Abstract
Previous studies have shown that α4β1 (very late activation antigen-4 [VLA-4]) and vascular cell adhesion molecule-1 (VCAM-1) play a major role in hematopoietic progenitor cell (HPC) homing to bone marrow (BM). However, the antibody used to block VLA-4 function in the mouse (hybridoma clone PS/2) is not specific to VLA-4 but inhibits both α4β1 and α4β7 integrins. Here we have evaluated the contribution of α4β7 in HPC homing to BM. LineagenegSca-1posc-kitpos cells from adult mouse BM and the factor-dependent cell progenitor (FDCP)—mix progenitor cell line express similar levels of α4β7 by flow cytometry. The α4β7 complex was functional since the chemokine CXCL12 enhanced the adhesion of FDCP-mix to immobilized mucosal addressin cell adhesion molecule-1 (MAdCAM-1) and this was completely abrogated by anti-α4β7 (hybridoma clone DATK32) or anti-α4 integrins (PS/2). BM intravital microscopy revealed that α4β7 plays a predominant role in initial tethering and rolling but not in firm adhesion of FDCP-mix cells. Using homing assays, we demonstrate that α4β7 on HPCs contributes to about half of all α4 integrin–mediated homing activity following BM transplantation. MAdCAM-1 is likely expressed since its inhibition significantly reduced HPC homing. Although there may be other α4β7 integrin ligands involved (eg, fibronectin and VCAM-1), these data thus suggest that α4β7 and its counterreceptor MAdCAM-1 represent a novel adhesion pathway mediating HPC homing to BM.
Introduction
Recent advances have partially elucidated the adhesion pathways that mediate hematopoietic progenitor cell (HPC) homing to the bone marrow (BM). Early studies have shown that very late activation antigen-4 (VLA-4; α4β1 integrin) and its counterreceptor vascular cell adhesion molecule-1 (VCAM-1) participate in HPC homing in the mouse since antibody blocking significantly inhibited progenitor homing.1,2 Subsequent studies revealed that the defect in HPC homing to BM was greater when VCAM-1 and both endothelial selectins (P- and E-selectins) were simultaneously inhibited.3 In addition, BM intravital microscopy studies revealed that both P- and E-selectins contribute to HPC rolling in the BM microvasculature and that the vast majority of rolling interactions were inhibited when all 3 pathways were blocked.4,5 These results indicate that HPC homing to BM is mediated by the concerted action of multiple HPC–endothelial adhesion receptor pairs, which are not selectively expressed on hematopoietic progenitor/stem cells.
Among the array of surface adhesion receptors expressed on HPCs,6 VLA-4, VLA-5, and CD18 have previously been suggested to participate in HPC homing using in vivo assays.1,2,7,8 In addition, we have recently shown that P-selectin glycoprotein ligand-1 (PSGL-1) participates in this process not only as a P-selectin ligand but, perhaps more importantly, also as one of the E-selectin ligands that cooperates with α4 integrin in vivo.9
Integrins are expressed as α- and β-chain heterodimers on the cell surface.10 In particular, the α4 chain can pair with only 2 known partners: α4β1 (VLA-4) and α4β7 (also called lymphocyte–Peyer patch adhesion molecule-1 [LPAM-1]). The α4β7 was identified on a subset of lymphocytes that specifically home to intestinal lymphoid tissues.11 This restricted migration into Peyer patches, mesenteric lymph nodes, and lamina propria venules is due in part to the specific expression of its endothelial counterreceptor, mucosal addressin cell adhesion molecule-1 (MAdCAM-1).12-14
It is notable that the effect of α4 integrin blockade is stronger than that of anti–VCAM-1 in reported in vivo HPC homing assays.1,3,9 This suggests that other integrin(s) or endothelial receptor(s) are involved in this process. In particular, the antibody used to block VLA-4 function (hybridoma clone PS/2) in the various in vivo HPC homing studies1,2,8,9 is not specific to VLA-4 but inhibits both α4 integrins.12,15
While, to our knowledge, there is no specific antibody against murine VLA-4 (α4β1), Andrew et al16 have generated a function-blocking antibody that specifically reacts with both chains of the α4β7 heterodimer (hybridoma clone DATK32). The specificity of DATK32 for α4β7 was further confirmed using β7-deficient mice in which lymphocytes still express high levels of α4β1 but did not bind to DATK32.17
Here, we show that α4β7 is expressed on murine hematopoietic progenitor/stem cells and mediates approximately 50% of all α4 integrin–mediated homing activity. We also demonstrate that its main counterreceptor MAdCAM-1 significantly contributes to the recruitment of hematopoietic progenitors in the BM following transplantation.
Materials and methods
Antibodies
Rat anti–mouse α4 integrin (clone PS/2), rat anti–mouse CD11b (clone M1/70), rat anti–mouse VCAM-1 (clone M/K2.7), and rat anti–mouse MAdCAM-1 (clone MECA367) were purified from supernatants of hybridoma cell lines (American Type Culture Collection, Manassas, VA) using a protein-G column (Amersham Pharmacia Biotech, Uppsala, Sweden). Potential endotoxin contamination was removed with polymixin-B endotoxin-removing gel (Pierce, Rockford, IL). For some flow cytometry experiments, PS/2 antibody was biotinylated using standard procedures. Control rat immunoglobulin G (IgG) was purchased from Sigma (St Louis, MO). Rat anti–mouse α4β7 (clone DATK32) was a gift from Dr J. Steven Alexander (Louisiana State University, Shreveport, LA) and purchased from BD Pharmingen (San Diego, CA). Rat anti–mouse CD16/CD32 (clone 2.4G2), rat anti–mouse Ter119, rat anti–mouse Gr-1 (clone RB6-8C5), rat anti–mouse B220 (clone RA3-6B2), peridinin chlorophyll A protein (PerCP) streptavidin, phycoerythrin (PE) rat anti–mouse c-kit (clone 2B8), fluorescein isothiocyanate (FITC) rat anti–mouse stem cell antigen-1 (Sca-1; clone E13-161.7), and biotin rat anti–mouse CXCR4 (clone 2B11) were from BD Pharmingen. Rat anti-CD3ϵ (clone C363.29B) and biotin rat anti–mouse α4β7 (DATK32) were from SouthernBiotech (Birmingham, AL). FITC-streptavidin, cyanin 5 (Cy5) mouse anti–rat IgG, Cy5 goat anti–human IgM, FITC goat anti–human IgM, Cy5-streptavidin, and biotin mouse anti–rat IgG were purchased from Jackson Immunoresearch (West Grove, PA). PE-Cy5-streptavidin was purchased from eBioscience (San Diego, CA).
Flow cytometry
P- or E-selectin human IgM chimeric proteins to detect cell surface selectin ligands were generated and used as previously described.5 For the analysis of surface molecules on linnegSca-1posc-kitpos hematopoietic stem/progenitor cell fraction (LSK cells), adult mouse BM cells were incubated in phosphate-buffered saline (PBS)/0.03% bovine serum albumin (BSA), after blockade of Fc receptors with 2.4G2, with monoclonal antibodies (Mabs) against lineage markers (Ter119, CD3ϵ, CD11b, B220, and Gr-1), followed by Cy5 anti–rat IgG. Potential nonspecific binding to Cy5 anti–rat IgG secondary antibody was blocked by rat IgG, and cells were further stained for FITC–Sca-1 and PE–c-kit together with biotin–anti-CXCR4, biotin–anti-α4 integrins, or biotin–anti-α4β7, followed by PE-Cy5-streptavidin. Erythrocytes were lysed in 0.8% NH4Cl lysis buffer and the remaining nucleated cells were washed twice in PBS/0.03% BSA. For the detection of selectin ligands on LSK cells, biotin–anti–rat IgG and PerCP-streptavidin were used for lineage markers. P- and E-selectin IgM chimeras were visualized by Cy5–anti–human IgM. For the detection of surface molecules on factor-dependent cell progenitor (FDCP)–mix, cells were incubated with biotin–anti-CXCR4, biotin–anti-α4 integrins, or biotin–anti-α4β7, followed by PE-Cy5-streptavidin. For the detection of selectin ligands on these cells, FITC–anti–human IgM was used. Analysis was performed on a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA).
Animals and cell line
E-selectin–deficient (E–/–) mice were generated by gene targeting18 and were backcrossed 7 generations into the C57BL/6 background. Wild-type (WT) C57BL/6 mice were purchased from National Cancer Institute (Frederick Cancer Research and Developmental Center, Frederick, MD). All animals used in this study were matched for sex and age (6-12 weeks). Mice were housed at Mount Sinai School of Medicine in the East Building barrier facility. Experimental procedures performed on the animals were approved by the Animal Care and Use Committee of Mount Sinai. FDCP-mix mouse progenitor cell line was purchased from DSMZ (German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany) and maintained in Iscoves modified Dulbecco medium (IMDM) supplemented with 20% fetal bovine serum (Hyclone, Logan, UT), 10% conditioned medium from WEHI3 cell line (containing interleukin 3 [IL-3]), and 10% conditioned medium from BHK/MKL (baby hamster kidney cell line stably transfected with an expression vector containing the cDNA encoding for the secreted form of murine stem cell factor).
Static adhesion assay
High-binding 96-well plates (Costar, Cambridge, MA) were coated overnight with 50 μL of recombinant murine MAdCAM-1–Fc (R&D Systems, Minneapolis, MN) resuspended at 6 μg/mL in carbonate buffer (0.1 M, pH 9.5) at 4° C. FDCP-mix cells were labeled with carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) for 20 minutes at 6° C, washed thrice in RPMI 1640, resuspended in adhesion medium (RPMI 1640 plus 0.5% BSA), and incubated with 10 μg/mL of either control rat IgG, DATK32, or PS/2 antibodies for 10 minutes at room temperature. An equal volume of recombinant human CXCL12 (R&D Systems) or medium only was then added to the cells to have a final concentration of 100 ng/mL and the cells were added in duplicate over the MAdCAM-1–Fc–coated wells. This concentration has previously been shown to be optimal for the migration of human CD34+ cells and FDCP-mix cells in vitro19 and binding of human CD34+ cells to immobilized VCAM-1 (data not shown). Adhesion was allowed to proceed for 30 minutes at 37° C, and unbound cells were removed by 3 washes with warm adhesion medium. Bound cells were lysed with 0.1% sodium dodecyl sulfate (SDS) in PBS and quantified using a fluorescence analyzer HTS 7000 Plus (Perkin Elmer, Norwalk, CT).
Bone marrow intravital microscopy
E–/– mice (6-8 weeks old) were irradiated (12 Gy) 3 hours before the recording and prepared for BM intravital microscopy exactly as described.9 Briefly, anesthetized animals were cannulated through the carotid artery to allow injection of cells, and the frontoparietal skull was exposed for recording of the vasculature of the parietal bone using a custom-designed intravital microscope.5 CFSE-labeled FDCP-mix cells were resuspended in RPMI 1640 containing control rat IgG, DATK32, or PS/2 antibodies (2 μg per 106 cells) at least 30 minutes before injecting into mice. Antibody-treated cells were sequentially injected into the same mice, waiting 15 to 20 minutes between each group of cells to permit complete clearance from the circulation. Hemodynamic parameters were calculated as described5 : average Vmax = 1154 ± 158 μm/s, vessel diameter = 37.7 ± 3.0 μm, wall shear rate = 161 ± 24 s–1 from 15 venules in 6 mice. Any cell traveling below the Vcrit was considered to be rolling on the vessel wall. Cells that remained stationary for at least 5 seconds were considered “arrested” cells.
Assays for hematopoietic progenitor homing
Donor BM cells were harvested from WT mice and were incubated with 2 μg/106 BM nucleated cell (BMNC) rat IgG as control, PS/2 (anti-α4 integrins), or DATK32 (anti-α4β7) in RPMI on ice for 30 minutes. Five million nucleated cells in 300 μL volume were injected into lethally irradiated (12 Gy, single dose) WT or E–/– recipient mice. For the blockade of endothelial adhesion molecules, 80 μg/mouse of control rat IgG or anti–MAdCAM-1 and/or anti–VCAM-1 antibodies were coinjected with 5 million donor BM nucleated cells in 300 to 400 μL volume. In the case of anti–MAdCAM-1 and anti–VCAM-1 double blockade, 160 μg/mouse of rat IgG was injected in the control group. Since data from control groups for single blockade (80 μg/mouse of rat IgG) and for double blockade (160 μg/mouse of rat IgG) were similar, all data from single blockade and double blockade were combined in Figure 5B. An IgG control group was included in each experiment testing adhesion-blocking antibodies. Three hours after injection, BM and/or spleen were harvested and transferred to colony-forming units in culture (CFU-Cs) assay. The number of homed CFU-Cs per femur was corrected to represent the whole BM (multiplied by 16.9 because one femur represents approximately 5.9% of the total murine BM20 ). Donor cells treated with antibodies were plated for CFU-C assay to assess the number of CFU-Cs injected (average input CFU-Cs: 18 155 ± 1278 for Figure 4, n = 7; 17 871 ± 556 for Figure 5B, n = 4). An irradiated mouse (age-, genotype-, and sex-matched) in each experiment did not receive a transplant so that the numbers of residual host-derived progenitors could be assessed. Very few background CFU-Cs were recovered after irradiation: out of 11 control mice used in experiments represented in Figures 4 and 5B, one background colony was observed in BM sample from 3 mice (2 WT and 1 E–/– mice; plated cellular content of 0.25 femur). For all other animals, the background colony in BM was 0. No background colonies were observed in the spleen from mice that did not receive transplants. Background colonies were subtracted from those that received BMNCs to calculate the percentage of homed HPCs to BM.
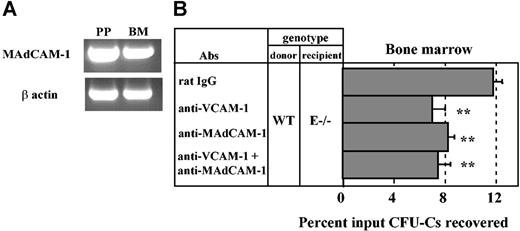
MAdCAM-1 is expressed in the bone marrow and mediates progenitor homing. (A) RT-PCR analysis of MAdCAM-1 mRNA expression in Peyer patch (PP) and bone marrow (BM) from a wild-type mouse. (B) Lethally irradiated E-selectin–deficient mice (E–/–) were injected with wild-type (WT) donor BM cells along with anti–MAdCAM-1 (MECA367) and/or anti–VCAM-1 (MK/2) antibodies. Three hours after injection, CFU-Cs were determined from the BM. **P < .01 compared with rat IgG control group, n = 5-11 per group. Data are presented as means ± SEM.
MAdCAM-1 is expressed in the bone marrow and mediates progenitor homing. (A) RT-PCR analysis of MAdCAM-1 mRNA expression in Peyer patch (PP) and bone marrow (BM) from a wild-type mouse. (B) Lethally irradiated E-selectin–deficient mice (E–/–) were injected with wild-type (WT) donor BM cells along with anti–MAdCAM-1 (MECA367) and/or anti–VCAM-1 (MK/2) antibodies. Three hours after injection, CFU-Cs were determined from the BM. **P < .01 compared with rat IgG control group, n = 5-11 per group. Data are presented as means ± SEM.
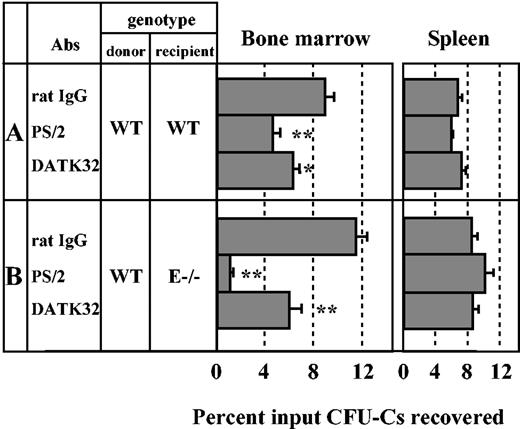
Role of α4β7 in progenitor homing to bone marrow. Lethally irradiated wild-type (WT) or E-selectin–deficient mice (E–/–) were injected with antibody-treated wild-type donor BM cells. CFU-Cs were determined from the recipient BM and spleen that were harvested 3 hours after injection. (A) Transplantation of donor cells treated with rat IgG, anti-α4 integrins (PS/2), or anti-α4β7 (DATK32) into WT recipient mice. n = 8-12 mice per group for BM and n = 6-8 for spleen. (B) Transplantation of antibody-treated donor cells into E–/– recipient mice. n = 6 per group. *P < .05; **P < .01 compared with rat IgG control group. Data are presented as means ± SEM.
Role of α4β7 in progenitor homing to bone marrow. Lethally irradiated wild-type (WT) or E-selectin–deficient mice (E–/–) were injected with antibody-treated wild-type donor BM cells. CFU-Cs were determined from the recipient BM and spleen that were harvested 3 hours after injection. (A) Transplantation of donor cells treated with rat IgG, anti-α4 integrins (PS/2), or anti-α4β7 (DATK32) into WT recipient mice. n = 8-12 mice per group for BM and n = 6-8 for spleen. (B) Transplantation of antibody-treated donor cells into E–/– recipient mice. n = 6 per group. *P < .05; **P < .01 compared with rat IgG control group. Data are presented as means ± SEM.
Isolation of cells and CFU-C assay
Reverse transcriptase–polymerase chain reaction (RT-PCR)
Total RNA was isolated from Peyer patches and BM of C57BL/6 mouse using TRIzol solution (Invitrogen, Carlsbad, CA) according to the supplier's instruction. The cDNA was reverse transcribed from 1 μg of total RNA in a 25-μL reaction volume using Reverse Transcription System (Promega, Madison, WI). The cDNA samples were subsequently stored at –20° C until use for PCR amplification. Nested PCR was performed to detect MAdCAM-1 mRNA expression. The outer primer set consisted of the forward primer (5′-AGAAGAGGAGATACAAGAGG-3′) and the reverse primer (5′-TAGTGTCTGGGCGAGGACC-3′). This primer set amplified 2 cDNA fragments of 701 base pairs (bp) and 269 bp, showing the presence of long and short MAdCAM-1 mRNAs.21 The amplification was carried out with 1 μL of cDNA template in a 25-μL reaction mixture. The cycle conditions used for the PCR reaction were as follows: an initial denaturation step of 5 minutes at 94° C, followed by 40 cycles of amplification (denaturation at 94° C for 30 s, annealing at 55° C for 30 s, elongation at 72° C for 1 minute), and a final elongation step at 72° C for 7 minutes. The inner primer set consisted of the forward primer (5′-ACTACAGAGCCAGACCTCAC-3′) and the reverse primer (5′-CCAGGAACATACAGATTGGTCAC-3′) and allowed amplification of a unique 411-bp cDNA fragment of the long MAdCAM-1 variant. To perform the nested PCR reaction, 1 μL of the first PCR product was used as a template in a 25-μL reaction mixture, with same cycle conditions as outer PCR. As a control for RNA quality, a 514-bp fragment of β-actin mRNA was amplified using the following primers: forward primer (5′-TGTGATGGTGGGAATGGGTCAG-3′) and reverse primer (5′-TTTGATGTCACGCACGATTTCC-3′). The PCR products were fractioned by electrophoresis on a 1.3% agarose gel containing ethidium bromide and were visualized by UV light.
Statistical analysis
All values are reported as mean ± SEM. For the comparison of multiple groups, analysis of variance (ANOVA) with Bonferroni correction was used. Significance was set at P less than .05.
Results
LineagenegSca-1posc-kitpos stem/progenitor cells and FDCP-mix cells express the α4β7 integrin complex
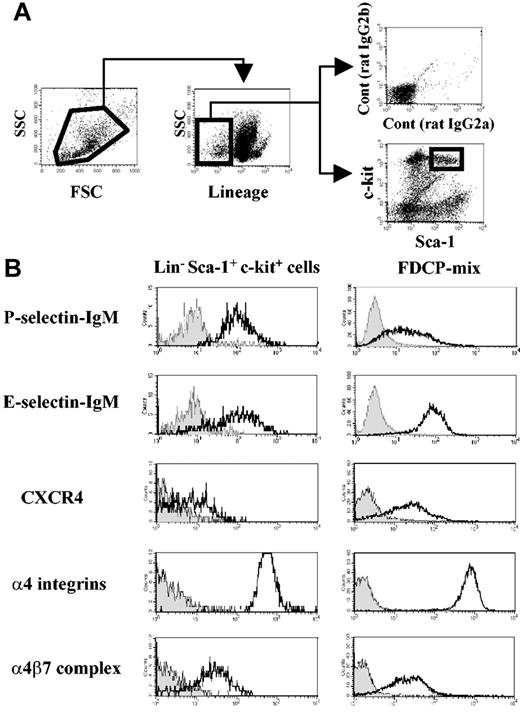
Four-color staining was performed to assess adhesion or chemokine receptor expression on LinnegSca-1posc-kitpos cells (Figure 1A) from adult mouse BM. As shown in Figure 1B, this fraction expresses high levels of functional P- and E-selectin ligands and α4 integrins, which are essential for HPC rolling on BM microvasculature and for homing into the BM.3-5,8,9 In addition, LSK cells clearly express the α4β7 integrin (Figure 1B).
Mouse hematopoietic progenitor cells express α4β7 integrin complex. (A) Flow cytometry gating of bone marrow–derived Lineageneg Sca-1pos c-kitpos (LSK) cells. (B) Selectin ligands, CXCR4, and α4 integrin expression on bone marrow LSK cells and on FDCP-mix mouse progenitor cell line. Filled histograms represent negative controls from isotype-matched staining or selectin chimeric protein staining in the presence of 5 mM EDTA (ethylenediaminetetraacetic acid). Geometric mean values obtained after subtraction of isotype-matched control values from 3 independent experiments were as follows: LSK cells, 576 ± 33 for α4 integrins and 31 ± 7 for α4β7 integrin; FDCP-mix cells, 656 ± 37 for α4 integrins and 18 ± 7 for α4β7.
Mouse hematopoietic progenitor cells express α4β7 integrin complex. (A) Flow cytometry gating of bone marrow–derived Lineageneg Sca-1pos c-kitpos (LSK) cells. (B) Selectin ligands, CXCR4, and α4 integrin expression on bone marrow LSK cells and on FDCP-mix mouse progenitor cell line. Filled histograms represent negative controls from isotype-matched staining or selectin chimeric protein staining in the presence of 5 mM EDTA (ethylenediaminetetraacetic acid). Geometric mean values obtained after subtraction of isotype-matched control values from 3 independent experiments were as follows: LSK cells, 576 ± 33 for α4 integrins and 31 ± 7 for α4β7 integrin; FDCP-mix cells, 656 ± 37 for α4 integrins and 18 ± 7 for α4β7.
FDCP-mix cells, previously derived from a mouse long-term BM culture, maintain unique features of immature hematopoietic cells, such as cytokine-dependent growth, colony formation in semisolid culture, and migration toward CXCL12 in vitro and in vivo.19,22 These cells express levels of P- and E-selectin ligands, α4 integrins, and α4β7 integrin similar to primary BM LSK cells (Figure 1B). Expression of CXCR4, the receptor for CXCL12 that mediates HPC migration,19,23 was also confirmed in FDCP-mix and LSK cells (Figure 1B). These results clearly show that the integrin α4β7 is expressed on hematopoietic stem cells (HSCs) and that FDCP-mix cells represent a useful cell line to study its role.
The α4β7 integrin on FDCP-mix is functional
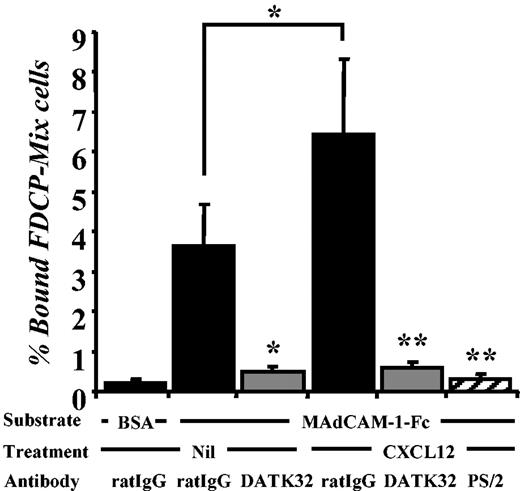
To further evaluate α4β7 function, we carried out static adhesion assays with FDCP-mix cells on MAdCAM-1–Fc. As shown in Figure 2, the adhesion of FDCP-mix to immobilized MAdCAM-1 was completely blocked by DATK32. Treatment of FDCP-mix cells with soluble CXCL12 significantly enhanced adhesion on MAdCAM-1, and adhesion was completely abrogated by either PS/2 or DATK32. These results (i) indicate that α4β7 is indeed functional on FDCP-mix cells; (ii) confirm that α4β7 expressed on hematopoietic progenitors, like lymphocyte α4β7, interacts with MAdCAM-1; and (iii) demonstrate that CXCL12 can enhance the affinity and/or avidity of α4β7 on progenitors for its natural counterreceptor MAdCAM-1.
The α4β7 integrin on FDCP-mix is functional. Fluorescently labeled FDCP-mix cells were preincubated with 10 μg/mL of control rat IgG, antibodies against the α4β7 integrin (clone DATK32), or α4 integrins (clone PS/2). Adhesion to MAdCAM-1–Fc was then allowed to proceed in the absence or presence of 100 ng/mL of CXCL12 for 30 minutes at 37°C. Bars represent mean percentage of adhesion ± SEM from 3 independent experiments. *P < .05; **P < .01 compared with the respective rat IgG controls or between indicated 2 groups. Irrelevant binding antibody did not influence adhesion, as anti–L-selectin (Mel-14) antibody-treated FDCP-mix cells were 105.7% ± 5.5% that of rat IgG-treated FDCP-mix cells (n = 3 experiments).
The α4β7 integrin on FDCP-mix is functional. Fluorescently labeled FDCP-mix cells were preincubated with 10 μg/mL of control rat IgG, antibodies against the α4β7 integrin (clone DATK32), or α4 integrins (clone PS/2). Adhesion to MAdCAM-1–Fc was then allowed to proceed in the absence or presence of 100 ng/mL of CXCL12 for 30 minutes at 37°C. Bars represent mean percentage of adhesion ± SEM from 3 independent experiments. *P < .05; **P < .01 compared with the respective rat IgG controls or between indicated 2 groups. Irrelevant binding antibody did not influence adhesion, as anti–L-selectin (Mel-14) antibody-treated FDCP-mix cells were 105.7% ± 5.5% that of rat IgG-treated FDCP-mix cells (n = 3 experiments).
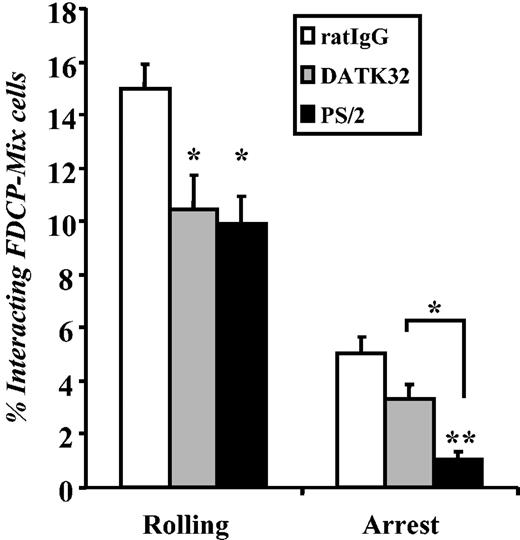
The integrin α4β7 plays a predominant role in initial tethering and rolling but not in firm adhesion
To evaluate the function of α4β7 in the initial interactions of transplanted HPCs with BM microvasculature in vivo, we carried out BM intravital microscopy experiments. Fluorescently labeled FDCP-mix cells were incubated with either anti-α4β7 or anti-α4 integrin antibody and were injected via a carotid artery catheter into lethally irradiated E–/– mice. E–/– mice were used in these experiments because the role of α4 integrins in HPC homing is more prominent in the absence of E-selectin.9 The interactions of FDCP-mix with parietal BM microvessels were recorded for subsequent analyses. As shown in Figure 3, FDCP-mix cell rolling was significantly inhibited by anti-α4β7 (DATK32; 31% reduction, P < .05) and anti-α4 integrins (PS/2; 34% reduction, P < .05), suggesting that the vast majority of α4 integrin–dependent cell rolling is mediated by α4β7. In contrast, the inhibition of both α4 integrins had a more dramatic effect than blockade of α4β7 alone on cell arrest (78% reduction for α4 integrins versus 34% reduction for α4β7 alone), suggesting that cell arrest requires α4β1. Thus, these data indicate that α4β7 plays a predominant role in the initial tethering and rolling steps but is not required for firm adhesion in the bone marrow microvasculature.
Role of α4 integrins in the interactions of the FDCP-mix progenitor cell line with the bone marrow microvasculature. Fluorescently labeled FDCP-mix cells preincubated with control rat IgG (□), anti-α4β7 integrin antibody (clone DATK32; ▦), or anti-α4 integrins (clone PS/2; ▪) were injected into lethally irradiated E-selectin–deficient mice. The fractions of cells rolling or arrested on the BM microvasculature were determined by analysis of video recordings from fluorescence intravital microscopy experiments. n = 6 mice; *P < .05; **P < .01 compared with the rat IgG control group or between indicated 2 groups. Data are presented as means ± SEM.
Role of α4 integrins in the interactions of the FDCP-mix progenitor cell line with the bone marrow microvasculature. Fluorescently labeled FDCP-mix cells preincubated with control rat IgG (□), anti-α4β7 integrin antibody (clone DATK32; ▦), or anti-α4 integrins (clone PS/2; ▪) were injected into lethally irradiated E-selectin–deficient mice. The fractions of cells rolling or arrested on the BM microvasculature were determined by analysis of video recordings from fluorescence intravital microscopy experiments. n = 6 mice; *P < .05; **P < .01 compared with the rat IgG control group or between indicated 2 groups. Data are presented as means ± SEM.
The integrin α4β7 on mouse hematopoietic progenitors plays a significant role in the homing to bone marrow
To assess the physiologic role of α4β7 integrin on HPCs, we carried out homing assays. BMNCs were harvested from WT mice and divided into 3 groups according to their antibody treatment: control rat IgG, anti-α4 integrins (PS/2), and anti-α4β7 (DATK32). As shown in Figure 4A, the percentage of injected HPCs that homed in the BM was on average 8.9% ± 0.8% in the rat IgG control group. Anti-α4 integrin treatment of donor BMNCs reduced homing of HPCs by 48% compared with rat IgG group (n = 8-11; P < .01) as previously reported by others and our own group.1,2,9 Interestingly, anti-α4β7 treatment significantly reduced homing of HPCs by 30% (n = 12; P < .05) compared with rat IgG control. We recently reported that α4 integrins (using PS/2 mAb) cooperate with E-selectin ligands in the process of HPC homing to BM.9 To test whether α4β7 cooperates with E-selectin ligands, the same 3 groups of antibody-treated WT BM cells were injected into lethally irradiated E–/– mice. Consistent with our previous observations,9 the percentage of HPCs homed in BM of the control rat IgG group was not reduced in E–/– mice compared with WT recipients, but it was drastically reduced when both α4 integrins were blocked (by more than 90%, n = 6, P < .01; Figure 4B). The inhibition of HPC homing by anti-α4β7 treatment was also greater in E–/– recipients (by 48%, n = 6, P < .01; Figure 4B) than WT mice (Figure 4A). These reductions were not due to effects of the antibodies on the growth or survival of progenitors since CFU-C counts and colony size from antibody-treated donor cells were similar among the 3 antibody treatment groups (no. of CFU-Cs/105 BMNCs: rat IgG, 363 ± 26; anti-α4 integrins [PS/2], 356 ± 36; anti-α4β7 [DATK32] 362 ± 26), and no alteration of HPC lodgment was observed in the spleen (Figure 4). Thus, these data suggest that α4β7 may cooperate with E-selectin ligands on HPCs and accounts for approximately half of the α4 integrin–mediated homing activity in spite of a lower expression level on the surface of stem/progenitor cells.
MAdCAM-1 is expressed in the bone marrow and plays a significant role in progenitor homing
Since we found a significant role for α4β7 in HPC homing to BM, we suspected that its main counterreceptor, MAdCAM-1, might be constitutively expressed in BM endothelium. We thus evaluated the expression of MAdCAM-1 by RT-PCR from bone marrow RNA extracts of wild-type C57BL/6 mice. Peyer patch RNA was extracted as positive control. As shown in Figure 5A, MAdCAM-1 mRNA was detected in both BM and Peyer patch extracts.
To investigate whether MAdCAM-1 contributed to the recruitment of HPCs in the BM, homing assays were carried out in lethally irradiated E–/– mice, in which the role of α4 integrins is prominent. Mice were treated with anti–MAdCAM-1 (MECA367) and/or anti–VCAM-1 (MK/2) or rat IgG control. As shown in Figure 5B, anti–MAdCAM-1 treatment reduced HPC homing to BM by 34% compared with the control group (n = 7-11; P < .01), whereas anti–VCAM-1 reduced homing by 44% (n = 5; P < .01) in E–/– recipients. Surprisingly, homing was not further reduced when both MAdCAM-1 and VCAM-1 were simultaneously blocked (37% reduction compared with rat IgG group, n = 5, P < .01; Figure 5B). These data suggest that MAdCAM-1 is expressed in the BM and plays a role in HPC homing but does not cooperate with VCAM-1.
Discussion
Recent studies suggest that the initial adhesion events mediating hematopoietic stem/progenitor cell homing to BM are not unique to multipotent cells. Indeed, accumulating evidence indicates that the adhesion molecules contributing to the recruitment of mature leukocytes also participate in the recruitment of immature precursors. However, the adhesion pathways that cooperate in the recruitment of blood cells into the BM parenchyma appear to be distinct. For example, E-selectin ligands closely cooperate with P-selectin ligands for the recruitment of neutrophils,18,24,25 whereas they synergize with α4 integrins instead of P-selectin ligands on stem cells.9 Although α4β1 (VLA-4) was assumed to represent the chief (or sole) functional α4 integrin on stem/progenitor cells, the potential role of the other α4 integrin, α4β7, had not been evaluated. We show here that α4β7 appears to be as important as α4β1 for HPC homing to the bone marrow after transplantation.
While both α4β1 and α4β7 can bind VCAM-1 and fibronectin,26 α4β7 can specifically interact with MAdCAM-1 expressed in intestinal and mesenteric high-endothelial venules (HEVs). This unique interaction is thought to confer specificity in the trafficking of α4β+7 lymphocytes in these areas.27 Our intravital microscopy experiments using FDCP-mix cell line suggest separate contributions of the 2 α4 integrins in that α4β7 plays a predominant role in initial tethering and rolling steps but not in firm adhesion. This finding is consistent with previous work indicating that α4β7 is expressed on microvilli and can mediate rolling interactions under flow.28 Owing to the lack of specific inhibitor of α4β1, however, we could not determine whether firm adhesion was mediated by α4β1 or whether it required the presence of both α4 integrins. It is possible that the interaction of α4β7 with MAdCAM-1 accounts for the differential contributions of α4 integrins in progenitor cell behavior in vivo.
A remarkable feature of the bone marrow endothelium is the constitutive expression of adhesion molecules that are otherwise found in inflamed areas. E-selectin and VCAM-1 are indeed constitutively expressed in the BM microvasculature, whereas they are expressed in other organs stimulated by inflammatory cytokines such as tumor necrosis factor α (TNF-α) and IL-1.4,24,29 Constitutive expression of E-selectin and VCAM-1 in the BM likely provides some selectivity in HPC homing. We suspected that MAdCAM-1 might also be expressed in the BM for 2 main reasons: (i) MAdCAM-1 expression was reported in organs subjected to chronic inflammatory diseases such as the pancreas in diabetes,30 central nervous system in chronic experimental autoimmune encephalomyelitis,31 and liver in primary sclerosing cholangitis and autoimmune hepatitis32 ; and (ii) MAdCAM-1, VCAM-1, and E-selectin appear to be similarly regulated in that the transcription of their respective genes is activated by nuclear factor–κB (NF-κB).33,34 Indeed, we found that MAdCAM-1 mRNA was expressed in the BM by RT-PCR (Figure 5A) and also found a significant contribution of MAdCAM-1 in HPC recruitment into the BM (Figure 5B). Our results differ from those of Vermeulen et al2 who showed no significant reduction of spleen colony-forming unit (CFU-S) homing into BM by injection of anti–MAdCAM-1 in WT mice. One explanation for this discrepancy might be a possible compensation by VCAM-1 since VCAM-1 and MAdCAM-1 have primary amino acid sequence homology35 and α4β7 can bind VCAM-1.36 To maximize the sensitivity of the assay, our experiments were carried out in E–/– recipients in which the vast majority of homing activity is mediated by α4 integrins/their receptors. Although we found a clear role for MAdCAM-1 in HPC recruitment, its expression in the BM, however, appears lower than that of mesenteric lymph nodes; we have not been able to detect MAdCAM-1 by conventional immunofluorescence staining of BM frozen sections or by the in vivo immunofluorescence staining method used by Mazo et al4 to detect VCAM-1 in BM, whereas mesenteric lymph nodes showed positive staining for MAdCAM-1 in both methods (Y.K. and P.S.F., unpublished results, March 2004). Additionally, the possibility that other α4β7 integrin ligands, such as fibronectin and/or VCAM-1, contribute to its activity in mediating HPC homing cannot be excluded.
The lack of additive effect of VCAM-1 and MAdCAM-1 suggests that these interactions do not represent 2 independent events whose effects might be additive in HPC homing but rather suggests a complete overlap in their functions. Since inactivation of both α4 integrins and E-selectin inhibit more than 90% HPC homing (Katayama et al9 and present results), we expected similar results by blockade of VCAM-1 and MAdCAM-1 in the E–/– recipient. The partial inhibition despite inactivation of all 3 endothelial receptors implicates the presence of other major ligand(s) for α4 integrins. One prime candidate is the fibronectin connecting segment-1 (CS-1) moiety since previous studies revealed that both α4β1 and α4β7 can bind this fragment.26,37-39 It has been reported that the treatment of donor HPCs with synthetic CS-1 peptide did not alter the homing to BM.40 Although van der Loo et al41 also showed that treatment of donor cells with either CS-1 or ARG-GLY-ASP (RGD) peptide (α5β1 integrin binding site on fibronectin) did not alter the level of engraftment after transplantation, incubation with a fibronectin fragment containing CS-1, the heparin binding site, and RGD sequence significantly reduced the level of engraftment. The role of fibronectin in HPC homing thus needs further evaluation.
The contribution of α4β7–MAdCAM-1 in stem/progenitor homing also has potential implications in lymphocyte trafficking in the bone marrow. For example, α4β+7 memory T cells that traffic in intestinal lymphoid tissues27 express CXCR4, respond to CXCL12,42 and may travel in the BM. The role, if any, of this subpopulation of lymphocytes in bone marrow transplantation biology is currently unknown. Since stem cells use overlapping adhesion pathways to home to BM and certain effector T cells can promote graft rejection43 or can enhance engraftment,44,45 it is conceivable that interference with α4β7–MAdCAM-1 might favorably modulate the graft without directly preventing the migration of hematopoietic stem/progenitor cells.
Prepublished online as Blood First Edition Paper, May 25, 2004; DOI 10.1182/blood-2003-12-4157.
Supported by the National Institutes of Health R01 DK56638 (P.S.F.).
Y.K. and A.H. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Steven Alexander for helpful advice and reagents.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal