopulating cells have been purified to a single cell with the use of CD34-, c-kit+, Sca-1+, Lin- cell markers,11 whereas identification of a human single cell phenotype capable of repopulating murine or fetal sheep xenograft models remains elusive.1,12,14 Second, human stem cell phenotype can be altered by cell cycle progression,20,21 ex vivo culture,14,19,22 and transplantation in vivo,12,15,16 indicating a dissociation between hematopoietic cell phenotype and repopulating cell function. Third, the phenotype of transplanted stem cells may remain constant despite reduced functional activity. Cytokinemobilized peripheral blood cells demonstrate increased CD34 expression without an increase in nonobese diabetic/severe combined immunodeficiency (NOD/SCID) repopulating ability.23,24 Finally, purification of human stem cells on the basis of hematopoietic markers may select cells with restricted developmental potential and may exclude cells with alternate stem cell or progenitor functions. Thus, methods to efficiently isolate human stem cells without relying entirely on phenotypic cell surface molecules are desirable.
One promising strategy is HSC isolation according to a conserved stem cell function rather than phenotype. In the murine system, lymphohematopoietic stem cells have been isolated according to the high expression of the detoxifying enzyme aldehyde dehydrogenase (ALDH).25-27 Cytosolic ALDH is also expressed at high levels in human hematopoietic progenitors,28 and increased ALDH activity confers resistance of HSCs to alkylating agents such as cyclophosphamide.29-31 Storms et al32 described a fluorescent substrate of ALDH (termed Aldefluor) that can be used to isolate cells with increased ALDH activity by fluorescence-activated cell sorting (FACS). The substrate is an amino acetaldehyde molecule conjugated to a BODIPY (4,4-difluoro-5,7-dimethyl-4-bora-3a,4adiaza-5-proprionic acid) fluorochrome that is metabolized by ALDH to an aminoacetate anion, retained within the cell because of its negative charge. Thus, the amount of fluorescent product that accumulates in viable cells correlates to ALDH activity, and cells with high ALDH activity can be selected from human umbilical cord blood (UCB) or mobilized peripheral blood by FACS.32,33 UCB cells isolated by using this strategy have demonstrated to be depleted of lineage committed hematopoietic cells and are enriched for primitive hematopoietic progenitors detected in clonogenic in vitro cultures.32
With the use of a 2-step strategy consisting of the depletion of cells that express mature lineage markers (Lin-) followed by the selection of cells with high and low ALDH activity (ALDHhiLin- and ALDHloLin-), we isolated a functional population of hematopoietic stem and progenitor cells from human UCB. This ALDHhi-Lin- population contained 22.6% ± 3.0% (n = 9) of the total Lin- population or 0.09% ± 0.012% (n = 5) of the total mononuclear cell (MNC) population, demonstrating that ALDHhiLin- cells were rare within UCB. ALDHhiLin- cells highly expressed primitive cell surface markers (CD34+CD38- and CD34+CD133+) previously associated with enhanced repopulating function.1-3 ALDHhiLin- cells demonstrated enhanced in vitro clonogenic progenitor capacity similar to that of lineage-depleted CD34+CD38- cells.2 Transplantation of purified ALDHhiLin- cells into immune deficient NOD/SCID and NOD/SCID β2 microglobulin null mice resulted in multilineage human cell engraftment in hematopoietic tissues, whereas ALDHloLin- cells were nearly devoid of repopulating ability. These data indicate that primitive human hematopoietic stem and progenitor cells can be efficiently isolated from UCB by the depletion of mature cells followed by fractionation according to ALDH activity.
Materials and methods
Human cells
Human UCB samples were obtained from the cord blood banking facility at Cardinal Glennon Children's Hospital, St Louis, MO. Use of these samples was approved by the local ethical and biohazard authorities at Washington University Medical School, St Louis, MO. Samples were diluted (1:2) in phosphate-buffered saline, and MNCs were isolated by Hypaque-Ficoll centrifugation (Pharmacia Biotech, Uppsala, Sweden). UCB MNCs were depleted of contaminating erythrocytes by red cell lysis in 0.8% ammonium chloride solution (Stem Cell Technologies, Vancouver, BC, Canada).
Cell purification by lineage depletion and aldehyde dehydrogenase activity
UCB MNCs were enriched for lineage depleted (Lin-) cells isolated as described previously.19,23 Briefly, mature cells were depleted by using a mixture of monoclonal antibodies against the cell surface markers: CD2, CD3, CD14, CD16, CD19, CD24, CD41, CD56, CD66b, Glycophorin A (Stem Cell Technologies). Addition of a secondary antibody conjugated to metal colloid particles allowed the selection of unlabeled cells (Lin-) by elution through a magnetized column. Labeled cells (Lin+) were washed from the column after removal of the magnetic field and retained for use as irradiated (1500 cGy) accessory cells for transplantation of purified cell populations at low cell densities into NOD/SCID mice.34 The resulting Lin- population was further fractionated according to ALDH activity by staining with Aldefluor reagent (StemCo Biomedical, Durham, NC) according to the manufacturer's specifications. Aldefluor substrate (0.625 μg/mL) was added to 1 to 5 × 106 Lin- cells/mL suspended in proprietary Aldefluor assay buffer and incubated for 20 to 30 minutes at 37°C to allow the conversion of Aldefluor substrate to a fluorescent product, retained within the cell because of its negative charge.32,33 For each experiment, an aliquot of Aldefluor-stained cells was immediately quenched with 5 μL of 1.5 mM diethylaminobenzaldehyde (DEAB), a specific ALDH inhibitor to serve as a negative control. The amount of intracellular fluorescence was measured by flow cytometry, and ALDHhiLin- or ALDHloLin- cells were selected by FACS (MoFlo; Cytomation, Denver, CO).
Phenotypic analysis of ALDHhiLin- and ALDHloLin- cells
Aldefluor substrate-labeled Lin- cells were costained with antihuman antibodies for human CD34-PE (phycoerythrin)-Cy7 and human CD45-APC (allophycocyanin), with either human CD38-PE, human CD31-PE, human CD117 (Becton Dickinson, San Jose, CA), or CD133-PE (Miltenyi Biotechnology, Bergish, Gladbach, Germany) and analyzed on a Coulter FC-500 flow cytometer with RXP analysis software (Beckman-Coulter, Miami, FL).
Clonogenic progenitor assays
Human clonogenic progenitor assays were performed by plating purified populations of cells at concentrations ranging from 2 × 102 to 2 × 103 (ALDHhiLin-), 2 × 104 to 1 × 105 (ALDHloLin-), or 1 × 103 to 5 × 103 (Lin-) into methylcellulose media (Methocult H4434; Stem Cell Technologies) containing 50 ng/mL recombinant human (rH) stem cell factor, 10 ng/mL rH granulocyte-macrophage colony-stimulating factor, 10 ng/mL rH interleukin-3, and 2 U/mL rH erythropoietin added 4 days after the initiation of culture. Colonies were evaluated for morphologic characteristics and enumerated under light microscopy (Zeiss, Muenchen, Germany) following incubation at 37°C, 5% CO2, for 14 to 17 days.
Transplantation of purified cells into NOD/SCID and NOD/SCID β2M null mice
Cells were transplanted by tail vein injection into 8- to 10-week-old, sublethally irradiated (300 cGy) NOD/LtSz-scid/scid (NOD/SCID) or NOD/SCID β2 microglobulin (β2M) null mice (Jackson Laboratories, Bar Harbor, ME). Mice that received transplants with low numbers of purified cells were coinjected with 105 irradiated (1500 cGy) Lin+ as accessory cells.34 Peripheral blood was collected from anesthetized mice (isoflurane; Halocarbon Laboratories, River Edge, NJ) by retroorbital eye bleed. The mice were killed at 4 or at 7 to 8 weeks after transplantation in accordance to local animal welfare protocols, and bone marrow (BM), spleen, and peripheral blood were collected for analysis of human chimerism by flow cytometry.
Flow cytometric analysis of murine bone marrow
To prepare cells for flow cytometry, femurs were flushed, and spleens were dissociated into a single-cell suspension. Contaminating red cells were lysed with an 0.8% ammonium chloride solution, and the remaining cells were resuspended in phosphate-buffered saline (PBS) with 5% fetal bovine serum. Samples were filtered (70 μm) to remove cellular debris, and approximately 0.5 to 1 × 106 cells were incubated for 30 minutes at 4°C with blocking solution and monoclonal antibodies for human pan-leukocyte specific marker anti-CD45-APC in combination with human anti-CD38-PE or isotype controls (Becton Dickinson). BM, spleen, and peripheral blood cells were analyzed by flow cytometry on a Coulter FC-500 flow cytometer with RXP analysis software (Beckman-Coulter). Low frequency (< 0.2% CD45+) of human engraftment in murine BM was confirmed by human Alu element-specific polymerase chain reaction (PCR).35 Analysis of multilineage engraftment was performed on BM from NOD/SCID or NOD/SCID β2M null mice that demonstrated a high level of human chimerism (5%-14% CD45+). Briefly, 1 × 105 to 2 × 105 cells were gated for human cells (anti-CD45-APC), and CD45+ cells were analyzed for B-lymphoid cells (anti-CD19-PE), myeloid cells (anti-CD33-PE, anti-CD15-FITC [fluorescein isothiocyanate]), T-lymphoid cells (anti-CD8-PE, anti-CD4-FITC), and primitive progenitor cells (anti-CD38-PE, anti-CD34-FITC, anti-CD133-PE; all antibodies from BD, except for anti-CD133-PE from Miltenyi Biotechnology).
Statistics
Levels of human engraftment were reported as the mean ± SEM for mice grouped according to transplanted cell numbers. A mouse was scored as positive (engrafted) when human CD45 and human CD38 were coexpressed on more than 0.1% of the total cells isolated. These data from several transplanted doses were grouped and analyzed to directly compare human repopulation data between the NOD/SCID and the more permissive NOD/SCID β2M null model. Statistical significance for colony-forming unit (CFU) data and the expression of cell surface markers were assessed by Student t test.
Results
Isolation of ALDHhiLin- and ALDHloLin- populations
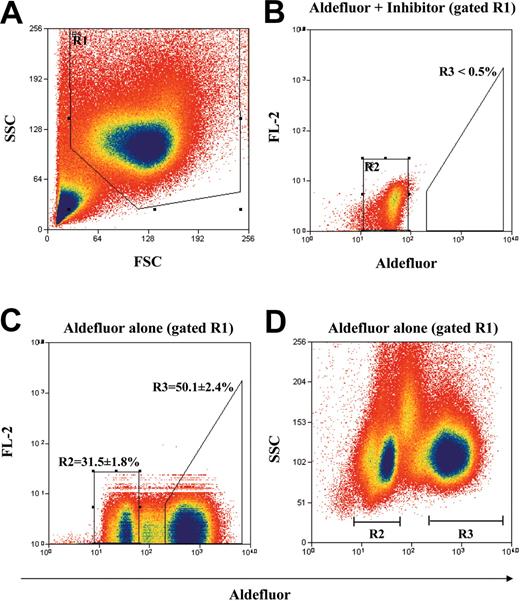
MNCs from human UCB were enriched for cells that do not express lineage-specific antigens by immunomagnetic depletion with a mixture of monoclonal antibodies directed against several lymphoid, myeloid, erythroid, and platelet-specific antigens.1 Figure 1 shows our strategy for the purification of the Lin- population according to ALDH activity by using the Aldefluor substrate and FACS. First, the Lin- mononuclear cell population was selected according to forward scatter (FSC) and side scatter (SSC) properties in R1 (Figure 1A) to reduce residual platelet, red cell, and granulocyte contamination. Aldefluor substrate was coincubated with DEAB, a specific inhibitor of ALDH according to manufacturer's instructions, and this sample was used to set the R2 gate (Figure 1B) representing cells with baseline cytosolic ALDH activity.32 With the use of this gating strategy, less than 0.5% of the total events fall within the ALDHhi region R3 in the presence of inhibitor. Incubation of the Lin- cells without inhibitor resulted in a right shift in fluorescence, defining the sorted ALDHhiLin- population in R3 (50.1% ± 2.4% of R1 cells) and the ALDHloLin- population in R2 (31.5% ± 1.8% of R1 cells; Figure 1C). Furthermore, the ALDHhi and ALDHlo selected populations represented 22.6% ± 3.0% and 16.1% ± 2.6% of the ungated Lin- population, respectively. The selected ALDHhiLin- and ALDHloLin- cells demonstrated equal SSC properties (Figure 1D) as predicted for nongranular primitive hematopoietic cells, whereas cells with intermediate fluorescence showed more variable side scatter properties representative of contaminating monocytes within the Lin- population.32 Cells with intermediate fluorescence were minimized in the sorted populations by using our stringent selection criteria. ALDHhiLin- and ALDHloLin- populations were reanalyzed after sorting and demonstrated more than 96% and more than 99% purity, respectively (data not shown). These sorted populations along with unsorted Lin- cells were assayed for in vitro progenitor function or transplanted into sublethally irradiated NOD/SCID or NOD/SCID β2M null mice to characterize in vivo repopulating function.
Identification and isolation of purified ALDHhiLin- and ALDHloLin- cell populations. Representative flow cytometric analysis of ALDH activity on lineage-depleted (Lin-) UCB cells. (A) Human UCB Lin- cells were selected according to forward scatter (FSC) and side scatter (SSC) properties using the gated region R1 to remove residual platelet and red cell contamination. (B) Lin- cells incubated with Aldefluor substrate and the specific inhibitor of ALDH, diethylaminobenzaldehyde (DEAB), were used to establish baseline fluorescence of these cells and to define the ALDHhi region (R3) as less than 0.5% of total events. (C) Incubation of Lin- cells with Aldefluor substrate in the absence of inhibitor induced a shift in FL1 fluorescence defining the ALDHlo (R2 = 31.5% ± 1.8%) and ALDHhi (R3 = 50.1% ± 2.4%) purified populations selected on the basis of intracellular ALDH conversion of Aldefluor substrate. (D) Lin- cells incubated with Aldefluor substrate were reanalyzed by using FL1 fluorescence versus SSC to ensure that mononuclear cells with equivalent side scatter properties were selected by sorting. These data represent the mean ± SEM on experiments performed with Lin- cells isolated from UCB samples from 9 different donors (n = 9).
Identification and isolation of purified ALDHhiLin- and ALDHloLin- cell populations. Representative flow cytometric analysis of ALDH activity on lineage-depleted (Lin-) UCB cells. (A) Human UCB Lin- cells were selected according to forward scatter (FSC) and side scatter (SSC) properties using the gated region R1 to remove residual platelet and red cell contamination. (B) Lin- cells incubated with Aldefluor substrate and the specific inhibitor of ALDH, diethylaminobenzaldehyde (DEAB), were used to establish baseline fluorescence of these cells and to define the ALDHhi region (R3) as less than 0.5% of total events. (C) Incubation of Lin- cells with Aldefluor substrate in the absence of inhibitor induced a shift in FL1 fluorescence defining the ALDHlo (R2 = 31.5% ± 1.8%) and ALDHhi (R3 = 50.1% ± 2.4%) purified populations selected on the basis of intracellular ALDH conversion of Aldefluor substrate. (D) Lin- cells incubated with Aldefluor substrate were reanalyzed by using FL1 fluorescence versus SSC to ensure that mononuclear cells with equivalent side scatter properties were selected by sorting. These data represent the mean ± SEM on experiments performed with Lin- cells isolated from UCB samples from 9 different donors (n = 9).
Phenotypic characterization of ALDHhiLin- and ALDHloLin- cells
To predict the repopulating function of cells sorted according to ALDH activity, ALDHhi and ALDHlo populations from lineage depleted UCB were costained for cell surface markers previously associated with hematopoietic repopulating function. Storms et al32 demonstrated that with the exception of some contaminating B cells the SSCloALDHbright population was devoid of cell surface markers for mature T cells, natural killer cells, myeloid cells, erythroid cells, and platelets. We hypothesized that lineage depletion prior to sorting would provide a more purified starting population by removal of most B cells and monocytes that contaminate the ALDHhi population. Lineage depletion resulted in a 443- ± 58-fold reduction in total nucleated cells (n = 5) and achieved nearly complete depletion of mature B cells (< 0.1% CD19+), T cells (< 0.2% CD3+), monocytes (< 0.2% CD14+), and natural killer cells (< 0.1% CD56+) prior to selection by ALDH activity.
Expression of stem cell surface molecules such as CD34 and CD133 are commonly used as markers to isolate primitive cells with in vitro progenitor and in vivo repopulating function. Expression of these molecules diminish with maturation and differentiation.1 Therefore, the ALDHhiLin- and ALDHloLin- populations were analyzed for the presence of primitive (CD34, CD133, CD117), hematopoietic (CD45, CD38), and putative hematopoietic or endothelial cell (CD133, CD31) markers. CD34+ and CD133+ cell populations contained 59.5% ± 6.4% and 34.6% ± 5.5% of the Lin- population, respectively (Table 1; n = 9). The ALDHhi-Lin- population was even further enriched for CD34 expression, with 91.0% ± 2.9% of the ALDHhiLin- cells positive for CD34. In contrast, only 37.8% ± 7.2% of the ALDHloLin- cells expressed CD34 (P < .001; Table 1). The pentaspan cell surface antigen CD133, previously shown to be an important molecule expressed on hematopoietic precursors from human UCB2,3 and on circulating UCB cells with endothelial cell capacity,36 was expressed on 70.9% ± 4.2% of ALDHhiLin- cells, as compared with 11.5% ± 4.5% of the ALDHloLin- cells (P < .001). In addition, the ALDHhiLin- population highly expressed the pan leukocyte marker CD45 (98.6% ± 0.7% CD45+), platelet-derived endothelial cell adhesion molecule-1 or CD31 (PECAM-1; 97.9% ± 0.8% CD31+), and the stem cell factor receptor c-kit or CD117 (83.1% ± 2.7% CD117+; Table 1). Thus, the ALDHhiLin- population contained a high frequency of cells that express CD34, CD133, and CD31, suggesting these primitive cells might possess hematopoietic, endothelial, or both progenitor characteristics.36
Analysis of ALDHhiLin− and ALDHloLin− cells for expression of primitive cell surface markers
. | Frequency of expression, % . | . | . | . | ||
|---|---|---|---|---|---|---|
| Cell type . | Lin− . | ALDHloLin− . | ALDHhiLin− . | P . | ||
| CD34 | 59.5 ± 6.4 | 37.8 ± 7.2 | 91.0 ± 2.9 | < .001 | ||
| CD133 | 34.6 ± 5.5 | 11.5 ± 4.5 | 72.9 ± 4.2 | < .001 | ||
| CD31 | 86.4 ± 2.8 | 66.7 ± 6.4 | 97.9 ± 0.8 | .001 | ||
| CD117 | 61.2 ± 7.0 | 41.5 ± 7.1 | 83.1 ± 2.7 | < .001 | ||
| CD34+CD38− | 13.8 ± 1.7 | 5.0 ± 1.3 | 25.5 ± 3.7 | .002 | ||
| CD34+CD133+ | 41.1 ± 6.0 | 12.0 ± 3.1 | 71.1 ± 4.6 | < .001 | ||
. | Frequency of expression, % . | . | . | . | ||
|---|---|---|---|---|---|---|
| Cell type . | Lin− . | ALDHloLin− . | ALDHhiLin− . | P . | ||
| CD34 | 59.5 ± 6.4 | 37.8 ± 7.2 | 91.0 ± 2.9 | < .001 | ||
| CD133 | 34.6 ± 5.5 | 11.5 ± 4.5 | 72.9 ± 4.2 | < .001 | ||
| CD31 | 86.4 ± 2.8 | 66.7 ± 6.4 | 97.9 ± 0.8 | .001 | ||
| CD117 | 61.2 ± 7.0 | 41.5 ± 7.1 | 83.1 ± 2.7 | < .001 | ||
| CD34+CD38− | 13.8 ± 1.7 | 5.0 ± 1.3 | 25.5 ± 3.7 | .002 | ||
| CD34+CD133+ | 41.1 ± 6.0 | 12.0 ± 3.1 | 71.1 ± 4.6 | < .001 | ||
Lineage-depleted human UCB MNCs were costained with Aldefluor and antibodies to various hematopoietic and primitive cell surface markers. The expression of each cell surface marker was enumerated and compared between the Lin−, ALDHhiLin−, and ALDHloLin− populations as outlined in “Materials and methods.” The ALDHhiLin− cells demonstrated increased expression of CD45, CD34, CD133, CD31 (PECAM-1), and CD117 (c-kit) cell surface molecules in comparison to the ALDHloLin− cells. The ALDHhiLin− cells were also enriched for CD34+ CD38− and CD34+CD133+ -expressing cells. Data represent mean ± SEM of 6 to 9 UCB samples, and P values were determined by Student t test comparing ALDHloLin− and ALDHhiLin− populations.
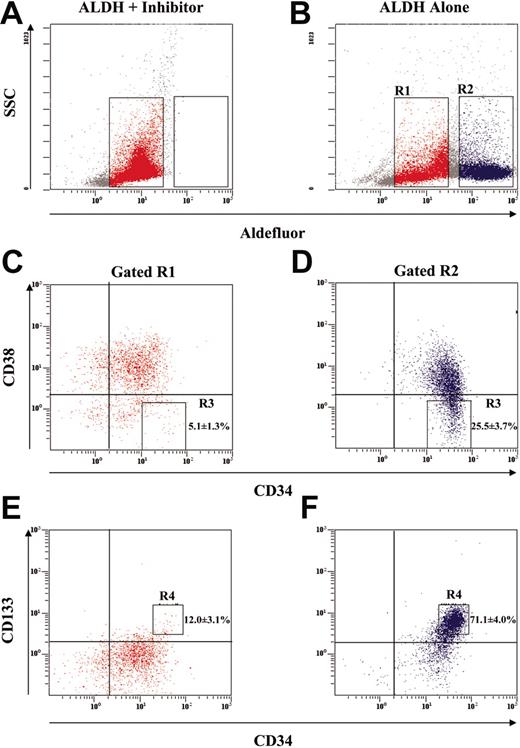
The most potent repopulating purified human cell phenotype reported to date is the CD34+CD38-Lin- cell.1 Flow cytometric analysis for CD34 expression with the absence of CD38 expression in cells purified according to on ALDH activity is shown in Figure 2. First, ALDHloLin- and ALDHhiLin- populations were selected according to Aldefluor fluorescence (Figure 2A-B). Aldefluor- and DEAB-treated cells were used to define gates R1 (red dots) and R2 (blue dots). Aldefluor staining without DEAB induced a shift in fluorescence from the ALDHhiLin- population previously defined (Figure 1). These gated populations were subsequently analyzed for CD34 and CD38 expression. As indicated (Figure 2C-D) 25.5% ± 3.7% of cells within the ALDHhiLin- population demonstrated CD34+CD38- expression, compared with only 5.1% ± 1.3% for the ALDHloLin- population (P < .005). Unselected Lin- cells included 13.8% ± 1.7% CD34+CD38- cells (P < .05). These data indicate that the ALDHhiLin- population consists of cells with phenotypic markers predicting high NOD/SCID repopulating ability in vivo.
Phenotypic analysis of purified ALDHhiLin- and ALDHloLin- populations. Human UCB Lin- cells were analyzed by flow cytometry for ALDH expression in combination with the expression of primitive hematopoietic markers. (A-B) Lin- cells were incubated with Aldefluor and an inhibitor of ALDH (DEAB) or with Aldefluor alone to establish R1 and R2 gates for ALDHloLin- and ALDHhiLin- cells, respectively. (C-D) ALDHhiLin- and ALDHloLin- populations were subsequently analyzed for the expression of CD34 with CD38. The frequency of CD34+CD38- cells (R3) was greater in the ALDHhiLin- cells than in the ALDHloLin- cells (P < .05). (E-F) ALDHhiLin- and ALDHloLin- cells were also analyzed for the coexpression of CD34 with CD133. The frequency of CD34+CD133+ cells (R4) was increased in the ALDHhiLin- cells compared with the ALDHloLin- cells (P < .001). These data represent the mean ± SEM on experiments performed with Lin- cells isolated from 6 UCB donors (n = 6). Numbers represent the frequency of events in the boxes.
Phenotypic analysis of purified ALDHhiLin- and ALDHloLin- populations. Human UCB Lin- cells were analyzed by flow cytometry for ALDH expression in combination with the expression of primitive hematopoietic markers. (A-B) Lin- cells were incubated with Aldefluor and an inhibitor of ALDH (DEAB) or with Aldefluor alone to establish R1 and R2 gates for ALDHloLin- and ALDHhiLin- cells, respectively. (C-D) ALDHhiLin- and ALDHloLin- populations were subsequently analyzed for the expression of CD34 with CD38. The frequency of CD34+CD38- cells (R3) was greater in the ALDHhiLin- cells than in the ALDHloLin- cells (P < .05). (E-F) ALDHhiLin- and ALDHloLin- cells were also analyzed for the coexpression of CD34 with CD133. The frequency of CD34+CD133+ cells (R4) was increased in the ALDHhiLin- cells compared with the ALDHloLin- cells (P < .001). These data represent the mean ± SEM on experiments performed with Lin- cells isolated from 6 UCB donors (n = 6). Numbers represent the frequency of events in the boxes.
Although the ligand and specific function of CD133 in stem cells are undefined at present, CD133 is a cell surface molecule associated with progenitor function in multiple cell types, including hematopoietic, endothelial, and neural tissues.2,7,36,37 In the hematopoietic system CD133+ hematopoietic progenitors from UCB highly coexpress CD34; therefore, an identical strategy was used to analyze ALDHhiLin- and ALDHloLin- populations for cells that coexpress CD34 and CD133. Unselected Lin- cells consisted of 41.1% ± 6.0% CD34+CD133+ cells, further demonstrating that this starting population was enriched for hematopoietic progenitors. After separating the Lin- population into ALDHhi and ALDHlo expressing groups, 72.7% ± 4.0% of ALDHhiLin- coexpressed CD34 and CD133, whereas only 12.0% ± 3.1% of the ALDHloLin- population were CD34+CD133+ (P < .001; Figure 2E-F). These data confirm that ALDHhiLin- cells highly express markers associated with stem and progenitor cell function.
Human ALDHhiLin- cells are enriched for hematopoietic progenitors. On the basis of cell surface phenotype analysis, we predicted that the ALDHhiLin- population would be highly enriched for cells with in vitro CFU ability and for cells with NOD/SCID repopulating capacity. Table 2 illustrates the hematopoietic CFU potential of Lin-, ALDHhiLin-, and ALDHloLin- cells isolated as described in Figure 1. Lineage-depleted human UCB cells demonstrated progenitor activity, producing erythroid, granulocyte or macrophage, and mixed colonies at a plating efficiency of 1 in 17 cells. ALDHhiLin- cells demonstrated significantly enhanced colony formation, yielding 248 colonies per 1000 cells plated, correlating to a plating efficiency of approximately 1 in 4 cells (Table 2). These colony-forming characteristics are equivalent to purified fractions of Lin- cells selected by CD34 expression.2 In contrast, the ALDHloLin- cells were nearly devoid of progenitor activity despite the presence of 37.8% ± 7.2% CD34-expressing cells (Table 1), with very few colonies produced at plating densities of up to 5 × 104 purified cells.
Human ALDHhiLin− cells are enriched for hematopoietic progenitors
. | No. of colonies per 103 cells . | . | . | . | Plating efficiency . | |||
|---|---|---|---|---|---|---|---|---|
| Cell population . | BFU-E . | CFU-MIX . | CFU-GM . | Total . | . | |||
| Lin− | 27.6 ± 5.4 | 2.4 ± 0.7 | 43.4 ± 5.3 | 73.4 ± 9.3 | 1 in 16.8 | |||
| ALDHloLin− | 0.2 ± 0.05 | 0.0 ± 0.0 | 0.3 ± 0.1 | 0.5 ± 0.1 | 1 in 3162 | |||
| ALDHhiLin− | 107.4 ± 10.1 | 10.7 ± 1.4 | 130.1 ± 14.8 | 248.3 ± 20.3 | 1 in 4.4 | |||
. | No. of colonies per 103 cells . | . | . | . | Plating efficiency . | |||
|---|---|---|---|---|---|---|---|---|
| Cell population . | BFU-E . | CFU-MIX . | CFU-GM . | Total . | . | |||
| Lin− | 27.6 ± 5.4 | 2.4 ± 0.7 | 43.4 ± 5.3 | 73.4 ± 9.3 | 1 in 16.8 | |||
| ALDHloLin− | 0.2 ± 0.05 | 0.0 ± 0.0 | 0.3 ± 0.1 | 0.5 ± 0.1 | 1 in 3162 | |||
| ALDHhiLin− | 107.4 ± 10.1 | 10.7 ± 1.4 | 130.1 ± 14.8 | 248.3 ± 20.3 | 1 in 4.4 | |||
Lineage depleted and purified ALDHhiLin− or ALDHloLin− cells were isolated and cultured in semisolid methylcellulose media (Methocult H4434) as described in “Materials and methods.” Human progenitor colonies of erythrocyte, granulocyte/macrophage and mixed lineages (BFU-E [erythroid burst-forming unit], CFU-GM [granulocyte-macrophage colony-forming unit], and CFU-MIX[mixed colony-forming unit]) were enumerated after 14 to 17 days of in vitro culture. Data represent the number of individual colonies produced per 1000 cells plated for doses ranging from 2 × 102 to 1 × 103 for ALDHhiLin− cells, 5 × 104 to 2 × 105 for ALDHloLin− cells, and 1 × 103 to 5 × 103 Lin− cells. Data are expressed as mean ± SEM for cells isolated from 4 to 5 fresh UCB samples (n = 4-5).
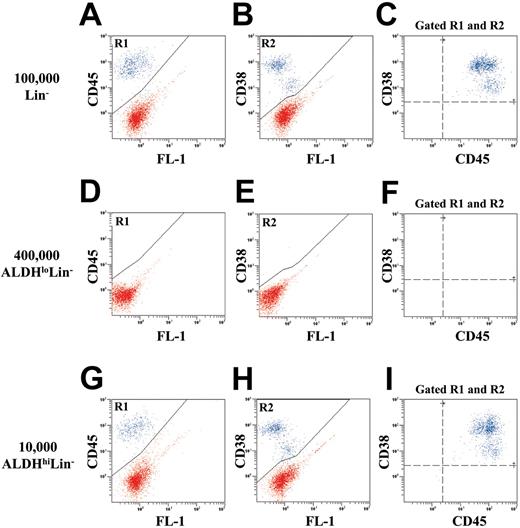
Human ALDHhiLin- cells are enriched for NOD/SCID β2M null and NOD/SCID repopulating cells. To test for in vivo repopulating function, we transplanted purified human Lin-, ALDHhiLin-, and ALDHloLin- cells into NOD/SCID β2M null and NOD/SCID mice. In addition, sublethally irradiated mice received transplants of 105 irradiated (1500 cGy) Lin+ cells as accessory cells to support the initial engraftment of low numbers of highly purified human hematopoietic cell populations.34 These irradiated accessory cells were mature and possessed no repopulating ability when transplanted alone (data not shown). Figure 3 shows a representative example of human cell engraftment in the BM of NOD/SCID β2M null mice 7 to 8 weeks after transplantation with unsorted Lin- (Figure 3A-C), ALDHloLin- (Figure 3D-F), or ALDHhiLin- cells (Figure 3G-I). Spleen and peripheral blood samples from each mouse were analyzed as described for BM (data not shown). Human hematopoietic cells were detected by the coexpression of human CD45 (R1) and human CD38 (R2; blue dots). Transplantation of 1 × 105 Lin- cells produced 15.3% ± 4.4% human engraftment (n = 4). Transplantation of 1 × 104 ALDHhiLin- cells resulted in similar levels of human engraftment (10.2% ± 4.8%, n = 4) in the mouse BM (P = .43). In contrast, transplantation of 40-fold more (4 × 105) ALDHloLin- cells produced no detectable human engraftment in the hematopoietic tissues analyzed (n = 4).
Flow cytometric detection of human cells in the bone marrow of NOD/SCID β2M null mice that received transplants with Lin-, ALDHloLin-, or ALDHhiLin- cells. Representative flow cytometric analysis of NOD/SCID β2M null mice that received transplants with (A-C) 1 × 105 Lin-, (D-F) 4 × 105 ALDHloLin-,or (G-I) 1 × 104 ALDHhiLin- cells. Mouse BM, spleen, and peripheral blood were extracted 7 to 8 weeks after transplantation, and human cells were detected by coexpression of the pan-leukocyte marker CD45 (R1) with CD38 (R2) in mice injected with ALDHhiLin- cells. Representative mice showed 18.0% human CD45+CD38+ cells after the transplantation of 105 Lin- cells and 16.5% human CD45+CD38+ cells after the transplantation of 104 ALDHhiLin- cells. Human cells were not detected in mice that received transplants with 4 × 105 ALDHloLin- cells.
Flow cytometric detection of human cells in the bone marrow of NOD/SCID β2M null mice that received transplants with Lin-, ALDHloLin-, or ALDHhiLin- cells. Representative flow cytometric analysis of NOD/SCID β2M null mice that received transplants with (A-C) 1 × 105 Lin-, (D-F) 4 × 105 ALDHloLin-,or (G-I) 1 × 104 ALDHhiLin- cells. Mouse BM, spleen, and peripheral blood were extracted 7 to 8 weeks after transplantation, and human cells were detected by coexpression of the pan-leukocyte marker CD45 (R1) with CD38 (R2) in mice injected with ALDHhiLin- cells. Representative mice showed 18.0% human CD45+CD38+ cells after the transplantation of 105 Lin- cells and 16.5% human CD45+CD38+ cells after the transplantation of 104 ALDHhiLin- cells. Human cells were not detected in mice that received transplants with 4 × 105 ALDHloLin- cells.
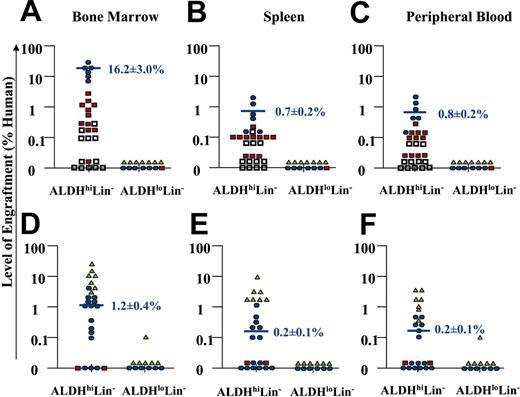
The majority of mice that received transplants (36 of 42 NOD/SCID, or 43 of 56 NOD/SCID β2M null mice) were analyzed for human engraftment between 7 to 8 weeks after transplantation. However, 6 NOD/SCID mice and 13 NOD/SCID β2M null mice were processed at 4 weeks in our initial experiments to screen for human engraftment. From the NOD/SCID β2M null cohort killed at 4 weeks, 6 of 6 mice that received transplants with 104 to 105 ALDHhiLin- cells showed human engraftment, whereas 0 of 7 mice that received transplants of 104 to 105 ALDHloLin- cells did not engraft. The mice killed at 4 weeks showed significantly decreased (P < .05) mean engraftment (4.9% ± 1.0%, n = 6) compared with mice that received transplants with equivalent cell doses and killed at 7 to 8 weeks after transplantation (16.2% ± 3.0%, n = 6) as described previously.38 Thus, only the mice killed at 7 to 8 weeks after transplantation were pooled to summarize the engraftment by ALDHhiLin- and ALDHloLin- cells transplanted into NOD/SCID β2M null and NOD/SCID mice as shown in Figure 4.
Summary of human cell repopulation in the BM, spleen, and peripheral blood of NOD/SCID and NOD/SCID β2M null mice that received transplants with ALDHhiLin- and ALDHloLin- cells. A summary of the level of human engraftment in the BM, spleen, and peripheral blood of NOD/SCID β2M null mice (n = 43) (A-C) or NOD/SCID mice (n = 36) (D-F) that received transplants with purified ALDHhiLin- and ALDHloLin- cells is shown. Mouse BM, spleen, and peripheral blood were extracted 7 to 8 weeks after transplantation, and human cells were detected by coexpression of the pan-leukocyte marker CD45 with CD38. Open squares represent mice injected with 2 × 102 to 1 × 103 purified cells, red squares represent mice injected with 2 × 103 to 1 × 104 purified cells, blue circles represent mice injected with 2 × 104 to 1 × 105 purified cells, and green triangles represent mice injected with 2 × 105 to 4 × 105 purified cells. Horizontal bars represent the average level of human engraftment (mean ± SEM) detected in the tissues of each mouse corresponding to the 2 × 104 to 105 dose range in each mouse strain. Human SCID-repopulating cells were observed exclusively within the ALDHhiLin- fraction. Human cell engraftment was consistently achieved with as few as 500 ALDHhiLin- cells in the NOD/SCID β2M null mouse, whereas more than 104 ALDHhiLin- cells were needed to engraft the parental NOD/SCID strain. Mice received transplants with the cells from 18 UCB samples. Purified cells from 2 UCB samples were pooled to achieve doses of 4 × 105 ALDHloLin- cells.
Summary of human cell repopulation in the BM, spleen, and peripheral blood of NOD/SCID and NOD/SCID β2M null mice that received transplants with ALDHhiLin- and ALDHloLin- cells. A summary of the level of human engraftment in the BM, spleen, and peripheral blood of NOD/SCID β2M null mice (n = 43) (A-C) or NOD/SCID mice (n = 36) (D-F) that received transplants with purified ALDHhiLin- and ALDHloLin- cells is shown. Mouse BM, spleen, and peripheral blood were extracted 7 to 8 weeks after transplantation, and human cells were detected by coexpression of the pan-leukocyte marker CD45 with CD38. Open squares represent mice injected with 2 × 102 to 1 × 103 purified cells, red squares represent mice injected with 2 × 103 to 1 × 104 purified cells, blue circles represent mice injected with 2 × 104 to 1 × 105 purified cells, and green triangles represent mice injected with 2 × 105 to 4 × 105 purified cells. Horizontal bars represent the average level of human engraftment (mean ± SEM) detected in the tissues of each mouse corresponding to the 2 × 104 to 105 dose range in each mouse strain. Human SCID-repopulating cells were observed exclusively within the ALDHhiLin- fraction. Human cell engraftment was consistently achieved with as few as 500 ALDHhiLin- cells in the NOD/SCID β2M null mouse, whereas more than 104 ALDHhiLin- cells were needed to engraft the parental NOD/SCID strain. Mice received transplants with the cells from 18 UCB samples. Purified cells from 2 UCB samples were pooled to achieve doses of 4 × 105 ALDHloLin- cells.
Transplanted cells were divided into 4 dose ranges; the lowest dose from 2 × 102 to 103 cells is represented by open squares, intermediate doses from 2 × 103 to 104 cells (red squares) or 104 to 105 cells (blue circles), and the highest dose from 2 × 105 to 4 × 105 cells is represented by green triangles. Consistent human engraftment was achieved in the BM of 10 of 11 NOD/SCID β2M null mice that received transplants with 2 × 103 to 104 ALDHhi-Lin- cells, for an average level of engraftment of 0.8% ± 0.3% human cells (Figure 4A). Transplantation of 2 × 104 to 105 ALDHhiLin- cells produced consistent engraftment in the BM of all mice that received transplants, with an average level of engraftment of 16.2% ± 3.0% human hematopoietic cells (Figure 4A). In this cohort, human cells were also consistently detected in the spleen (0.7% ± 0.2%) and peripheral blood (0.8% ± 0.2%) of mice (Figure 4B-C). For comparison, transplantation of 105 unsorted Lin- cells produced similar levels of engraftment in the BM (12.3% ± 5.8%), spleen (2.0% ± 1.2%), and peripheral blood (1.4% ± 0.3%) of NOD/SCID β2M null mice (n = 4). Human engraftment was also observed in 2 NOD/SCID β2M null mice that received transplants with 500 ALDHhiLin- cells. In contrast, transplantation of up to 4 × 105 ALDHloLin- cells produced no detectable human engraftment in any of the hematopoietic tissues, 7 to 8 weeks after transplantation in 14 mice (Figure 4A-C). Therefore, ALDHloLin- cells were unable to repopulate the NOD/SCID β2M null mouse and did not possess significant progenitor function in vitro. These data suggest that nearly all of the progenitor function and NOD/SCID β2M repopulating ability contained within the Lin- population was associated with cells having high ALDH activity, despite the presence of CD34+ cells and CD34+CD38- cells within the ALDHloLin- population.
The NOD/SCID β2M null model is comparatively more permissive to human cell repopulation than the NOD/SCID model because of reduced natural killer (NK) cell function conferred by the additional mutation in β2-microglobulin.39 NOD/SCID β2M null mice have been previously shown to engraft distinct human progenitor populations of short-term (myeloid) and long-term (lymphoid or myeloid) repopulating hematopoietic cells, neither of which efficiently engraft the NOD/SCID mouse.40 Therefore, a similar analysis was performed after the transplantation of ALDHhi-Lin- and ALDHloLin- populations into conventional NOD/SCID mice to determine whether the ALDHhiLin- population contained cells that were primarily committed progenitors with short-term repopulating ability or cells with unrestricted NOD/SCID repopulating potential (Figure 4). Consistent with previous results the ALDHloLin- population showed little repopulating ability in the NOD/SCID mouse with only 1 of 12 mice engrafting the BM after transplantation of up to 4 × 105 ALDHloLin- cells (Figure 4D). However, human UCB ALDHhiLin- cells demonstrated human cell repopulation in the BM of 12 of 15 mice NOD/SCID mice that received transplants with 2 × 104 to 105 (mean engraftment of 1.2% ± 0.4% human cells, 12 of 15 mice), or 2 × 105 to 4 × 105 cells (mean engraftment of 14.6% ± 4.6% human cells, 7 of 7 mice). A lower frequency of human hematopoietic cells was detected in the spleen (Figure 4E) and peripheral blood (Figure 4F) of most of these mice. In comparison to transplantations performed in the NOD/SCID β2M null mouse, 10-fold increased cell doses were needed by using the NOD/SCID mouse as a host to achieve a similar level and frequency of engraftment (Figure 4A-F). Taken together, these data indicate that human UCB ALDHhiLin- cells represent a heterogeneous mixture of cells with varying progenitor and long-term repopulating function. The ALDHhiLin- population is enriched for committed progenitors with in vitro CFU function and efficient repopulating ability in NOD/SCID β2M null mice. In addition, this population includes more primitive repopulating cells capable of engrafting the NOD/SCID mouse.
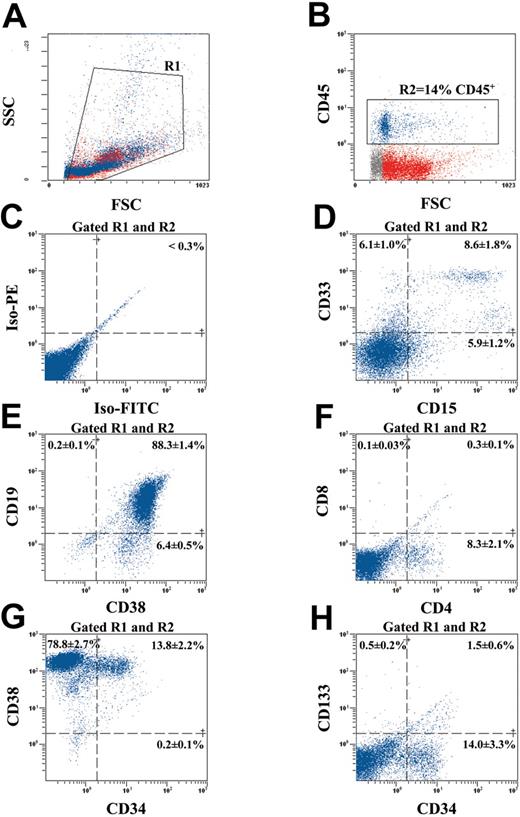
Human ALDHhiLin- cells differentiate into multiple hematopoietic lineages in vivo. To examine the differentiative and proliferative capacity of human ALDHhiLin- cells after transplantation into the NOD/SCID β2M null mouse, we analyzed engrafted mouse BM for the acquisition of lineage restricted, mature cell surface markers, for the retention of primitive cell surface phenotype, or both after 8 weeks repopulation in vivo (Figure 5). Human hematopoietic cells in the BM of mice that received transplants were selected by forward and side scatter (R1; Figure 5A) and gated for the expression of the human-specific pan leukocyte marker CD45 (R2; Figure 5B). The representative mouse BM shown in Figure 5 was engrafted at 14% CD45+ cells, and the gated human cells demonstrated low levels of nonspecific fluorescence (Figure 5C). Without accounting for engraftment to other bones, the number of human cells harvested represents at least a 50-fold expansion in the number of human cells transplanted, indicating that ALDHhiLin- cells demonstrate extensive proliferative capacity after 8 weeks in vivo.
Multilineage differentiation of human ALDHhiLin- cells in NOD/SCID β2M null mice. BM from a highly engrafted mouse that received transplants with 104 ALDHhiLin- cells was stained with human-specific antibodies for markers expressed on mature hematopoietic lineages and on primitive progenitor cells. (A) Mononuclear cells were first selected (gated R1) according to forward and side scatter properties. (B) Human hematopoietic cells were selected by the expression of CD45 (gated R2) and analyzed for background staining (C), mature myeloid cell markers CD33 and CD15 (D), mature B-cell markers CD19 and CD38 (E), and mature T-cell markers CD4 and CD8 (E). CD45+ human hematopoietic cells were also analyzed for the maintenance of human progenitors by CD34 and CD38 expression (G) and primitive repopulating cells by CD34 and CD133 (H) coexpression. Data represent the mean ± SEM expression of various markers on the CD45+ human cells derived from the BM of 5 human engrafted NOS/SCID β2M null mice (n = 5).
Multilineage differentiation of human ALDHhiLin- cells in NOD/SCID β2M null mice. BM from a highly engrafted mouse that received transplants with 104 ALDHhiLin- cells was stained with human-specific antibodies for markers expressed on mature hematopoietic lineages and on primitive progenitor cells. (A) Mononuclear cells were first selected (gated R1) according to forward and side scatter properties. (B) Human hematopoietic cells were selected by the expression of CD45 (gated R2) and analyzed for background staining (C), mature myeloid cell markers CD33 and CD15 (D), mature B-cell markers CD19 and CD38 (E), and mature T-cell markers CD4 and CD8 (E). CD45+ human hematopoietic cells were also analyzed for the maintenance of human progenitors by CD34 and CD38 expression (G) and primitive repopulating cells by CD34 and CD133 (H) coexpression. Data represent the mean ± SEM expression of various markers on the CD45+ human cells derived from the BM of 5 human engrafted NOS/SCID β2M null mice (n = 5).
Gated human cells also demonstrated the presence of mature myeloid cells by CD33 expression and granulocytes by CD15 expression (Figure 5D). Mature B-lymphoid cells were detected by the expression of the mature pan B-cell marker CD19 that was highly coexpressed with the differentiation marker CD38 (Figure 5E). Murine BM was almost devoid of CD8 expression and only expressed a small amount of CD4 protein (8.3% ± 2.1%; Figure 5F) because of the poor capacity of the NOD/SCID strains to support complete human T-cell maturation in vivo.39 Human engraftment in NOD/SCID BM (n = 2) demonstrated identical surface molecule expression pattern (data not shown). The presence of multiple lineages of myeloid and lymphoid cells in the engrafted mice indicates that ALDHhiLin- cells possess extensive differentiative capacity in vivo. Analysis of primitive cell surface markers on transplanted human cells engrafting the murine BM demonstrated the retention of a significant number of cells with a primitive phenotype (13.8% ± 2.2% CD34+CD38+; Figure 5G). Primitive repopulating CD34+CD38- cells were detected but rare (0.2% ± 0.1%) after 8 weeks in vivo. In addition, CD34+ human cells isolated from the chimeric BM did not significantly coexpress CD133 (1.5% ± 0.6% CD34+CD133+; Figure 5H) even though ALDHhiLin- cells were highly enriched for CD34 and CD133 coexpression at the time of transplantation (72.7% ± 4.0% CD34+CD133+; Figure 2F). Similar engraftment patterns were observed after the transplantation of de novo isolated or ex vivo cultured human CD34+ cells from UCB and mobilized peripheral blood.1,14,19,23 Taken together, these analyses suggest that human cell engraftment is maintained after the transplantation of human ALDHhiLin- UCB cells and that most of these cells differentiate into mature cell lineages in the murine BM microenvironment.
Discussion
In this report we described an alternative strategy for the isolation of purified human hematopoietic progenitor and stem cells by using the depletion of cells expressing mature lineage markers followed by the selection of cells that possess high ALDH activity. This method allows the purification of repopulating cells that are based primarily on stem cell function rather than a traditional cell surface phenotype such as CD34 expression.1,4,5,12 ALDHhiLin- cells represented approximately 22.6% ± 3.0% of the lineage-depleted population or 0.09% ± 0.012% of total MNCs from UCB samples. These cells were highly enriched for cells expressing surface phenotypes, such as CD34+CD38- and CD34+CD133+ cells previously associated with primitive repopulating and progenitor function.2 Functional comparisons of ALDHhiLin- and ALDHlo-Lin- populations confirmed that in vitro multilineage human hematopoietic progenitor function was exclusive to the ALDHhi-Lin- population, whereas the ALDHloLin- population had little in vitro colony-forming ability. These findings are in agreement with previous findings that SSCloALDHbright cells isolated from unprocessed UCB mononuclear cells contained an equal frequency of human progenitors as SSCloCD34+ purified cells.32 In addition, ALDHhiLin- cells possessed significant repopulating ability after transplantation into NOD/SCID and NOD/SCID β2M null mice and also demonstrated multilineage differentiative potential, a characteristic of purified CD34+ hematopoietic progenitor repopulation in the NOD/SCID model. These findings demonstrate, for the first time, that cells with high ALDH activity from human UCB possess robust in vivo repopulating function in murine xenotransplantation models.
Despite the presence of 40% CD34-expressing cells and 5% CD34+CD38- cells within the ALDHloLin- subset, this population was nearly devoid of NOD/SCID reconstituting capacity and possessed low colony-forming ability in vitro. Thus, human CD34-expressing cells that do not considerably contribute to functional repopulation after transplantation can be identified within the ALDHloLin- population. These data strongly suggest there is functional heterogeneity within the CD34+ cell population and that further purification of human stem or progenitor cells with or without repopulating ability may be achieved through the analysis of ALDH activity.
Many potential clinical applications for cellular therapy, such as purified stem cell transplantation or gene therapy strategies, rely on efficient isolation of hematopoietic stem cells capable of long-term repopulation and self-renewal, as well as highly proliferative short-term repopulating cells that rapidly generate granulocytes, platelets, and erythroid cells able to reduce early posttransplantation complications following high-dose chemotherapy.41,42 CD34+Lin- cells exhibit short-term and long-term repopulating characteristics in the NOD/SCID mouse.37 Similarly, we showed that ALDHhiLin- cells exhibit vigorous progenitor production in vitro and consistent repopulation in NOD/SCID and NOD/SCID β2M null mice, capable of supporting the survival and proliferation of more committed progenitor cells as well as primitive stem cells.40 Thus, the ALDHhiLin- population represents a heterogeneous mixture of primitive repopulating cells and a large proportion of more committed progenitor cells that are primarily but not exclusively CD34+. These observations support previous findings that the human hematopoietic stem cell compartment consists of cells with varying phenotypes, including both CD34+ and CD34- cells, capable of stem and progenitor function12-14,16,43 and suggest that analysis of mobilized peripheral blood, BM, or UCB samples for ALDH activity may provide a functional alternative to CD34 enumeration during clinical transplantation procedures. In fact, in patients with cancer that had autologous peripheral blood stem cell transplantation, the number of infused SSCloALDHbright cells infused per kilogram highly correlated with the time to neutrophil and platelet engraftment.33 Thus, the high progenitor content and long-term repopulating ability characteristic of ALDHhiLin- UCB cells may represent a mixture of hematopoietic cells that quickly engraft to produce neutrophils and platelets, and, in addition, provide ample long-term repopulating cells to sustain hematopoiesis after purified cell transplantation.
Isolation of stem and progenitor cells based on a surrogate function such as ALDH activity also represents a novel tool to explore the heterogeneity of selected stem cells. Our data revealed that CD34-expressing cells exist within the ALDHloLin- population that possessed minimal progenitor function and were unable to consistently engraft the NOD/SCID model. These data suggest that not all CD34+ cells within the Lin- population possess stem or progenitor cell function. Furthermore, systematic subfractioning of the ALDHhi population by using a variety of previously described or novel cell surface markers may prove a powerful tool to prospectively isolate human stem cells with hematopoietic or alternative differentiative potential from human bone marrow or UCB sources.
The selection of stem cells according to high ALDH activity following lineage depletion is ideally suited to clinical application and has several advantages over traditional phenotypic isolation. First, the staining and selection methods are efficient, highly reproducible and can easily be adapted for clinical applications without excessive cellular manipulation. In addition, our in vivo data suggest that the Aldefluor substrate is nontoxic to human cells and does not alter the repopulating function of human cells under common xenotransplantation procedures. The reagent does not intercalate into DNA and is a substrate for the multidrug resistance pump,38 allowing efficient clearance from labeled cells. Consequently, ALDH selection appears to be relatively safe compared with selection with dyes that bind DNA.43 Because high ALDH expression is an intrinsic property to a variety of stem cells,44 modification of this strategy may provide a standard method to purify stem cells from alternate tissues without using uncharacterized or variable cell surface molecules that may be lineage restricted. Future experimentation and trials are needed to determine whether the selection of cells by ALDH activity is feasible for clinical applications. In addition, the ALDH activity of leukemic cell types needs to be thoroughly explored for the development of purging strategies according to ALDH activity for stem cell transplantation procedures. Further studies that use ALDH activity as an analytical measure of stem cell function33 or to isolate primitive hematopoietic cells for gene marking and in vivo xenotransplantation studies are definitely warranted. In summary, cellular fractionation according to lineage depletion and ALDH activity provides a nontoxic, highly reproducible method to purify human stem and progenitor cells based primarily on a conserved stem cell function rather than a hematopoietic cell surface marker. Functional isolation according to ALDH activity may be considered as a potential alternative to CD34 cell selection in future clinical transplantation procedures.
Prepublished online as Blood First Edition Paper, June 3, 2004; DOI 10.1182/blood-2004-02-0448.
Supported by the National Institutes of Health (NIH) National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK; R01DK61848-01) and National Heart, Lung, and Blood Institute (NHLBI; 1RO1HL073256-01), D.A.H. is supported by a fellowship award from the Canadian Institutes of Health Research (CIHR).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank W. Eades and J. Hughes from the Siteman Cancer Center flow cytometry core facility for their technical expertise in the development of the ALDH cell sorting assay, and C. Johnson from the Cardinal Glennon Children's Hospital cord blood banking facility for providing human umbilical cord blood samples.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal