Abstract
Despite major advances, multiple myeloma (MM) remains an incurable malignancy. Recently we have found that disease stabilization was achieved in 64% of patients with advanced MM treated with the farnesyltransferase inhibitor R115777 (Zarnestra) in a phase 2 clinical trial. In order to enhance R115777 antitumor activity in MM, we examined the combination of this novel agent with other anticancer drugs in MM cell lines. In this study, R115777 was found to synergize with paclitaxel and docetaxel, but not with other chemotherapy agents, including doxorubicin, 5-fluorouracil, cisplastin, melphalan, mitoxantrone, and dexamethasone. R115777 synergized with paclitaxel to inhibit MM cell proliferation and to induce apoptosis. Synergism in the induction of apoptosis was accompanied by increase in cytochrome c release and caspase-3 activation. Furthermore, flow cytometry analysis also showed that paclitaxel and R115777 synergized to induce G2/M cell-cycle arrest. Importantly, synergism was observed in taxane- and R115777-resistant MM cells. In the human severe combined immunodeficient (SCID-hu) bone model of myeloma growth, the ability of paclitaxel to inhibit tumor growth in vivo was enhanced by R115777. Combination of paclitaxel or docetaxel with R115777 in the treatment of MM cells from patients with multiple myeloma was more beneficial than treatment with single agents. Our results provide the basis for combination therapy clinical trials with paclitaxel or docetaxel with R115777 in MM patients. (Blood. 2005;105:4759-4766)
Introduction
Multiple myeloma (MM) is a hematologic malignancy characterized by a clonal expansion of plasma cells in bone marrow, accounting for 1% of all malignancies and 10% of hematologic malignancies.1 Despite recent advances in treatment with the development of new targeted therapies, it remains an incurable disease with median survival time ranging from 3 to 6 years. A challenge in drug development for myeloma is the absence of a genetic abnormality that uniformly affects all patients with the disease. In contrast, the disease is characterized by its heterogeneity, and it is most likely that combination therapy targeting several pathways responsible for myeloma malignancy will be needed for optimal disease control.
The small guanosine triphosphatase (GTPase) Ras is an important target in myeloma, as mutations of this gene are commonly encountered in myeloma and are associated with disease progression and decreased survival.2,3 Because Ras and other proteins require farnesylation, a lipid posttranslational modification, for malignant transformation activity, several investigators developed farnesyltransferase inhibitors (FTIs) as potential anticancer drugs.4 One of the FTIs, R115777, has been studied extensively in the clinic, and as a single agent has shown some clinical activity in both solid tumors such as breast5 and hematologic diseases such as acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS).6 We conducted a phase 2 trial of R115777 in patients with advanced myeloma, and found R115777 to induce disease stabilization in 64% of the patients.7 Although some patients clearly benefited from treatment with R115777, only disease stabilization, but not actual responses, was observed. Therefore, we explored the use of this drug in combination with other anticancer agents with the goal of identifying drugs capable of enhancing the antitumor activity of R115777.
To this end, we examined the effects on MM cells of combinations of R115777 with several cytotoxic agents such as taxanes, doxorubicin, cisplatin, 5-fluorouracil (5-FU), melphalan, mitoxantrone, or dexamethasone and found that only taxanes synergized with R115777. The combination of paclitaxel and R115777 was very effective at inhibiting proliferation and at inducing mitotic arrest and apoptosis. Importantly, MM cells that are resistant to R115777 still responded to the combination treatment in a synergistic fashion. In the human severe combined immunodeficient (SCID-hu) bone model that is permissive for engraftment and growth of MM,8,9 treatment with the combination was more beneficial than monotherapy. Combination of paclitaxel or docetaxel with R115777 in bone marrow mononuclear cells (MNCs) from patients with multiple myeloma was also more effective than monotherapy. The results provide the basis for investigating the use of the combination of paclitaxel or docetaxel with R115777 in MM patients.
Materials and methods
Cell lines and chemicals
RMPI8226/S cells, U266 cells, and H929 cells were purchased from American Type Culture Collection (ATCC, Manassas, VA). R115777-resistant RPMI8226 cells (RPMI8226/R5) were obtained by selecting RMPI8226/S cells in the presence of micromolar levels of R115777. RMPI8226 cells and U266 cells were cultured in RMPI1640 medium with 10% fetal bovine serum (FBS). H929 cells were cultured in RMPI 1640 medium (ATCC), 10% FBS, and 50 μM β-mercaptoethanol. Cells were maintained at 37°C in a humidified incubator with the supply of 5% CO2. Chemicals used to treat cells were dissolved in dimethyl sulfoxide (DMSO) and stored at -80°C. R115777 was obtained from Johnson and Johnson Pharmaceutical (Raritan, NJ). Docetaxel was from Aventis Pharmaceuticals (Bridgewater, NJ). All other chemotherapeutic agents were purchased from Sigma (St Louis, MO).
MTT assays
Cell suspensions at 5 to 10 × 103 cells per 200 μL medium each well were aliquoted in 96-well plates. Drugs were diluted with medium and added to the plates to achieve various drug concentrations and equal amount of solvent (DMSO) among all wells. The cells were then cultured for 48 to 72 hours. Methyl-thiazol tetrazolium (MTT, 100 μL; Sigma) at the concentration of 2 mg/mL in 1 x phosphate-buffered saline (PBS) was added to each well. The plates were incubated in the incubator for another 3 hours. The medium was carefully taken away, and 150 μL DMSO was added to each well. The plates were then shaken gently for 10 minutes at room temperature, and absorbance at 540 nm was measured. The viability of untreated cells was set as 100%, and viability in other groups was calculated by comparing the optical density (OD) readings with the control.
TUNEL assays and caspase assays
RPMI8226/S cells (2 × 105/mL) were treated with 1 nM paclitaxel or 50 nM R115777 or both for 36 hours. Cells were then collected and slides were made with Cytospin (Thermo-Forma, Pittsburgh, PA). Deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) assays were performed using In Situ Cell Death Detection kit, AP, from Roche Molecular Biochemicals (Indianapolis, IN) according to the manufacturer's instruction. At least 300 cells from 3 vision fields were counted from each slide. Images were captured with a Leica model IV/00 inverted microscope and 40×/0.17 NA objective lens (Leica Microsystems, Bannockburn, IL) and a Spot Insight camera, model 3.2.0 (Diagnostic Instruments, Sterling Heights, MI). PAX-IT v.5 (PAXcam, Franklin Park, IL) and Adobe Photoshop CS software (Adobe, San Jose, CA) were used.
For caspase activity assays, RPMI8226/S cells (2 × 105/mL) were treated with 2 nM paclitaxel or 100 nM R115777 or both for 48 hours. Cells were collected and lysed. Caspase-3 activity was assayed using Caspase-3 Colorimetric Assay kit from BD Pharmingen (San Diego, CA), and caspase-8 activity was assayed using Caspase-8 Activity Assay kit from Chemicon International (Temecula, CA) according to manufacturer's instructions.
Flow cytometric analysis
For cell-cycle analysis, RPMI8226/S cells (1 × 105/mL) were treated with drugs for 48 hours. Cells were collected by spinning at 805 g for 5 minutes. The cells were then fixed with cold ethanol for 20 minutes on ice, treated with RNase A (0.5 mg/mL in 1.12% sodium citrate) for 15 minutes at 37°C, and stained with propidium iodine (0.05 mg/mL in 1.12% sodium citrate) for 30 minutes at room temperature. Fluorescence staining was analyzed by a flow cytometer.
Western blot analysis
RPMI8226/S cells (2 × 105/mL) were treated with drugs for 72 hours. Western blot analysis was performed as previously described.10 Polyclonal antibodies against human proteins were from Santa Cruz Biotechnology (H-Ras, sc-520; B-cell lymphoma 2 [Bcl-2]-antagonist of cell [Bad], sc-7869; Bcl-2-associated X protein [Bax], sc-7480; bak, sc-832; Santa Cruz, CA), BD Pharmingen (bcl-2, no. 554160; bcl-2 XL, no. 556361), and Upstate Biotechnology (pBad(Ser155), no. 07138; Lake Placid, NY). Monoclonal antibodies against human proteins were from BD Pharmingen (cytochrome c, no. 556433) and Sigma (β-actin, A-5441). All secondary antibodies were from Jackson ImmunoResearch Laboratories (Western Grove, PA). For cytochrome c release assays, cells were lysed in lysis buffer (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.5], 10 mM KCl, and 1 mM EDTA [ethylenediaminetetraacetic acid]) with protease inhibitor cocktail (no. 8340; Sigma), frozen and thawed 3 times, and spun at 2000g for 5 minutes, and the supernatant was further centrifuged at 60 000g for 30 minutes at 4°C. The supernatant was analyzed for cytochrome c content by Western blot analysis. All Western blot analysis experiments were repeated at least twice with similar results.
SCID-hu in vivo myeloma model study
C.B-17 mice homozygous for both severe combined immunodeficiency (scid) and beige (bg) mutations (C.B-1 7/GbmsTac-Prkdcscid Lystbg, herein referred to as SCID; Taconic, Germantown, NY) were used. Fetal tissues of 18 to 23 weeks of gestation were obtained from Advance Bioscience Resource (Alameda, CA) in compliance with the regulations issued by the state and federal governments. SCID mice 6 to 8 weeks old received transplants subcutaneously with fetal human bones (humerus, femur, or tibia) as described previously.11,12 At 6 weeks after implantation, 5 × 104 RPMI8226/S human MM cells in PBS were injected directly into the implanted bones. At 6 to 8 weeks after cells were implanted, mice were given R115777, 25 mg/kg per day, by gastric gavage for 2 weeks; paclitaxel, 12.5 mg/kg, was given intraperitoneally for 2 weeks, every 4 days. At the end of the treatment, mice were humanely killed and implanted bones were removed for histology. Images were captured using a Leica Model IV/00 inverted microscope and 40× objective lens (Leica Microsystems, Bannockburn, IL), a Spot Insight Camera model 3.2.0 (Diagnostic Instruments, Sterling Heights, MI), and PAX-IT v.5 (Franklin Park, IL), and Adobe Photoshop CS (Adobe, San Jose, CA) software.
Apoptotic analysis of bone marrow MNCs from MM patients
Bone marrow aspirates were obtained from patients diagnosed with MM with patients' consent. Fresh bone marrow (BM) samples were used to prepare MNCs using Ficoll-Paque Plus from Amersham Bioscience (Arlington Heights, IL) according to the manufacturer's protocol. Isolated MNCs were cultured in a 12-well plate (1-3 × 105 MNCs/well) and treated with drugs for 72 hours. Cells were then washed with PBS, stained with CD138-phycoerythrin (PE), and then with annexin V-allophycocyanin (APC) and 7-aminoactinomycin D (7-AAD; BD Pharmingen), and subjected to flow cytometric analysis. Samples from myeloma patients were collected under the approved protocol MCC 13715, University of South Florida Institutional Review Board, Tampa, FL.
Statistical analysis
The large disparity in the median inhibitory concentration (IC50) dose range among R115777 (0-200 nM), docetaxel (0-0.6 nM), and paclitaxel (0-2.5 nM) considered in the isobologram analysis of additivity led us to conclude that the method of Tallarida13 was not applicable since the relative fractions of the latter 2 compounds relative to R115777 would be very small and result in a statistical test with low power. We applied the following alternative: Let Y0 and X0, respectively, represent the IC50 doses for R115777 alone and docetaxel (or paclitaxel) alone. Let Yt and Xt, respectively, represent doses of R115777 and docetaxel (or paclitaxel) achieving IC50 in combination. The null hypothesis of additivity implies that Y0/X0 = (Y0 - Yt)/Xt. Hence, additivity can be tested by testing H0: ln(Y0/X0)-ln((Y0 - Yt)/Xt) = 0. We used the Delta Method14 to estimate the standard error of the associated statistic and based P values on the t-statistic since the test statistic is a function of sample means. The Bonferroni method15 was used to control type I error in the multiple testing of additivity for the 9 combinations considered. Hence, P values less than .05/9 = .006 are considered statistically significant in the testing of additivity across the multiple combinations.
Results of statistical analysis
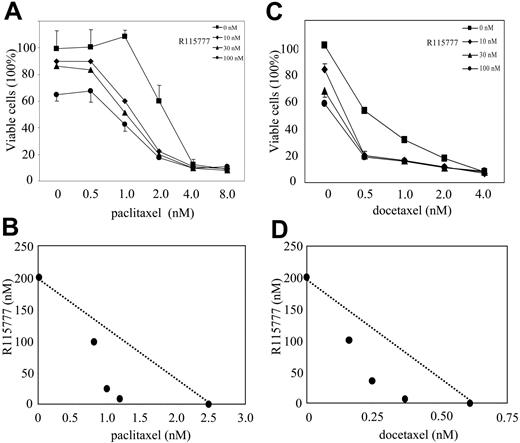
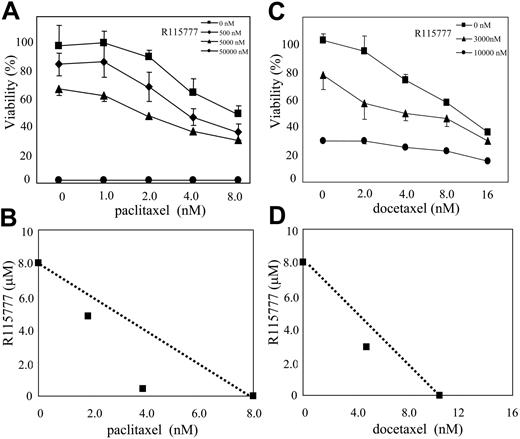
After adjusting for multiple testing, 6 combinations were found to be significantly different (P < .006) from that expected under additivity. One combination (5 nM R115777 and 2 nM of paclitaxel) was significant only at the unadjusted P less than .05 level. From the isobolograms (Figures 1B,D and 5B,D), we conclude that the significant combinations were synergistic.
Results
R115777 synergizes with paclitaxel or docetaxel, but not with other anticancer drugs to inhibit MM cell proliferation
A mechanism by which cancer cells become resistant to chemotherapy is by activating oncogenic and tumor survival signal transduction pathways. We therefore evaluated whether the antisignaling agent R115777 could reverse this resistance. In an effort to find chemotherapeutic agents whose antitumor effects can be enhanced by FTI R115777, we treated MM cell lines with the combination of R115777 and a group of commonly used chemotherapeutic agents. We first used MTT assays to identify anticancer drugs that would synergize with R115777 at inhibiting cell proliferation. As shown in Table 1, R115777 greatly enhanced the antiproliferative effect of paclitaxel and docetaxel in RPMI8226/S cells. In contrast, R115777 at concentrations as high as 100 nM had little effect on the ability of doxorubicin, cisplatin, and 5-FU to inhibit RPMI8226/S cell proliferation (Table 1). Furthermore, R115777 did not enhance the ability of 3 drugs commonly used in the treatment of MM (melphalan, dexamethasone, and mitoxantrone) to inhibit RPMI8226/S cell proliferation (data not shown). We next examined the effects of the combination treatment on the proliferation of 3 different MM cell lines. As shown in Table 2, the enhancement of antiproliferative effects of paclitaxel or docetaxel by R115777 was significant in RPMI8226/S cells and U266 cells, but not in H929 cells. The reason for the lack of enhancement with H929 cells is not known. However, it is conceivable that taxanes cause resistance to H929 cells by mechanisms not involving signal transduction pathways that are suppressed by R115777. Figure 1A and 1C show that in the absence of R115777, paclitaxel and docetaxel inhibited the proliferation of RPMI8226/S, with IC50 values of 2.5 and 0.6 nM, respectively. In the presence of increasing concentrations of R115777, the IC50 values of paclitaxel and docetaxel decreased significantly. Isobologram analysis13 of the data demonstrated synergism between R115777 and paclitaxel and docetaxel (Figure 1B,D). After controlling for multiple testing, 2 combinations of R115777 and paclitaxel and 3 combinations of R115777 and docetaxel were determined to be statistically different from additivity (ie, P < .006) (see “Materials and methods” for statistical details).
IC50 values of different drugs in the presence of R115777 in RPMI8226/S cells
R115777, nM . | Paclitaxel, nM . | Docetaxel, nM . | Gemcitibine, nM . | Doxorubicin, nM . | 5-FU, μM . | Cis-platinum, μM . |
|---|---|---|---|---|---|---|
| 0 | 2.5 | 0.60 | 10 | 100 | 5.0 | 8.0 |
| 10 | 1.2 | 0.35 | 9.0 | 100 | 5.0 | 7.8 |
| 30 | 1.0 | 0.25 | 8.0 | 100 | 5.0 | 7.5 |
| 100 | 0.8 | 0.20 | 7.0 | 70 | 5.0 | 7.0 |
R115777, nM . | Paclitaxel, nM . | Docetaxel, nM . | Gemcitibine, nM . | Doxorubicin, nM . | 5-FU, μM . | Cis-platinum, μM . |
|---|---|---|---|---|---|---|
| 0 | 2.5 | 0.60 | 10 | 100 | 5.0 | 8.0 |
| 10 | 1.2 | 0.35 | 9.0 | 100 | 5.0 | 7.8 |
| 30 | 1.0 | 0.25 | 8.0 | 100 | 5.0 | 7.5 |
| 100 | 0.8 | 0.20 | 7.0 | 70 | 5.0 | 7.0 |
Data obtained with MTT assays with 72-hour incubation of drugs.
IC50 values of paclitaxel or docetaxel in the presence of R115777 in MM cell lines
. | RPMI8226/S . | . | U266 . | . | H929 . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| R115777, nM . | P . | D . | P . | D . | P . | D . | |||
| 0 | 2.5 | 0.6 | 5.5 | 8.0 | 3.0 | 8.0 | |||
| 10 | 1.2 | 0.35 | 4.0 | — | 2.8 | — | |||
| 30 | 1.0 | 0.25 | 4.0 | 8.0 | 2.5 | 8.0 | |||
| 100 | 0.8 | 0.2 | 3.0 | 5.0 | 2.0 | 6.0 | |||
. | RPMI8226/S . | . | U266 . | . | H929 . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| R115777, nM . | P . | D . | P . | D . | P . | D . | |||
| 0 | 2.5 | 0.6 | 5.5 | 8.0 | 3.0 | 8.0 | |||
| 10 | 1.2 | 0.35 | 4.0 | — | 2.8 | — | |||
| 30 | 1.0 | 0.25 | 4.0 | 8.0 | 2.5 | 8.0 | |||
| 100 | 0.8 | 0.2 | 3.0 | 5.0 | 2.0 | 6.0 | |||
Data obtained with MTT assays with 72-hour incubation of drugs. Taxane concentrations are labeled in nM.
P indicates paclitaxel; D, docetaxel; —, not determined.
Synergism between R115777 and paclitaxel or docetaxel to inhibit MM cell proliferation. RPMI8226/S cells were treated with drugs for 72 hours before MTT assays. Results represent means and standard deviations of 4 independent experiments. (A,C) R115777 enhanced the cytotoxic effects of paclitaxel or docetaxel. (B,D) Isobologram based on data from panels A and C, showing synergism between R115777 and paclitaxel or docetaxel.
Synergism between R115777 and paclitaxel or docetaxel to inhibit MM cell proliferation. RPMI8226/S cells were treated with drugs for 72 hours before MTT assays. Results represent means and standard deviations of 4 independent experiments. (A,C) R115777 enhanced the cytotoxic effects of paclitaxel or docetaxel. (B,D) Isobologram based on data from panels A and C, showing synergism between R115777 and paclitaxel or docetaxel.
R115777 synergizes with paclitaxel to induce apoptosis
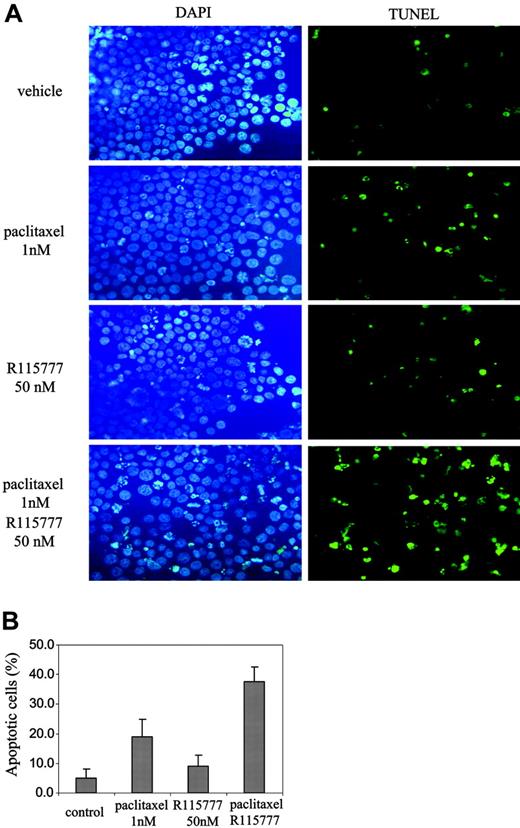
We further investigated whether R115777 enhances the ability of paclitaxel to induce apoptosis. Figure 2 shows that treatment with paclitaxel (1 nM) alone increased the percentage of apoptotic cells from 5.1% plus or minus 2.9% to 19.1% plus or minus 5.8%. R115777 (50 nM) treatment alone increased apoptosis from 5.1% plus or minus 2.9% to 9.0% plus or minus 3.8% only. In contrast, the combination treatment of R115777 (50 nM) and paclitaxel (1 nM) increased apoptosis to 37.6% plus or minus 5.0%, significantly higher than R115777 (50 nM) or paclitaxel (1 nM) alone (P < .01). Thus, although R115777 had little effect on its own, it greatly enhanced the ability of paclitaxel to induce apoptosis in RPMI8226/S MM cells (Figure 2).
R115777 synergizes with paclitaxel to induce apoptosis. RPMI8226/S cells were treated with paclitaxel (1 nM), or R115777 (50 nM), or both for 36 hours before TUNEL assays. (A) Representative pictures of TUNEL slides of cells with different drug treatments are shown. (B) Means and standard deviations of 3 independent experiments.
R115777 synergizes with paclitaxel to induce apoptosis. RPMI8226/S cells were treated with paclitaxel (1 nM), or R115777 (50 nM), or both for 36 hours before TUNEL assays. (A) Representative pictures of TUNEL slides of cells with different drug treatments are shown. (B) Means and standard deviations of 3 independent experiments.
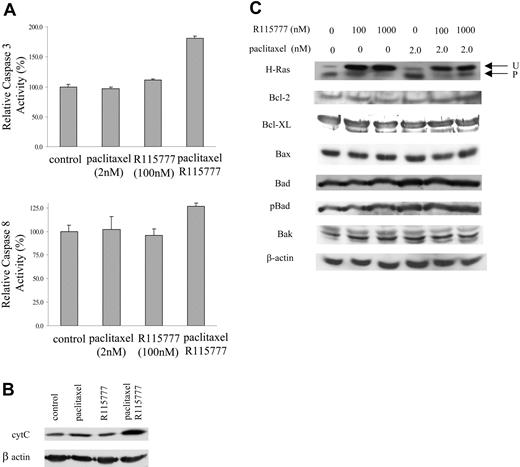
One of the possible mechanisms by which paclitaxel and R115777 could synergistically induce apoptosis is by the activation of the protease cascade of caspases. To this end, we treated RPMI8226/S cells with either R115777 or paclitaxel, alone or together, and examined caspase-3 and caspase-8 activity in the cells. As shown in Figure 3A, treatment of cells with both paclitaxel and R115777 caused significant (P < .01) caspase-3 activation, while neither alone showed any significant change. Caspase-8 activity was slightly higher in cells treated with both paclitaxel and R115777, but the difference between monotherapy and combination was not statistically significant. The activation of caspase-3 suggested that in MM cells treated with both paclitaxel and R115777, the apoptosis enhancement could be mediated at least in part through cytochrome c release from the mitochondria. To this end, we examined the effects of R115777 and paclitaxel treatments on cythochrome c release. Figure 3B shows that treatment with R115777 or paclitaxel each alone did not result in significant accumulation of cytochrome c in the cytosol. However, the combination of the 2 resulted in a significant increase in cytosolic cytochrome c. Cytochrome c release to the cytoplasm could be the result of an increase in proapoptotic Bcl2 family members or a decrease in prosurvival Bcl2 family members. Figure 3C shows that neither agent alone or in combination caused any significant change in the levels of Bcl2, Bcl-XL, Bax, Bak, phospho-Bax, or Bad, suggesting other mechanisms might be involved in the release of cytochrome c from mitochondria. As expected, R115777 inhibited the processing of farnesylated H-Ras (Figure 3D).
R115777 synergizes with paclitaxel to arrest cells at mitosis
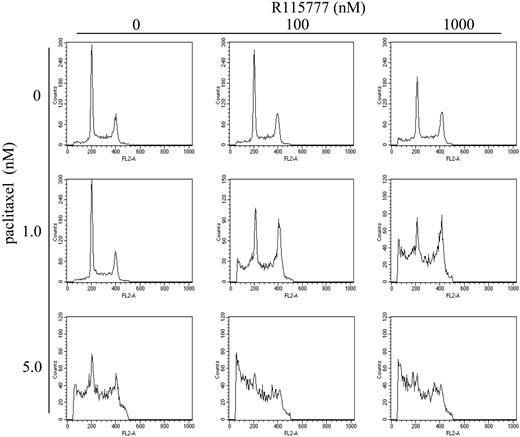
Paclitaxel stabilizes microtubules and causes mitotic arrest, which is important in paclitaxel-induced apoptosis.16,17 We therefore examined the effect of combination of paclitaxel and R115777 on cell-cycle progression. As shown in Figure 4 and in Table 3, 1 nM paclitaxel had no effect on cell-cycle distribution. Similarly, R115777 (100 nM) alone had little effect (Table 3). In contrast, combination treatment with paclitaxel (1 nM) and R115777 (100 nM) significantly decreased the proportion of RPMI8226/S cells in the G0/G1 phase (from 43.1% ± 0.48% to 24.39% ± 1.63%, P < .01) and in parallel doubled the proportion of cells in mitosis (from 21.27% ± 0.05% to 41.22% ± 1.67%, P < .01) (Figure 4; Table 3). Similarly, combination of 100 nM R115777 and 1 nM paclitaxel increased significantly the proportion of cells with hypodiploid DNA and/or cell debris, from 9.18% to 40.94%, further demonstrating synergism between R115777 and paclitaxel to induce apoptosis and cell death (Figure 4; Table 3).
Effect of paclitaxel and R115777 on cell-cycle progression
Cell-cycle parameters, % . | R115777 . | . | . | ||
|---|---|---|---|---|---|
| . | 0 nM . | 100 nM . | 1000 nM . | ||
| Paclitaxel, 0 nM | |||||
| G0/G1 | 43.10 ± 0.48 | 41.19 ± 2.01 | 33.14 ± 2.74 | ||
| S | 35.62 ± 0.53 | 31.94 ± 1.68 | 36.75 ± 0.64 | ||
| G2/M | 21.27 ± 0.05 | 26.87 ± 0.53 | 30.11 ± 2.18 | ||
| Debris | 9.18 ± 2.30 | 14.25 ± 1.45 | 18.88 ± 0.81 | ||
| Paclitaxel, 1.0 nM | |||||
| G0/G1 | 41.23 ± 4.67 | 24.39 ± 1.63 | 22.48 ± 2.67 | ||
| S | 35.86 ± 3.48 | 34.40 ± 0.57 | 31.26 ± 6.95 | ||
| G2/M | 22.90 ± 2.20 | 41.22 ± 1.67 | 46.26 ± 9.29 | ||
| Debris | 9.58 ± 1.21 | 40.94 ± 0.23 | 59.24 ± 3.04 | ||
| Paclitaxel, 5.0 nM | |||||
| G0/G1 | 25.27 ± 0.88 | 20.01 ± 0.12 | 15.44 ± 4.75 | ||
| S | 44.14 ± 4.44 | 33.98 ± 3.97 | 33.74 ± 10.15 | ||
| G2/M | 30.59 ± 3.65 | 46.01 ± 4.09 | 50.82 ± 6.11 | ||
| Debris | 45.51 ± 4.69 | 68.51 ± 2.56 | 73.91 ± 2.04 | ||
Cell-cycle parameters, % . | R115777 . | . | . | ||
|---|---|---|---|---|---|
| . | 0 nM . | 100 nM . | 1000 nM . | ||
| Paclitaxel, 0 nM | |||||
| G0/G1 | 43.10 ± 0.48 | 41.19 ± 2.01 | 33.14 ± 2.74 | ||
| S | 35.62 ± 0.53 | 31.94 ± 1.68 | 36.75 ± 0.64 | ||
| G2/M | 21.27 ± 0.05 | 26.87 ± 0.53 | 30.11 ± 2.18 | ||
| Debris | 9.18 ± 2.30 | 14.25 ± 1.45 | 18.88 ± 0.81 | ||
| Paclitaxel, 1.0 nM | |||||
| G0/G1 | 41.23 ± 4.67 | 24.39 ± 1.63 | 22.48 ± 2.67 | ||
| S | 35.86 ± 3.48 | 34.40 ± 0.57 | 31.26 ± 6.95 | ||
| G2/M | 22.90 ± 2.20 | 41.22 ± 1.67 | 46.26 ± 9.29 | ||
| Debris | 9.58 ± 1.21 | 40.94 ± 0.23 | 59.24 ± 3.04 | ||
| Paclitaxel, 5.0 nM | |||||
| G0/G1 | 25.27 ± 0.88 | 20.01 ± 0.12 | 15.44 ± 4.75 | ||
| S | 44.14 ± 4.44 | 33.98 ± 3.97 | 33.74 ± 10.15 | ||
| G2/M | 30.59 ± 3.65 | 46.01 ± 4.09 | 50.82 ± 6.11 | ||
| Debris | 45.51 ± 4.69 | 68.51 ± 2.56 | 73.91 ± 2.04 | ||
RPMI8226/S cells were treated for 48 hours before being stained with PI for cell-cycle analysis.
Paclitaxel and docetaxel sensitize R115777-resistant RPMI8226 and MM1 multiple myeloma cells
We next determined whether R115777-resistant multiple myeloma cells are sensitized to paclitaxel or docetaxel. To this end, we treated the R115777-resistant cell lines RPMI8226/R5 and MM1 with R115777, paclitaxel, or docetaxel, alone or in combination. The IC50 of R115777 for RPMI8226/R5 is 5 μM compared with 100 nM for RPMI8226/S cells (Robert Buzzeo, Steven Enkemann, Rama Nimmanapalli, M.A., M.G.L., W.S.D., and D.M.B., manuscript submitted, April 2005). In MTT analysis, we found that the RPMI8226/R5 cells were also resistant to paclitaxel (IC50 at 8 nM, compared with 2.5 nM for RPMI8226/S cells) or docetaxel (IC50 at 10 nM, compared with 0.6 nM for RMPI8226/S cells). Although the cells were resistant to R115777 and paclitaxel, 2 combinations showed synergistic effect at the unadjusted P less than .05 level. One of these combinations remained significant at the adjusted P < .001 level (Figure 5). In contrast, the combination of R115777 and docetaxel was not determined to be significantly different from additivity (P = .09). Similar results were also observed with another resistant multiple myeloma cell line, MM1. The IC50 values of R115777, paclitaxel, and docetaxel in this cell line are 10 μM, 20 nM, and 13 nM, respectively. In presence of R115777, the IC50 values of paclitaxel and docetaxel were much lower, 2.5 nM and 2.0 nM, respectively. Thus, in highly resistant multiple myeloma cell lines, combination treatment of R115777 with paclitaxel or docetaxel was more beneficial than monotherapy.
R115777 enhances the ability of paclitaxel to inhibit tumor growth in the MM SCID-hu in vivo model
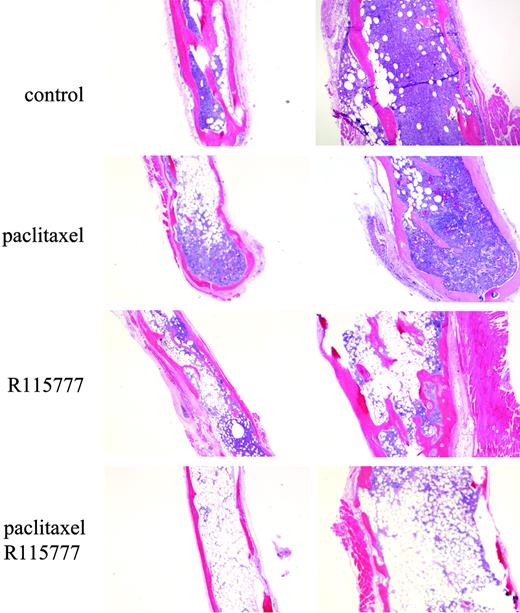
To determine whether the combination is effective in vivo, we studied the antitumor effects of paclitaxel and R115777 in SCID mice implanted with human fetal bones and injected with MM cells. To this end, RPMI8226/S cells were transplanted into the fetal bone grafts, and 4 weeks later the mice were treated with vehicle, R115777 alone, paclitaxel alone, or the combination of both drugs as described in “Materials and methods.” After 2 weeks of treatment, the fetal bones were removed for histology. As shown in Figure 6, paclitaxel or R115777 each alone had limited effect in eliminating proliferating MM cells from bone marrow in the fetal bone grafts. In contrast, the combination of paclitaxel and R115777 had a significant effect on inhibiting MM tumor burden (Figure 6). These in vivo results confirm our results in cultured MM cells.
R115777 synergizes with paclitaxel to induce cytochrome c (cyt C) and caspase-3. (A) R115777 (100 nM) synergized with paclitaxel (2 nM) in caspase-3 activation. RPMI8226/S cells were treated with drugs for 48 hours before caspase-3 and caspase-8 activities were assayed. Results represent means and standard deviation of 3 independent experiments. (B) R115777 (100 nM) synergized with paclitaxel (2 nM) in causing cytochrome c release. RPMI8226/S cells were treated with drugs for 72 hours before harvesting for Western blot analysis. (C) Effect of treatment of paclitaxel and R115777 on apoptotic and prosurvival Bcl2 family members. RPMI8226/S cells were treated with drugs at indicated concentrations for 72 hours before harvesting for Western blot analysis.
R115777 synergizes with paclitaxel to induce cytochrome c (cyt C) and caspase-3. (A) R115777 (100 nM) synergized with paclitaxel (2 nM) in caspase-3 activation. RPMI8226/S cells were treated with drugs for 48 hours before caspase-3 and caspase-8 activities were assayed. Results represent means and standard deviation of 3 independent experiments. (B) R115777 (100 nM) synergized with paclitaxel (2 nM) in causing cytochrome c release. RPMI8226/S cells were treated with drugs for 72 hours before harvesting for Western blot analysis. (C) Effect of treatment of paclitaxel and R115777 on apoptotic and prosurvival Bcl2 family members. RPMI8226/S cells were treated with drugs at indicated concentrations for 72 hours before harvesting for Western blot analysis.
R115777 synergizes with paclitaxel to cause mitotic arrest. RPMI8226/S cells were treated with drugs for 48 hours before being stained with PI and analyzed by flow cytometry. Representative cell-cycle profiles of cells with different drug treatments are shown.
R115777 synergizes with paclitaxel to cause mitotic arrest. RPMI8226/S cells were treated with drugs for 48 hours before being stained with PI and analyzed by flow cytometry. Representative cell-cycle profiles of cells with different drug treatments are shown.
R115777 enhances the ability of paclitaxel and docetaxel to induce apoptosis in bone marrow MNCs from MM patients
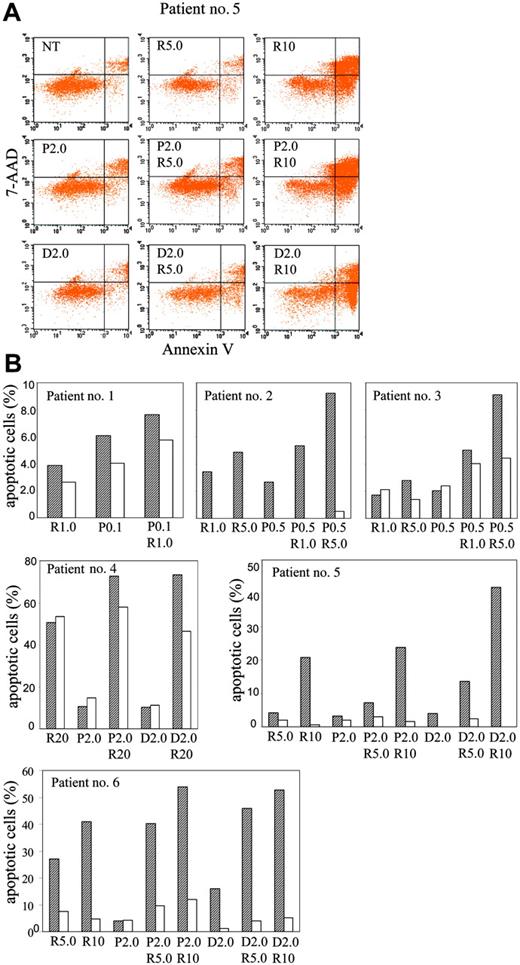
To explore the effectiveness of combination therapy of paclitaxel or docetaxel with R115777 in freshly isolated myeloma cells ex vivo, we examined the induction of apoptosis by R115777 and paclitaxel or docetaxel in bone marrow MNCs from 6 MM patients. We found that combination treatment with paclitaxel/docetaxel and R115777 is more beneficial than treatment with single agents. Contrary to MM cell lines, much higher concentrations of drugs are required to induce significant apoptosis in MNCs from these MM patients. Figure 7 shows results from flow cytometric analysis. Initially, we treated MNCs from 3 patients with low concentrations (R115777, 1 μM; paclitaxel, 100 to 500 nM). Although the induction of apoptosis was low, combination was still better than single-agent treatment in all 3 patient samples (Figure 7B). In samples from a fourth patient (no. 4), where the MNCs were treated with high concentrations (R115777, 20 μM; paclitaxel, 2 μM; docetaxel, 2 μM), the percentages of apoptotic CD138+ MM cells in the R115777-, paclitaxel-, and docetaxel-treated groups were 50.7%, 10.5%, and 10.3%, respectively (Figure 7B). In combination groups (paclitaxel/R115777 and docetaxel/R115777), the percentages were 72.8% and 73.3%, respectively. In CD138- cells, R115777, paclitaxel, and docetaxel induced 53.3%, 14.8%, and 11.2% apoptosis, respectively. The combination of R115777/paclitaxel and R115777/docetaxel induced 58.0% and 46.5% apoptosis, respectively (Figure 7B). In MNCs from a fifth patient, single treatment of R115777 alone (5 and 10 μM), paclitaxel alone (2 μM), and docetaxel alone (2 μM) resulted in 4.1%, 20.8%, 3.3%, and 4.0%, respectively, of CD138+ cells that are apoptotic (Figure 7A-B). Combination treatment resulted in 7.3% (R115777, 5 μM) and 13.1% (paclitaxel [2 μM] or docetaxel [2 μM]). Combination of R115777 (10 μM) and paclitaxel (2 μM) or docetaxel (2 μM) resulted in 23.9% and 41.8%, respectively, apoptotic CD138+ cells. In CD138- cells, less apoptosis was observed and no benefit from combination was observed (Figure 7B). Samples from a sixth patient treated at the same doses as those of samples from patient no. 5 showed similar results (Figure 7B). Taken together, these results suggest that paclitaxel and docetaxel enhanced the ability of R115777 to induce apoptosis in malignant MM cells at concentrations that are similar to those achieved in plasma from patients treated with paclitaxel (0.8 to 13 μM),18 docetaxel (1.92 to 3.34 μM),19 and R115777 (.35 to 3.76 μM).20 The concentration of R115777 in patients' bone marrow was found to be 4.16 to 7.48 μM.20
Synergism between R115777 and paclitaxel or docetaxel in R115777-resistant MM cells. RPMI8226/R5 cells were treated with drugs for 72 hours before MTT assays. Results represent means and standard deviations of 4 independent experiments. (A,C) R115777 enhanced the cytotoxic effects of paclitaxel or docetaxel. (B,D) Isobologram based on data from panels A and C, showing synergism between R115777 and paclitaxel or docetaxel.
Synergism between R115777 and paclitaxel or docetaxel in R115777-resistant MM cells. RPMI8226/R5 cells were treated with drugs for 72 hours before MTT assays. Results represent means and standard deviations of 4 independent experiments. (A,C) R115777 enhanced the cytotoxic effects of paclitaxel or docetaxel. (B,D) Isobologram based on data from panels A and C, showing synergism between R115777 and paclitaxel or docetaxel.
R115777 enhances paclitaxel antitumor activity in the SCID-hu bone mouse model of MM growth. SCID mice were implanted with human fetal bones injected with RPMI8226/S cells. After the tumors formed, the mice were treated with paclitaxel or R115777 or both for 2 weeks. The implanted bones were then analyzed by histology staining. Representative pictures from each group are shown.
R115777 enhances paclitaxel antitumor activity in the SCID-hu bone mouse model of MM growth. SCID mice were implanted with human fetal bones injected with RPMI8226/S cells. After the tumors formed, the mice were treated with paclitaxel or R115777 or both for 2 weeks. The implanted bones were then analyzed by histology staining. Representative pictures from each group are shown.
Combination of paclitaxel or docetaxel with R115777 is beneficial in the induction of apoptosis in MM patient bone marrow MNCs. MNCs were isolated from bone marrow aspirates and incubated with paclitaxel (P), docetaxel (D), and R115777 (R) at different concentrations (μM) for 48 to 72 hours. Cells were then stained with CD138-PE, annexin V-APC, and 7-AAD, and subjected to flow cytometric analysis as described in “Materials and methods.” Apoptosis in CD138+ and CD38- cells was then analyzed. (A) Dot graphs showing annexin-V staining profiles of cells from patient no. 5 treated with different drugs at different concentrations. CD138+ cells were gated for analysis. (B) Summary of annexing staining results of MNCs from 6 patients after treatment with different drugs at different concentrations. Net increases in apoptotic cells after subtracting the apoptotic cells in the untreated group from CD138+(▨) and CD138- (□) cells are shown.
Combination of paclitaxel or docetaxel with R115777 is beneficial in the induction of apoptosis in MM patient bone marrow MNCs. MNCs were isolated from bone marrow aspirates and incubated with paclitaxel (P), docetaxel (D), and R115777 (R) at different concentrations (μM) for 48 to 72 hours. Cells were then stained with CD138-PE, annexin V-APC, and 7-AAD, and subjected to flow cytometric analysis as described in “Materials and methods.” Apoptosis in CD138+ and CD38- cells was then analyzed. (A) Dot graphs showing annexin-V staining profiles of cells from patient no. 5 treated with different drugs at different concentrations. CD138+ cells were gated for analysis. (B) Summary of annexing staining results of MNCs from 6 patients after treatment with different drugs at different concentrations. Net increases in apoptotic cells after subtracting the apoptotic cells in the untreated group from CD138+(▨) and CD138- (□) cells are shown.
Discussion
Clinical investigations with the farnesyltransferase inhibitor R115777 demonstrated clinical activity in some cancers but not others. For example, in patients with high-risk leukemia20 and MDS21 response rates on the order of 30% were reported; lower response rates were seen with metastatic breast cancer (14%)5 and recurrent metastatic glioma (10%).37 However, no benefits were found for metastatic colorectal or pancreatic cancer.4 Recently, we have shown that in advanced multiple myeloma patients, R115777 as a single agent did not result in clinical responses but induced disease stabilization in a significantly large percentage (64%) of patients.7 This prompted us to investigate whether combining R115777 with other anticancer drugs would be more effective. The present preclinical study demonstrated that among all anticancer drugs studied (doxorubicin, paclitaxel, 5-fluoruracil, cisplatin, melphalan, dexamethasone, and mixozantrone), paclitaxel or docetaxel was most beneficial when combined with R115777 at inhibiting the proliferation of MM cells. The reason why only the taxanes synergize with R115777 is not known. One possible explanation is that both taxanes and R115777 are known to prevent progression through the mitotic phase of the cell division cycle. The synergy could be the result of R115777 and taxanes affecting different but critical steps during mitosis. The fact that taxanes synergize with R115777 is consistent with other reports that showed that combination of FTIs and paclitaxel is more effective than single-agent treatment in other cell lines from other cancers.22,23 Unlike this study with MM, however, FTI combinations with other anticancer drugs were found to be effective with other cancer types. For example, FTI combinations with paclitaxel, cisplatin, or gemcitabine were more effective than the corresponding monotherapies for lung tumor xenografts in nude mice.23
The mechanism by which FTIs inhibit tumor growth is not known partly because not all the farnesylated proteins critical to malignant transformation have been identified.24 Although the farnesylated GTPase Ras is clearly not the only target, it has been suggested that inhibition of the Ras/phosphatidylinositol 3-kinase (PI3K)/Akt pathway plays a pivotal role in the mechanism by which FTIs induce apoptosis in some human cancer cells10,25 ; paclitaxel has been shown to inhibit Akt activation26,27 and increase the activation of extracellular signal-related kinase (Erk),28,29 however, there is no indication that such effects contribute to the mechanism of antitumor activity of paclitaxel. We found that R115777 at 100 nM inhibited the activation of Erk in RPMI8226/S cells, but 2 nM paclitaxel did not have significant effect on Erk activation (data not shown). Phospho-Akt was barely detected in RPMI8226/S cells.
In addition to the PI3K/Akt and mitogen-induced extracellular kinase (Mek)/Erk pathways, the Janus kinase (JAK)/signal transducer and activator of transcription 3 (STAT3) pathway is another oncogenic pathway that is important for MM tumor survival.30,31 Furthermore, interleukin-6 (IL-6), an important paracrine factor for MM malignancy, activates STAT3, and R115777 at high concentrations has been shown to inhibit IL-6 activation of STAT3 and Erk.32 In contrast, in our studies R115777 at the low concentration (100 nM) that is effective at synergizing with paclitaxel did not inhibit IL-6-stimulated activation of STAT3 in RPMI8226 MM cells (data not shown). Similarly, neither paclitaxel nor R115777, alone or in combination, inhibited the expression levels of vascular endothelial growth factor (VEGF), another critical paracrine factor for MM malignancy31 (data not shown).
Our data also suggest that the observed synergistic interaction between R115777 and paclitaxel to inhibit MM proliferation can be due to at least 2 mechanisms. One involves cell-cycle arrest at mitosis, which was clearly demonstrated by showing that concentrations of R115777 (100 nM) and paclitaxel (1 nM) that had little effect on their own, significantly induced mitotic arrest when combined. The other involves a synergistic interaction to induce programmed cell death as demonstrated by cytochrome c release, caspase activation, and TUNEL staining. Although paclitaxel has previously been shown to induce mitotic arrest,33 the demonstration of synergy with R115777 is novel and suggests that the ability of this combination to induce potent apoptosis and tumor growth inhibition in the MM animal model used is at least in part due to this mitotic arrest.
In our experiments, the combination of R115777 and paclitaxel or docetaxel is more beneficial than monotherapy even in MM cells that are highly resistant to R115777 and taxanes. This is a critical finding as tumor resistance (either intrinsic or acquired) is one of the major mechanisms of failure to cancer therapy.34-36 Here we demonstrated that RPMI8226/R5 cells that are 50-fold more resistant to R115777, and 3- to 10-fold more resistant to paclitaxel or docetaxel than RPMI8226/S cells, were sensitized by combination treatment. Similar results were obtained with the highly resistant MM1 multiple myeloma cell line. These findings have major implications for future clinical trials as they suggest that the R115777/taxanes combination may broaden the spectrum of MM patients that can benefit from such treatment.
Taken together, our results demonstrate that the combination of paclitaxel with the FTI inhibitor R115777 synergizes to induce mitotic arrest and apoptosis. The beneficial effects that were demonstrated in MM cells from patients and in highly resistant MM cells coupled with the results from MM cells grown in vivo in a human bone marrow microenvironment give strong preclinical proof-of-concept for investigating this combination clinically in MM patients.
Prepublished online as Blood First Edition Paper, February 22, 2005; DOI 10.1182/blood-2004-11-4307.
Supported by National Cancer Institute grant no. CA83978 (S.M.S.). W.G.K. is the Newman Scholar of the Leukemia and Lymphoma Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr David End from Johnson and Johnson for providing us with R115777. We would also like to thank the H. Lee Moffitt Cancer Center & Research Institute Flow Cytometry, Statistics and Pathology core facilities.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal