Abstract
Donor lymphocyte infusions (DLIs) induce effective graft-versus-tumor responses in patients with multiple myeloma who relapse after allogeneic hematopoietic stem-cell transplantation. The graft-versus-myeloma response is presumably mediated primarily by donor T cells, but recent studies have also demonstrated the presence of antibodies specific for a variety of myeloma-associated antigens in patients who achieve complete remission after DLI. One of the B-cell antigens identified in these studies was B-cell maturation antigen (BCMA), a transmembrane receptor of the tumor necrosis factor (TNF) superfamily that is selectively expressed by mature B cells. The present studies were undertaken to characterize the functional significance of antibodies to BCMA in vivo. Using transfected cells expressing BCMA, antibodies in patient serum were found to react with the cell-surface domain of BCMA. Post-DLI patient serum was able to induce complement-mediated lysis and antibody-dependent cellular cytotoxicity (ADCC) of transfected cells and primary myeloma cells expressing BCMA. BCMA antibodies were only found in post-DLI responders and not in other allogeneic transplant patients or healthy donors. These results demonstrate that BCMA is a target of donor B-cell immunity in patients with myeloma who respond to DLI. Antibody responses to cell-surface BCMA may contribute directly to tumor rejection in vivo.
Introduction
Allogeneic hematopoietic stem-cell transplantation (HSCT) has been shown to be an effective therapy for patients with multiple myeloma.1,2 In part, the efficacy of allogeneic HSCT derives from the ability of donor lymphocytes to target both normal and malignant recipient hematopoietic cells and establish full donor chimerism. In instances where recipient myeloma cells persist or relapse after transplantation, single infusions of donor lymphocytes without other therapy can induce partial or complete remissions in approximately 30% to 40% of patients.3,4 Donor T cells are presumed to be the primary effector cells that mediate tumor immunity after allogeneic HSCT,5,6 but other immunologic mechanisms may also contribute to the elimination of residual tumor cells in this setting. In fact, recent reports have suggested that donor natural killer (NK) cells are also capable of mediating significant tumor immunity after allogeneic HSCT.7
Previous studies from our laboratory examined immune reconstitution in patients with myeloma who received T-cell–depleted HSCT followed by infusion of CD4+ donor lymphocytes.8 Donor lymphocyte infusions (DLIs) induced further tumor responses in all patients with evidence of persistent myeloma and was often associated with the development of normal polyclonal plasma cell infiltrates in the marrow.9,10 Concurrent examination of peripheral lymphocytes also demonstrated the polyclonal expansion of donor CD20+ B cells after DLI when compared with similar patients who did not receive DLI.10 This led us to consider whether B-cell responses to myeloma-associated antigens might also contribute to tumor rejection after allogeneic HSCT.
To identify B-cell antigens associated with tumor rejection we screened a myeloma cDNA expression library with post-DLI serum from patients who achieved a complete response after DLI using SEREX (serological analysis of recombinant cDNA expression libraries).11-13 From this screening we identified 13 gene products that were reactive with post-DLI serum but negative with pre–bone marrow transplantation (pre-BMT) and pre-DLI serum.14 One of the proteins identified in these experiments was B-cell maturation antigen (BCMA). This protein is a member of the tumor necrosis factor (TNF) receptor superfamily and contains well-defined intracellular, transmembrane, and extracellular domains.15-18 BCMA, transmembrane activator and calcium modulator and cyclophilin ligand factor (TACI), and B-cell activating factor-receptor (BAFF-R) are 3 distinct receptors for 2 ligands, BAFF and proliferation-inducing ligand (APRIL).19-23 These 2 ligands and their associated receptors play important roles in the development of B-cell immunity and maintenance of B-cell homeostasis.24 Several recent studies have also documented the expression of BCMA in malignant as well as normal plasma cells and have shown that BAFF signaling through BCMA can result in myeloma cell proliferation in vitro.18,25-27 Taken together, these studies suggested that BCMA is commonly expressed in myeloma and signaling through BCMA may also contribute to the expansion of myeloma cells in vivo.
To evaluate the significance of antibodies to BCMA in DLI responders, we undertook studies to further characterize the specificity of the antibody response as well as the functional effects of these antibodies. These experiments demonstrated that the antibody response in vivo was at least in part directed against the extracellular domain of BCMA. Antibodies to BCMA in post-DLI patient serum were also able to induce complement-mediated lysis and ADCC of BCMA-positive cell lines and primary myeloma tumor cells. These results demonstrate that the antibody response to BCMA can contribute directly to the elimination of myeloma cells in vivo and suggest that B-cell responses to tumor-associated antigens can play an important role in tumor rejection following allogeneic HSCT.
Materials and methods
Patient samples and treatment
Serum samples were obtained after informed consent from healthy donors and patients enrolled on clinical trials of allogeneic HSCT. All patients received myeloablative therapy followed by infusion of marrow stem cells from HLA-identical donors. Clinical characteristics of the 2 DLI responders who developed high titer antibodies against BCMA are summarized in Table 1. Clinical protocols were approved by the institutional review board of the Dana-Farber/Harvard Cancer Center, and informed consent was obtained from each patient.
Clinical characteristics of multiple myeloma DLI responders who had antibody response to BCMA
Patient no. . | Patient age at BMT/sex . | Donor age/sex . | Conditioning regimen . | Disease at BMT . | Acute GVHD after BMT, grade . | Chronic GVHD after BMT . | Response after BMT . | Acute GVHD after DLI, grade . | Chronic GVHD after DLI . | Response after DLI . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 51/M | 60/F | CTX/TBI | PR | 0 | - | PR | 1 | Yes, EXT | CR |
| 2 | 37/M | 46/F | CTX/TBI | PR | 0 | - | Relapse | 0 | Yes, EXT | CR |
Patient no. . | Patient age at BMT/sex . | Donor age/sex . | Conditioning regimen . | Disease at BMT . | Acute GVHD after BMT, grade . | Chronic GVHD after BMT . | Response after BMT . | Acute GVHD after DLI, grade . | Chronic GVHD after DLI . | Response after DLI . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 51/M | 60/F | CTX/TBI | PR | 0 | - | PR | 1 | Yes, EXT | CR |
| 2 | 37/M | 46/F | CTX/TBI | PR | 0 | - | Relapse | 0 | Yes, EXT | CR |
CTX indicates cyclophosphamide; TBI, total body irradiation; PR, partial response; -, none; CR, complete response; EXT, extensive.
BCMA gene expression
BCMA gene expression was measured by high-density oligonucleotide microarrays (U95Av2 Gene Chip; Affymetrix, Santa Clara, CA) using standard methods in a series of primary tumor samples from patients with myeloma and B-cell lineage leukemias.14,28 Purified CD138+ plasma cells from normal bone marrow and patients with monoclonal gammopathy of undetermined significance (MGUS) were also tested. CEL files were imported into d-Chip public software (http://www.dchip.org) and normalized values were obtained.
BCMA plasmid construction and transfection
A cDNA fragment encoding full-length BCMA with HindIII and BamH1 restriction sites was generated by polymerase chain reaction (PCR) using Pfu Turbo DNA polymerase (Stratagene, La Jolla, CA). A His-Tag was inserted at the 3′ end and the PCR product was ligated into the PcDNA3 vector (Invitrogen, Carlsbad, CA). DNA sequence of the constructed plasmid was verified and used to transfect human 293 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). To establish a stable cell line expressing BCMA, 293 cells were selected in the presence of neomycin (G418, Cellgro; Mediatech, Herndon, VA). Resistant cells expressing high levels of BCMA were purified by cell sorting using anti–BCMA antibody (R&D Systems, Minneapolis, MN) and expanded in vitro. Cell sorting and in vitro expansion of 293 cells expressing BCMA was repeated 4 times to establish a stable BCMA-293 cell line expressing high levels of membrane antigen. Expression of BCMA was confirmed by flow cytometry and Western blot with anti–BCMA and anti–His-Tag antibodies (Qiagen, Valencia, CA).
Western blot and immunoprecipitation
Transfected and nontransfected 293 cells (10 × 106) were solubilized with RIPA lysis buffer with protease inhibitors (Sigma-Aldrich, St Louis, MO). Total protein (500 μg) was precleared with 50 μL protein A beads (Amersham Pharmacia Biotech, Piscataway, NJ) for 1 hour at 4°C and incubated with 5 μg anti–BCMA antibody or control immunoglobulin G (IgG) for 3 hours followed by 35 μL 1:1 slurry of protein A beads (Amersham Pharmacia) for 1 hour. The beads were washed 5 times with lysis buffer, and the precipitates were subjected to protein gel electrophoresis using 8% to 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) with Tris-glycine buffer and blotted on a nitrocellulose membrane. Membranes were stained with either anti–His-Tag or patient serum (1:200 dilution) followed by a secondary antibody and visualized using chemiluminescence.
Flow cytometry
Transfected BCMA-293 cells (5 × 105) were incubated with goat anti–BCMA antibody followed by RPE-conjugated donkey anti–goat IgG (Jackson ImmunoResearch, West Grove, PA). Identical cells were incubated with human serum (1:10 dilution) for 3 hours followed by R-phycoerythrin (RPE)–conjugated goat anti–human IgG (Jackson ImmunoResearch). In the same experiments, nontransfected 293 cells or 293 cells transfected with the empty vector (vector-293) were stained with the same antibodies or human serum to determine the level of nonspecific background fluorescence. A minimum of 15 000 gated cells was acquired using a Coulter EPICS XL flow cytometer (Beckman Coulter, Hialeah, FL) and data analyzed using EXPO software (Beckman Coulter). Mean fluorescence intensity (MFI) was determined for each test sample. BCMA-specific fluorescence for each sample was calculated as the MFI for BCMA-293 minus MFI for vector-293.
Complement-mediated cytotoxicity and ADCC
Specific cell lysis with antibody and complement was measured using a standard 2-hour 51Cr release assay. Rabbit complement dilutions ranging between 1:2 and 1:40 without additional antibody were tested to determine nonspecific complement toxicity and a 1:4 dilution was chosen for all assays. Cells incubated with complement only or with serum only were used as a negative control and cells incubated with polyclonal anti–BCMA antibody were used as a positive control. For ADCC, peripheral blood mononuclear cells (PBMCs) from healthy donors were used as effector cells in a 4-hour 51Cr release assay. Radiolabeled BCMA-293 and vector-293 cells or primary myeloma cells were first incubated with different dilutions of patient serum; effector PBMCs were then added at different effector-to-target (E/T) ratios: 15:1, 60:1, and 100:1. Spontaneous release was determined by incubating target cells with media alone and maximum release was obtained by lysing cells in 10% NP-40. Percent specific cytotoxicity was calculated by the formula: (experimental release - spontaneous release) / (maximum release - spontaneous release) × 100.
Statistical analysis
The upper limit of nonspecific fluorescence in the flow cytometry assay for BCMA antibodies was defined as less than or equal to 0.2 MFI units. This was determined by the 95th percentile of MFI in 40 healthy donors. To examine whether the mean intensity fluorescence of BCMA antibodies differed among healthy donors, DLI responders, DLI nonresponders, patients with chronic graft-versus-host disease (cGVHD), patients with T-cell–depleted bone marrow transplantation (TCD-BMT), and patients treated with nonmyeloablative therapy, the Wilcoxon rank-sum test was used for continuous BCMA antibody measures. No adjustment was made for multiple 2-way comparisons.
Results
Expression of BCMA in normal plasma cells and myeloma cells
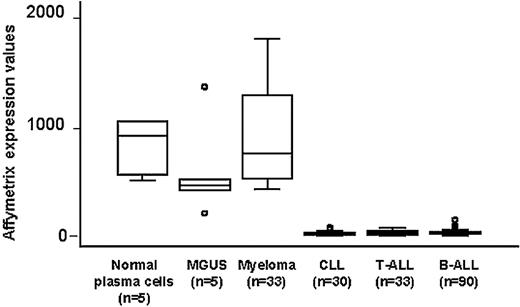
BCMA is known to be preferentially expressed in mature B cells.15,17 To examine the potential role of BCMA as a myeloma-associated antigen, BCMA gene expression was further characterized using high-density oligonucleotide microarrays in 33 primary myelomas. Gene expression in human myeloma cells was compared with purified plasma cells from patients with MGUS and from normal bone marrow as well as with primary tumor cells from B-cell lineage leukemias. As shown in Figure 1, the BCMA gene was highly expressed in all myeloma samples. Although purified plasma cells from patients with MGUS had lower expression of BCMA, there was no significant difference when compared with the expression found in normal plasma cells or myeloma cells. In contrast, BCMA expression was significantly lower in B-cell chronic lymphocytic leukemia (CLL) and pre-B-acute lymphocytic leukemia (ALL) (P < .001). The very low levels of BCMA gene expression in CLL and pre–B-ALL were similar to the level of BCMA in T-ALL. These results confirmed the selective expression of BCMA in late stages of B-cell maturation and also suggested that expression of BCMA in myeloma primarily reflects the derivation of these tumor cells from normal plasma cells.29
BCMA Western blot with patient serum
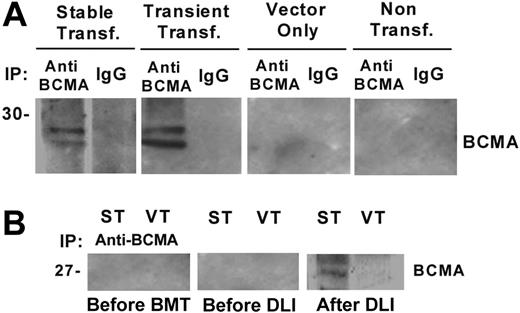
To further characterize the antibody response to BCMA following DLI we transfected human 293 cells with the full-length BCMA gene to create a stable cell line expressing BCMA (BCMA-293). As shown in Figure 2A, Western blotting with control anti–BCMA antibody revealed 2 positive bands in BCMA-293 cell lysate as well as in lysate from 293 cells transiently transfected with BCMA plasmid. No BCMA signal was detected in vector-293 cells or in nontransfected 293 cells. As shown in Figure 2B, antibodies specific for BCMA were also detected by Western blot in patient serum obtained 4 months after DLI. In contrast, pre-BMT and pre-DLI serum samples from the same patient were negative by Western blot. These results confirmed that the antibody response post-DLI was specifically directed at epitopes contained in human BCMA protein.14
Quantitative expression of the BCMA gene in primary myeloma cells, plasma cells from patients with MGUS and normal bone marrow, CLL, T-ALL, and pre-B-ALL measured by Affymetrix U95Av2 microarray. Box plots define the median values, 25% to 75% of values around the median and the range of values. ○ indicates outliers.
Quantitative expression of the BCMA gene in primary myeloma cells, plasma cells from patients with MGUS and normal bone marrow, CLL, T-ALL, and pre-B-ALL measured by Affymetrix U95Av2 microarray. Box plots define the median values, 25% to 75% of values around the median and the range of values. ○ indicates outliers.
Western blot analysis with patient serum. (A) Western blot analysis of immune precipitates from BCMA-293 stable transfectants, BCMA-293 transient transfectants, empty vector-293, and nontransfected 293 cells. (B) Immune precipitates (IP) from BCMA-293 (ST) and vector-293 (VT) cell lysates were blotted with patient serum (1:200 dilution) obtained before BMT, before DLI, and 4 months after DLI.
Western blot analysis with patient serum. (A) Western blot analysis of immune precipitates from BCMA-293 stable transfectants, BCMA-293 transient transfectants, empty vector-293, and nontransfected 293 cells. (B) Immune precipitates (IP) from BCMA-293 (ST) and vector-293 (VT) cell lysates were blotted with patient serum (1:200 dilution) obtained before BMT, before DLI, and 4 months after DLI.
Post-DLI serum is reactive with the cell-surface domain of BCMA
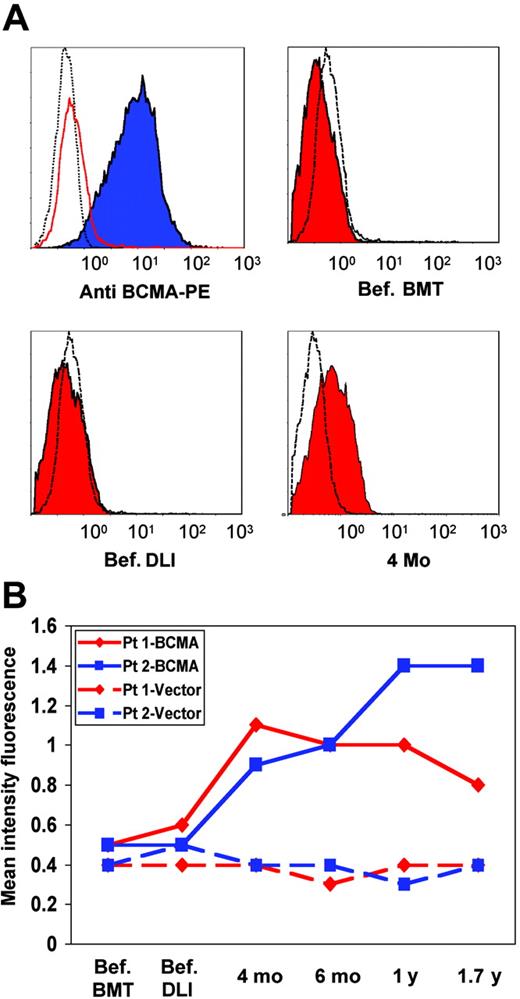
BCMA-293 cells were next used to determine whether the antibody response was directed against the extracellular domain of BCMA. BCMA-293 cells were incubated with pre-BMT, pre-DLI, and post-DLI serum from 2 patients previously found to be positive when screening the myeloma cDNA library. As shown in Figure 3A, BCMA-293 cells were strongly positive when stained with the anti–BCMA control antibody. Significant reactivity with BCMA-293 was also found with patient serum obtained after DLI (Figure 3A) compared with vector-293 control cells. In contrast, pre-BMT and pre-DLI serum (Figure 3A) had no BCMA reactivity compared with vector-293 cells. Serial serum samples obtained before and after DLI from both patients were then tested by flow cytometry. As shown in Figure 3B, antibodies specific for BCMA were first detectable 4 months after DLI and remained detectable 1.7 years after DLI in both patients.
Flow cytometric analysis with patient serum. (A) Flow cytometric analysis of BCMA-293 (blue filled histogram), vector-293 (red open histogram), and nontransfected 293 cells (black open histogram) with control anti–BCMA antibody. BCMA-293 (red filled histograms) and vector-293 cells (black open histograms) incubated with pre-BMT (Bef. BMT), pre-DLI (Bef. DLI), and 4 months post-DLI serum (4 mo). PE indicates phycoerythrin. (B) Mean fluorescence intensity (MFI) of serial patient samples tested for reactivity against BCMA-293 (—) and vector-293 control cells (–). ♦ indicates patient 1; ▪, patient 2.
Flow cytometric analysis with patient serum. (A) Flow cytometric analysis of BCMA-293 (blue filled histogram), vector-293 (red open histogram), and nontransfected 293 cells (black open histogram) with control anti–BCMA antibody. BCMA-293 (red filled histograms) and vector-293 cells (black open histograms) incubated with pre-BMT (Bef. BMT), pre-DLI (Bef. DLI), and 4 months post-DLI serum (4 mo). PE indicates phycoerythrin. (B) Mean fluorescence intensity (MFI) of serial patient samples tested for reactivity against BCMA-293 (—) and vector-293 control cells (–). ♦ indicates patient 1; ▪, patient 2.
Functional effects of anti-BCMA antibodies
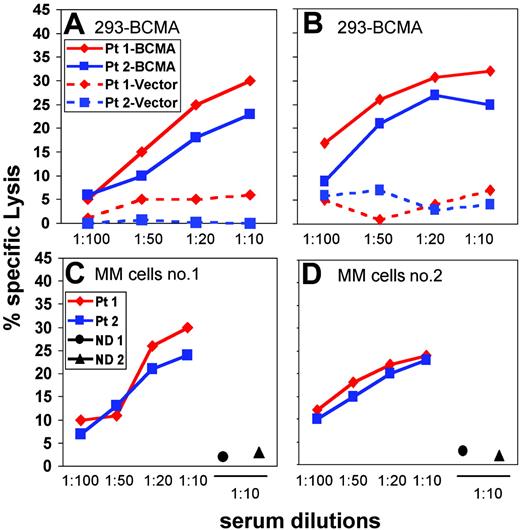
Having determined that patients with myeloma who respond to DLI can develop antibody responses directed against the cell-surface domain of BCMA we next determined whether these antibodies could mediate lysis of BCMA-positive target cells. As shown in Figure 4A, post-DLI serum from 2 patients was able to initiate complement-mediated lysis of BCMA-293 target cells in a dose-dependent manner. This lytic effect was specific for BCMA since control vector-293 cells were not affected by the same serum samples.
We also tested the ability of patient serum to mediate ADCC. Testing different E/T ratios ranging from 15:1 to 100:1, the percent lysis increased in a dose-dependent manner (data not shown) and a final E/T ratio of 100:1 was used for all subsequent experiments. As shown in Figure 4B, increasing concentrations of post-DLI serum from both patients mediated increased levels of ADCC when tested against BCMA-293 target cells. ADCC activity was also specific for BCMA since control vector-293 cells were not lysed by the same serum samples. The functional activity of patient serum was also tested against primary BCMA-positive myeloma cells. As shown in Figure 4C-D, post-DLI serum from both patients induced similar levels of ADCC against 2 different BCMA-positive allogeneic myeloma cell targets. For comparison, no ADCC was noted when serum from 2 healthy donors was tested in the same assay against these primary tumor cells. When tested against 2 primary myeloma samples that did not express cell-surface BCMA, post-DLI serum showed no cytotoxicity (data not shown), confirming the specificity of the ADCC response for BCMA.
Complement-mediated cytotoxicity and ADCC using patient serum. (A-B) — indicates BCMA-293 cells; –, vector-293 cells; (A-D) ♦, patient 1; ▪, patient 2. (A) Complement-mediated cytotoxicity of post-DLI patient serum against BCMA-293 or vector-293 target cells. (B) ADCC mediated by post-DLI patient serum against BCMA-293 or vector-293 target cells. (C-D) ADCC mediated by post-DLI patient serum against BCMA-positive multiple myeloma (MM) cells. Serum from 2 healthy donors (• and ▴) was also tested at 1:10 dilution.
Complement-mediated cytotoxicity and ADCC using patient serum. (A-B) — indicates BCMA-293 cells; –, vector-293 cells; (A-D) ♦, patient 1; ▪, patient 2. (A) Complement-mediated cytotoxicity of post-DLI patient serum against BCMA-293 or vector-293 target cells. (B) ADCC mediated by post-DLI patient serum against BCMA-293 or vector-293 target cells. (C-D) ADCC mediated by post-DLI patient serum against BCMA-positive multiple myeloma (MM) cells. Serum from 2 healthy donors (• and ▴) was also tested at 1:10 dilution.
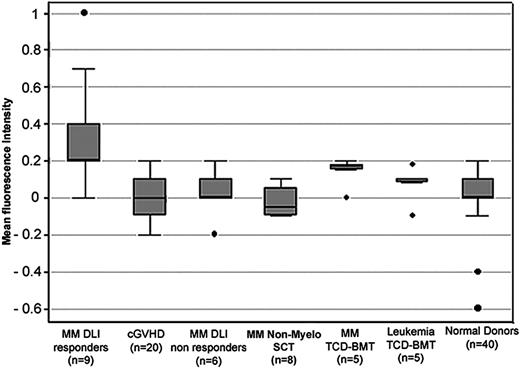
Detection of BCMA antibodies in posttransplantation patient serum compared with serum from healthy donors. BCMA-specific fluorescence was measured by flow cytometry. Box plots describe the median values, 25% to 75% values around the median, and the range of values for each group. • indicates outliers.
Detection of BCMA antibodies in posttransplantation patient serum compared with serum from healthy donors. BCMA-specific fluorescence was measured by flow cytometry. Box plots describe the median values, 25% to 75% values around the median, and the range of values for each group. • indicates outliers.
Flow cytometric screening for BCMA antibodies
Having established that flow cytometric testing of BCMA transfectants provided a sensitive method for detecting antibodies specific for BCMA in patient samples, we used this assay to test serum samples from 53 patients with myeloma and other hematologic malignancies who had undergone allogeneic HSCT and 40 healthy donors (Figure 5). Using this assay, 4 of 9 myeloma patients who responded to DLI had significant reactivity with BCMA-293. This included 2 patients who had not been positive in previous assays as well as the 2 known positive patients. This difference is likely due to the use of less diluted serum in the flow cytometry assay (1:10 compared with 1:200 or 1:500 dilution). At higher serum concentrations, the sensitivity of the assay was increased and lower levels of BCMA antibodies could be detected in additional DLI responders. As shown in Figure 5, the mean level of BCMA-specific fluorescence was significantly higher in the 9 myeloma DLI responders compared with 40 healthy donors (P < .001), 6 myeloma patients who did not respond to DLI (P = .01), 20 patients with chronic GVHD (P < .001), 5 patients with different leukemias who underwent allogeneic HSCT with T-cell–depleted marrow (P = .04), and 8 myeloma patients who underwent allogeneic HSCT after nonmyeloablative conditioning (P < .001). In this latter group, 6 of the 8 patients had persistent disease after transplantation and did not respond to treatment. In contrast, BCMA-specific fluorescence was not significantly different in myeloma DLI responders compared with myeloma patients in remission for more than 5 years after allogeneic HSCT with T-cell–depleted marrow (P = .30). Interestingly, BCMA-specific fluorescence was also significantly higher in myeloma patients in long-term remission compared with healthy donors (P = .01). Although the presence of BCMA antibodies in this last group of patients was not confirmed using other methods, these observations suggest that BCMA antibodies may occur more generally in myeloma patients who maintain long-term remissions after allogeneic HSCT.
Discussion
Although donor T cells are presumed to be the primary mediators of tumor immunity after allogeneic HSCT, recent studies from our laboratory have shown that donor B cells are also capable of mounting antibody responses to a variety of tumor-associated antigens.9,14,30 The development of antibodies specific for tumor-associated antigens correlate with tumor responses and high titer IgG antibodies persist at high levels for prolonged periods of at least several years in patients who remain in remission following allogeneic HSCT. However, in almost all cases, these antibody responses have been found to target intracellular proteins and the mechanisms whereby these antibodies might contribute to tumor immunity in vivo have not been established. Since these proteins are intracellular targets, antibodies could facilitate presentation of these antigens to dendritic cells and augment T-cell responses to peptide epitopes presented by major histocompatibility complex molecules. Similar mechanisms have been demonstrated for anti-body responses to intracellular antigens associated with autoimmunity,31 but experimental evidence for productive interactions between B- and T-cell immunity to tumor-associated antigens have not yet been demonstrated. In contrast, the experiments in this report demonstrate that antibody responses in vivo are also directed against cell-surface molecules. Since these antibodies are able to mediate lysis of myeloma tumor cells through both complement activation and ADCC, this represents the first demonstration that antibody responses to tumor-associated antigens after allogeneic HSCT can contribute directly to tumor cell lysis in vivo.
Although BCMA is expressed on normal plasma cells as well as myeloma cells, the targeting of cell-surface antigens expressed on normal B cells as well as B-cell tumors can result in the selective depletion of tumor cells in vivo. Thus, despite the fact that CD20 antigen is expressed on all mature B lymphocytes, the expression of this surface antigen on B-lymphoma cells renders these cells susceptible to lysis by rituximab in vivo.32 As a result, rituximab is commonly used alone or in combination with other agents in the treatment of various B-cell lymphomas. Although the primary mechanism of action of rituximab remains controversial, there is strong evidence that both complement activation and ADCC contribute to tumor cell lysis in vivo.33,34 In our analysis, antibodies specific for BCMA developed in several DLI responders. Although the derivation of these antibodies was not directly examined, they are presumably derived from donor B cells since these patients also converted to complete donor hematopoiesis after DLI.10 Like exogenous humanized monoclonal antibodies specific for CD20, these endogenous antibodies specific for BCMA are also able to mediate complement lysis and ADCC of primary myeloma tumor cells and transfectants expressing BCMA surface antigen. Control cells not expressing BCMA were not affected.
Despite the persistence of BCMA antibodies for prolonged periods, neither of the 2 patients with high titer antibodies developed hypogammaglobulinemia after DLI. These results suggest that normal plasma cells may be relatively resistant in vivo to the effects of anti-BCMA compared with myeloma cells. Alternatively, BCMA protein is known to be expressed in intracellular compartments as well as on the cell surface during B-cell maturation. Although levels of BCMA RNA expression are similar in normal plasma cells and myeloma cells, the level of BCMA protein expression of the cell surface of normal cells may not be sufficient to mediate lysis by circulating antibody. Although the effects of BCMA antibodies on normal plasma cells have not been directly examined, extensive clinical experience with rituximab also suggests that normal levels of polyclonal immunoglobulin can be maintained despite prolonged treatment and elimination of CD20+ lymphoma cells and normal B cells.35 The mechanisms for maintaining normal production of immunoglobulins during rituximab therapy have not been clearly established and further studies will be needed to examine the effects of anti-BCMA on normal plasma cell numbers and function in vivo.
Finally, it is important to note that IgG specific for BCMA persisted for over 1 year after DLI in both of our index patients. The present studies have focused on the functional significance of BCMA antibodies that developed in these individuals. However, the long-term persistence of high-titer IgG antibodies also suggests that this represents a coordinated immune response involving both B- and T-cell immunity to BCMA. Similar coordinated responses against cell membrane antigens have recently been demonstrated in patients with autoimmune thyroiditis.36 Further studies will be needed to determine whether BCMA-derived peptides can be presented by major histocompatibility complex molecules and whether BCMA peptides can also elicit specific T-cell responses. If BCMA is found to have also elicited specific T-cell immunity in these individuals, this would provide additional support for exploiting BCMA as a new therapeutic target in patients with multiple myeloma.
Prepublished online as Blood First Edition Paper, February 3, 2005; DOI 10.1182/blood-2004-11-4463.
Supported by National Institutes of Health grants CA078378 and AI29530 and the Ted and Eileen Pasquarello Research Fund.
An Inside Blood analysis of this article appears at the front of the issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal