Abstract
Acute myeloid leukemia (AML) cells are characterized by a constitutive and abnormal activation of the nuclear factor-κB (NF-κB) transcription factor. This study, conducted in vitro on 18 patients, shows that targeting the IKB kinase 2 (IKK2) kinase with the specific pharmacologic inhibitor AS602868 to block NF-κB activation led to apoptosis of human primary AML cells. Moreover, AS602868 potentiated the apoptotic response induced by the current chemotherapeutic drugs doxorubicin, cytarabine, or etoposide (VP16). AS602868-induced cell death was associated with rupture of the mitochondrial transmembrane potential and activation of cellular caspases. NF-κB inhibition did not affect normal CD34+ hematopoietic precursors, suggesting that it could represent a new adjuvant strategy for AML treatment.
Introduction
Acute myeloid leukemia (AML) is characterized by invasion of the bone marrow by leukemic myeloid blasts arrested at various maturation steps.1 Despite advances in diagnosis of the different subtypes of AML and progress in therapeutic approaches, current chemotherapies produce only initial remission. Relapse often occurs, and AML is finally fatal in more than 70% of cases.2 Dysregulation of malignant hematopoietic stem/progenitor cells (leukemic stem cells [LSCs]) produces blast cells with differentiation defects. LSCs, which are quiescent or slowly cycling and therefore less sensitive to chemotherapy, are responsible for disease relapse and represent the target for future innovative therapies.3 LSCs are both phenotypically and biologically similar to normal hematopoetic stem cells (HSCs), making discrimination and targeting difficult. One interesting difference is a constitutive activation of the nuclear factor-κB (NF-κB) transcription factor in LSC and AML blasts but not in HSCs.4 Such abnormal constitutive NF-κB activation has been detected in 2 other hematopoietic cancers: Hodgkin lymphoma5 and acute lymphoid leukemia (ALL)6 as well as in various solid tumors.7 Transcription factors of the NF-κB family are regulators of cell proliferation and survival8 and control expression of several genes relevant to the tumorigenic process.7 For instance, NF-κB promotes cell survival through expression of genes coding for antiapoptotic proteins (cellular inhibitor of apoptosis protein-1 [c-IAP1], c-IAP2, bfl-1, and bcl-xl). NF-κB is also known to stimulate cell proliferation via induction of growth factors (interleukin-2 [IL-2], granulocyte-macrophage colony-stimulating factor [GM-CSF]) or cell cycle regulators (cyclin D1, c-myc). NF-κB could participate in the resistance of tumor cells to treatments after expression of the multidrug resistance (MDR) protein. NF-κB could promote metastasis through induction of the extracellular matrix-degrading enzymes matrix metalloproteinase 9 (MMP9) and urokinase-type plasminogen activator (uPA).9-11 In addition, most actual antineoplastic drugs also activate NF-κB, an event that interferes with the treatments.
It has therefore been proposed that inhibition of NF-κB could be an adjuvant therapy for cancer.10 NF-κB dimers are maintained inactive in the cytosol by inhibitory subunits of the IκB family. Upon cell triggering by a wide spectrum of stimuli,12 IκB molecules are phosphorylated on 2 critical serine residues by 2 highly related specific kinases, IKK1, or α, and IKK2, or β, which together with the scaffold protein IKKγ/NF-κB essential modulator (NEMO) form the IKK complex that integrates signals for NF-κB activation.13 Serine phosphorylation results in polyubiquitination of IκB and its subsequent degradation by the proteasome.13 If transfection of a superrepressor form of the IκB-α inhibitory molecule is highly specific to block NF-κB, its use is restrained to cell lines.9,14 Besides, highly specific pharmacologic inhibitors of the NF-κB pathway are still missing.15 Promising results in clinical trials to treat multiple myeloma, characterized by constitutive NF-κB activation, have been obtained with proteasome inhibitors, but these molecules exert actions largely beyond NF-κB.16 In this study, we used a small molecule inhibitor of the IKK2 kinase (AS602868) that was previously shown to block tumor necrosis factor-α (TNF-α)-induced NF-κB activation and to reveal the apoptotic potential of the cytokine in Jurkat leukemic cells.17
We show here that AS602868 blocks constitutive NF-κB activation in primary AML blasts and induces a strong apoptotic response associated with mitochondrial transmembrane potential failure and activation of caspases. Killing of AML blasts in vitro by blocking NF-κB was as efficient as doxorubicin, cytarabine, or etoposide (VP16). Moreover, AS602868 increased the efficiency of these drugs. On the contrary, normal HSCs from cord blood were not affected by the IKK2 inhibitor. These results strongly suggest that pharmacologic inhibition of the NF-κB pathway could be an interesting adjuvant approach in future treatments for AML.
Materials and methods
Characteristics of AS602868
AS602868 is an anilinopyrimidine derivative and adenosine triphosphate (ATP) competitor selected for its inhibitory effect in vitro on IKK2ee, a constitutively active version of IKK2. The compound is covered by the patent application no. PCT WO 02/46171. AS602868 has an in vitro inhibitory concentration of 50% (IC50) of 60 nM toward purified IKK2 and no effect on IKK1 (IC50 = 14 μM) or on a large panel of recombinant kinases. It has some inhibitory effect on JNK2 (IC50 = 600 nM). AS602868 blocked phosphorylation of IκB and subsequent NF-κB activation in various cell lines. AS602868 also blocked production of TNF-α (IC50 = 300 nM) in lipopolysaccharide (LPS)-stimulated THP-1 cells as well as intercellular adhesion molecule-1 (ICAM-1) (IC50 = 800 nM) but not ICAM-2 or vascular cell adhesion molecule (VCAM) expression in TNF-stimulated human umbilical vein endothelial cells (HUVECs) (data not shown). AS602868 revealed the apoptotic potential of TNF-α in Jurkat cells.17
Cell isolation and culture
AML cells were obtained from peripheral blood or bone marrow of patients at the Hematology Department; CD34+ cells were obtained from umbilical cord blood cells at the Obstetric Department, both at the CHU-Nice (Centre Hospitalier Universitaire, Nice, France). Samples were obtained after approval by the institutional review boards and appropriate informed consent from patients.
Blasts were purified by Ficoll-Paque-Plus (Amersham Biosciences, Buckinghamshire, United Kingdom) density gradient and resuspended in RPMI 1640 medium containing 2 mM glutamine, 50 U/mL penicillin, 50 mg/mL streptomycin, 1 mM pyruvate, 100 μM β-mercaptoethanol, and 10% fetal calf serum before use. Hematopoietic progenitors from cord blood samples were enriched using the RosetteSep kit (StemCell Technologies, Vancouver, BC) before isolation of mononucleated cells by Ficoll-Paque-Plus.
Purification of CD34+ hematopoietic progenitors and CD34+ AML cells
Mononuclear AML and cord blood cells were labeled with CD34 microbeads isolated by magnetic positive selection (MACS cell isolation kit; Miltenyi Biotec, Paris, France). CD34+ cells were resuspended in RPMI 1640 medium containing 2 mM glutamine, 50 U/mL penicillin, 50 mg/mL streptomycin, 1 mM pyruvate, 100 μM β-mercaptoethanol, and 10% fetal calf serum before use.
Measurement of apoptosis by annexin V analysis
Apoptosis was measured using 2 different staining procedures. Samples with less than 90% of blasts were successively stained with anti-CD34-fluorescein isothiocyanate (FITC) antibody (Miltenyi Biotec) for 30 minutes at 4°C followed by annexin V-phycoerythrin (PE) (BioVision, Palo Alto, CA) for 15 minutes in the dark. Samples with more than 90% of AML blasts were stained with annexin V-FITC and propidium iodide (CALTAG Laboratories, Burlingame, CA). Apoptosis was recorded using FL-1 and FL-2 channels on a FACScan (Becton Dickinson, Cowley, United Kingdom).
Apoptosis quantification
For samples with more than 90% of blasts, apoptosis was characterized by annexin-positive propidium iodide (PI)-positive and annexin-positive/PI-negative populations. For samples with less than 90% of blasts, apoptosis was characterized by an annexin-positive/CD34+ population.
The percentage of drug-specific apoptosis is as follows: (drug-induced apoptosis - apoptosis in medium) × 100/(100 - apoptosis in medium).
Statistical analysis
Analysis of variance (paired ANOVA) and the protective least significant difference (PLSD) Fisher test were performed. A probability of less than .05 was considered significant.
Determination of subdiploid DNA content
Cell cycle analysis was performed by quantifying DNA content using propidium iodide staining, according to Vindelov et al.18
Electrophoretic mobility shift assays (EMSAs)
Cellular extracts and mobility shift assays were performed as described.17
Mitochondrial membrane depolarization
AML cells (0.5 × 106/mL) were stimulated, harvested, washed with phosphate-buffered saline (PBS), and incubated with 50 nM 3,3-dihexyloxacarbocyanine (DiOC6(3)) (Molecular Probes, Eugene, OR) and propidium iodide (15 minutes, 37°C). Cells were centrifuged and resuspended in PBS containing 2.5 mM CaCl2. DiOC6(3) and PI fluorescence were measured by fluorescence-activated cell sorting (FACS).
Measurement of caspase activity by flow cytometry
AML blasts were permeabilized (4°C, 20 minutes) before staining with antiactive caspase-3 antibody (BD Biosciences, Pont de Claix, France) and cytometry analysis.
Western blot analysis
Total cell extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on polyacrylamide gels and blotted on Immobilon membranes.17 Anti-poly(adenosine diphosphate-ribose) polymerase (anti-PARP), anti-hsp60, anti-IKK2, and anti-p-IKK2 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) were revealed with a secondary peroxidase-conjugated antibody (DakoCytomation, Glostrup, Denmark) followed by enhanced chemiluminescence (ECL) detection (Amersham Pharmacia, Saclay, France).
Evaluation of NF-κB activation by RTQ-PCR
IκB-α mRNA expression is used as a reporter of NF-κB activation.19
Real-time quantitative polymerase chain reaction (RTQ-PCR) analysis was performed on an ABI PRISM 7000 Sequence Detection System using SYBR Green I Dye (Applied Biosystems, Foster City, CA). Total RNA was prepared with the Absolutely RNA Microprep Kit (Stratagene, La Jolla, CA). A total of 150 ng RNA was reverse transcribed using SuperScript II reverse transcriptase (Invitrogen, Amsterdam, The Netherlands) following manufacturer's instructions and resuspended in 150 μL final volume. Complementary DNAs (5 μL) or water as control were amplified in duplicate by real-time PCR in a final volume of 25 μL using the SYBR Green Master Mix reagent (Eurogentec, Angers, France) and 300 nM forward and reverse primers. Primers were designed using PRIMER Express Software (Applied Biosystems), and sequences are available upon request. Fluorescence differences were collected by the 7000 ABI thermal cycler during each 60°C cycle. Semilogarithmic plots were constructed of delta fluorescence versus cycle number. For analysis, a threshold was set for change in fluorescence at a point in the linear PCR amplification phase (Ct). To calculate IκB-α mRNA levels, a standard curve prepared with serial dilutions of a plasmid containing the IκB target sequence (number of copies of plasmid DNA: 106 to 1/μL) was used.
Results
This study was conducted on fresh primary cells purified from blood or bone marrow (1 sample) from 18 patients with AML. Table 1 shows clinical characteristics of patients from M0, M1, M2, and M4 subtypes according to the French-American-British (FAB) classification. Twelve patients were analyzed immediately upon diagnosis (nos. 1 to 9 and 16 to 18) while 6 (nos. 10 to 15) were already under therapy. In these latter cases, all experiments were performed at distance from treatments.
Clinical characteristics of patients with AML
Patient no. . | Sex . | Age, y . | FAB category . | CD34 blasts, % . | Karyotype . |
|---|---|---|---|---|---|
| 1 | F | 52 | M1 | 56 | 46 |
| 2 | F | 44 | M1 | 36 | 48,(21) |
| 3 | F | 65 | M4 | 17 | 46,inv(16) |
| 4 | F | 61 | M2 | > 90 | 46 |
| 5 | M | 53 | M4 | 60 | 46 |
| 6 | M | 64 | M2 | > 90 | 46 |
| 7 | M | 35 | M1 | > 90 | 46,del(13)(q13q22) |
| 8 | M | 60 | M4 | > 90 | ND |
| 9 | F | 78 | M1 | > 90 | ND |
| 10 | F | 38 | M2 | 9-35 | 46,t(8;21) |
| 11 | M | 10 | M4 | > 90 | ND |
| 12 | M | 72 | CML/M2 | 80 | 47,(+13) |
| 13 | F | 71 | M4 | 20 | 46 |
| 14 | M | 81 | M2 | 43 | 47,(+11) |
| 15 | F | 62 | M2 | > 90 | 46,t(8;21) |
| 16 | M | 85 | M0 | > 90 | ND |
| 17 | M | 74 | MD | > 90 | ND |
| 18 | M | 38 | M0 | > 90 | ND |
Patient no. . | Sex . | Age, y . | FAB category . | CD34 blasts, % . | Karyotype . |
|---|---|---|---|---|---|
| 1 | F | 52 | M1 | 56 | 46 |
| 2 | F | 44 | M1 | 36 | 48,(21) |
| 3 | F | 65 | M4 | 17 | 46,inv(16) |
| 4 | F | 61 | M2 | > 90 | 46 |
| 5 | M | 53 | M4 | 60 | 46 |
| 6 | M | 64 | M2 | > 90 | 46 |
| 7 | M | 35 | M1 | > 90 | 46,del(13)(q13q22) |
| 8 | M | 60 | M4 | > 90 | ND |
| 9 | F | 78 | M1 | > 90 | ND |
| 10 | F | 38 | M2 | 9-35 | 46,t(8;21) |
| 11 | M | 10 | M4 | > 90 | ND |
| 12 | M | 72 | CML/M2 | 80 | 47,(+13) |
| 13 | F | 71 | M4 | 20 | 46 |
| 14 | M | 81 | M2 | 43 | 47,(+11) |
| 15 | F | 62 | M2 | > 90 | 46,t(8;21) |
| 16 | M | 85 | M0 | > 90 | ND |
| 17 | M | 74 | MD | > 90 | ND |
| 18 | M | 38 | M0 | > 90 | ND |
Samples 1 to 9 and 16 to 18 were obtained at diagnosis; numbers 10 to 15 were patients under treatment.
FAB indicates French-American-British classification; ND, not determined; CML, chronic myeloid leukemia; MD, AML with multilineage dysplasia.
Analysis of AML cells survival after treatment with VP16 or AS602868
We first analyzed effect of the IKK2 inhibitor on survival of AML cells. Cells were purified from blood or bone marrow (patient 5) using Ficoll-Paque-Plus and centrifugation. Survival/apoptosis was measured by annexin V staining. Results obtained with 15 patients with AML are summarized in Table 2. Interestingly, all patients, whether at diagnosis (patients 1 to 9) or already under therapeutic treatment (patients 10 to 15), showed an apoptotic response upon incubation for 72 hours with 10 μM AS602868. The effects of AS602868 were in general higher than those obtained with the topoisomerase poison and chemotherapeutic drug etoposide (VP16), except for patients 5, 7, 12, 14, and 15. Importantly, in all cases a potentiation of the apoptotic response was observed upon combination of AS602868 with VP16. IC50 for AS602868 ranged from 0.6 μM (patient 9) to 14 μM (patients 5 and 6). Three patients (7, 12, and 15) were low responders for AS602868 (IC50 more than 30 μM). Nevertheless, combining AS602868 with VP16 produced slightly higher apoptosis in these patients.
Annexin V analysis of apoptotic responses in 15 AML patients
Patient no. . | Incubation time, h . | AS602868, 10 μM, % . | VP16, 10 μM, % . | AS602868, 10 μM; VP16, 10 μM, % . | IC50, μM; AS602868 . |
|---|---|---|---|---|---|
| 1 | 72 | 87 | 1 | 89 | 4 |
| 2 | 72 | 73 | 7 | 93 | 1.4 |
| 3 | 72 | 63 | –16 | 75 | 2.2 |
| 4 | 72 | 71 | 64 | 78 | 1 |
| 5 | 48 | 45 | 85 | 94 | 14 |
| 6 | 72 | 34 | 16 | 59 | 14 |
| 7 | 72 | 17 | 79 | 86 | > 30 |
| 8 | 72 | 78 | 28 | 87 | 2.8 |
| 9 | 72 | 92 | 58 | 97 | 0.6 |
| 10 | 72 | 92 | ND | ND | 2.8 |
| 11 | 72 | 39 | 31 | 66 | 5 |
| 12 | 72 | 36 | 39 | 44 | > 30 |
| 13 | 72 | 65 | 1 | 79 | 8 |
| 14 | 72 | 64 | 77 | 87 | 7.1 |
| 15 | 72 | 23 | 60 | 77 | > 30 |
Patient no. . | Incubation time, h . | AS602868, 10 μM, % . | VP16, 10 μM, % . | AS602868, 10 μM; VP16, 10 μM, % . | IC50, μM; AS602868 . |
|---|---|---|---|---|---|
| 1 | 72 | 87 | 1 | 89 | 4 |
| 2 | 72 | 73 | 7 | 93 | 1.4 |
| 3 | 72 | 63 | –16 | 75 | 2.2 |
| 4 | 72 | 71 | 64 | 78 | 1 |
| 5 | 48 | 45 | 85 | 94 | 14 |
| 6 | 72 | 34 | 16 | 59 | 14 |
| 7 | 72 | 17 | 79 | 86 | > 30 |
| 8 | 72 | 78 | 28 | 87 | 2.8 |
| 9 | 72 | 92 | 58 | 97 | 0.6 |
| 10 | 72 | 92 | ND | ND | 2.8 |
| 11 | 72 | 39 | 31 | 66 | 5 |
| 12 | 72 | 36 | 39 | 44 | > 30 |
| 13 | 72 | 65 | 1 | 79 | 8 |
| 14 | 72 | 64 | 77 | 87 | 7.1 |
| 15 | 72 | 23 | 60 | 77 | > 30 |
Statistical analysis by paired ANOVA and PLSD Fisher test (a probability of < .05 is considered significant).
ND indicates not determined.
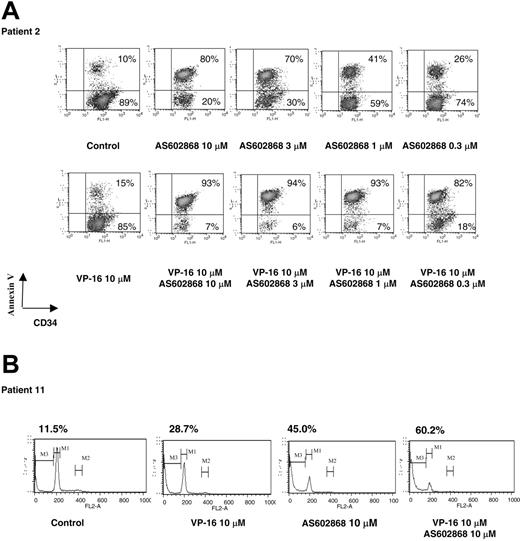
A detailed analysis performed on patient 2 (M1, 36% CD34+ blasts) is displayed in Figure 1A. Each experiment was repeated twice with similar results. The results presented for patient 2 are representative of responses obtained in patients analyzed at diagnosis. Cells from patient 2 displayed a low spontaneous apoptosis in culture (10%, Figure 1A). Incubation with 10 μM AS602868 led to a maximal apoptotic response (80% of annexin-positive cells among CD34+ blasts). A dose-dependent decrease in AS602868 concentration to 0.3 μM lowered cell death to 26%. Under similar culture conditions, this patient had a low response to VP16 (15%). Combination of VP16 with AS602868 had an additive effect (93%), and decreasing the AS602868 concentration to 0.3 μM only minored the apoptotic response (82%).
The IKK2 inhibitor AS602868 induces apoptosis of AML blasts. (A) Cells from patient 2 (36% CD34+ blasts) were incubated for 72 hours with indicated doses of AS602868 and VP16 before staining with anti-CD34-FITC antibody and annexin V-PE. Cells were gated on the CD34+ compartment. The percentages of apoptotic cells are displayed. (B) Sub-G1 analysis of apoptotic blasts. Blasts from patient 11 (more than 90% blasts) were stained with propidium iodide. The percentage of apoptotic blasts in sub-G1 (gate M3) is displayed.
The IKK2 inhibitor AS602868 induces apoptosis of AML blasts. (A) Cells from patient 2 (36% CD34+ blasts) were incubated for 72 hours with indicated doses of AS602868 and VP16 before staining with anti-CD34-FITC antibody and annexin V-PE. Cells were gated on the CD34+ compartment. The percentages of apoptotic cells are displayed. (B) Sub-G1 analysis of apoptotic blasts. Blasts from patient 11 (more than 90% blasts) were stained with propidium iodide. The percentage of apoptotic blasts in sub-G1 (gate M3) is displayed.
A cell cycle analysis was then performed on blasts from patient 11 (M4, more than 90% CD34+ blasts) (Figure 1B). Dead cells were defined as cells with a lower amount of DNA (sub-G1). Addition of 10 μM AS602868 to the cell culture for 72 hours led to 45% of cell death compared with 28.7% induced by VP16. Combination of the 2 compounds had greater effects (60.2%).
A statistical analysis (paired ANOVA; Tables 3 and 4) and a PLSD Fisher test were performed and demonstrated that the effect of AS602868 is not statistically higher (P = .06) than that of VP16 (mean = 56.21% ± 23.78% of apoptotic cells versus 37.85% ± 33.07%). By contrast, the combination of AS602868 with VP16 (79.35% ± 14.7%) is statistically more efficient than AS602868 alone (P = .02) or VP16 alone (P = .0002).
Comparison of AS602868 and VP16 apoptotic responses
Treatment . | Mean of apoptotic responses . |
|---|---|
| AS602868 | 56.21 ± 23.78 |
| VP16 | 37.85 ± 33.07 |
| AS602868 + VP16 | 78.35 ± 14.7 |
Treatment . | Mean of apoptotic responses . |
|---|---|
| AS602868 | 56.21 ± 23.78 |
| VP16 | 37.85 ± 33.07 |
| AS602868 + VP16 | 78.35 ± 14.7 |
Values are means of fluorescence intensities +/- standard deviation.
Statistical analysis of apoptotic responses to AS602868 and VP16
Statistical analysis . | P . |
|---|---|
| AS602868 > VP16 | .0613 |
| AS602868 + VP16 > AS602868 | .0206 |
| AS602868 + VP16 > VP16 | .0002 |
Statistical analysis . | P . |
|---|---|
| AS602868 > VP16 | .0613 |
| AS602868 + VP16 > AS602868 | .0206 |
| AS602868 + VP16 > VP16 | .0002 |
> indicates means more efficient than.
AS602868 blocked autophosphorylation of IKK2
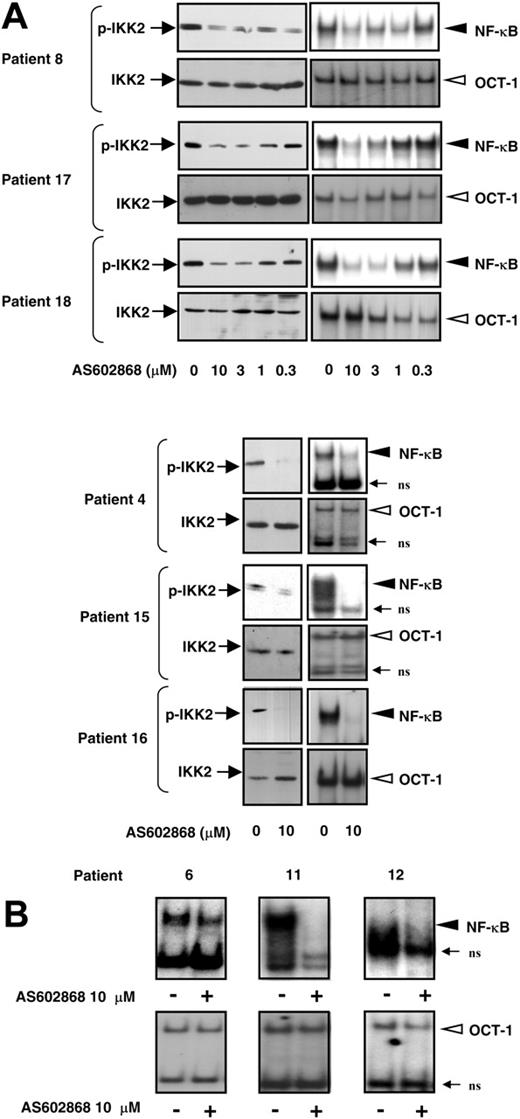
We then checked the capacity of AS602868 to interfere with IKK2 activity. During activation, IKK2 is autophosphorylated in its activation loop on serines 177 and 181. Replacement of these serines by alanines impaired IKK2 activation, whereas substitution by glutamic acids produced constitutively active IKK2.20 We analyzed the phosphorylation status of IKK2 in 6 patients using antibodies specific for these 2 phosphorylation sites. As shown in Figure 2A, constitutive phosphorylated IKK2 could be detected in AML blasts of patients 4, 8, 15, 16, 17 and 18. Incubation of leukemic blasts with AS602868 for 18 hours led to a dose-dependent decrease in IKK2 phosphorylation in patients 8, 17, and 18 (Figure 2A, left column, upper panels). A maximal effect of AS602868 was observed for 10 μM. Similarly, an 18-hour treatment of blasts from patients 4, 15, and 16 (Figure 2A) with 10 μM AS602868 induced an almost complete fading of phospho-IKK2 signals. This decreased phosphorylation was not due to a change in kinase levels (Figure 2A, left column, lower panels). These results demonstrate that, in AML blasts, AS602868 could block constitutive phosphorylation of IKK2, an event that reflects its kinase activity. The inhibitory effect of AS602868 on IKK2 in patients 16, 17, and 18 was associated with induction of apoptosis (data not shown).
AS602868 inhibits constitutive activation of IKK2 and NF-κB in AML blasts. Blasts from patients 8, 17, and 18 were incubated with indicated doses of AS602868 for 18 hours. Blasts from patients 4, 6, 11, 12, 15, and 16 were incubated with 10 μM AS602868 for 18 hours or left untreated. (A, left column) AS602868 blocked autophosphorylation of IKK2 in AML blasts. Cell lysates were resolved by SDS-PAGE and transferred to Immobilon membranes. The autophosphorylation status of IKK2 was analyzed with antibodies against phosphorylated serines 177 and 181, and anti-IKK2 antibodies show equal amounts of the kinase in the different conditions. AS602868 inhibits NF-κB activation (Panel A, right column; and panel B). Total cell extracts were incubated with radioactive probes encompassing the NF-κB or OCT-1 DNA binding sequences. Complexes were separated by nondenaturing electrophoresis followed by autoradiography (EMSA). ns indicates nonspecific.
AS602868 inhibits constitutive activation of IKK2 and NF-κB in AML blasts. Blasts from patients 8, 17, and 18 were incubated with indicated doses of AS602868 for 18 hours. Blasts from patients 4, 6, 11, 12, 15, and 16 were incubated with 10 μM AS602868 for 18 hours or left untreated. (A, left column) AS602868 blocked autophosphorylation of IKK2 in AML blasts. Cell lysates were resolved by SDS-PAGE and transferred to Immobilon membranes. The autophosphorylation status of IKK2 was analyzed with antibodies against phosphorylated serines 177 and 181, and anti-IKK2 antibodies show equal amounts of the kinase in the different conditions. AS602868 inhibits NF-κB activation (Panel A, right column; and panel B). Total cell extracts were incubated with radioactive probes encompassing the NF-κB or OCT-1 DNA binding sequences. Complexes were separated by nondenaturing electrophoresis followed by autoradiography (EMSA). ns indicates nonspecific.
The IKK2 inhibitor abolished constitutive activation of NF-κB in AML cells
The ability of AS602868 to interfere with NF-κB activation was analyzed in parallel to its blocking effect on IKK2. As visualized by electrophoretic mobility shift assay (EMSA), constitutive NF-κB was detected in patients 8, 17, and 18 (Figure 2A), which was decreased in a dose-dependent manner after an 18-hour incubation with AS602868 (Figure 2A, right column, upper panels). AS602868 appeared to be most efficient at a dose of 10 μM, which is consistent with the apoptotic responses and inhibition of IKK2 induced by the molecule. In cells from patients 4, 15, and 16, AS602868 (10 μM) blocked NF-κB activation in parallel to IKK2 phosphorylation. As a control, AS602868 had no effect on octamer binding protein-1 (OCT-1) DNA binding (Figure 2A, right column, bottom panels).
Because of sample size limitation, it has not been possible to always evaluate in parallel the action of AS602868 on both IKK2 phosphorylation and NF-κB activation. Nevertheless, the inhibitory effect of 10 μM AS602868 on constitutive NF-κB activation is further shown on cells from patients 6, 11, and 12 (Figure 2B). We have thus shown that AS602868 could block constitutive NF-κB activation in 9 of 9 patients who could be analyzed.
We then measured the transactivation potential of NF-κB through a real-time PCR analysis of IκB-α mRNA, which is rapidly induced by NF-κB to terminate NF-κB activation.19
IκB-α mRNA levels in patient samples were compared with levels in normal peripheral blood leukocyte (PBL) (11 molecules of IκB-α mRNA per cell) Jurkat cells19 or in the NF-κB constitutive Hodgkin cell line HDLM-2 (558 × 103; Table 6). Heterogeneous levels were measured (Table 5). Patients 9, 1, and 13 displayed levels in the range of HDLM-2 cells: 400 × 103, 230 × 103, and 158 × 103, respectively. Most of patients have levels that are above PBL or Jurkat values except patients 3 and 12 (6.8 and 7.3, respectively). In all cases, incubation with 10 μM AS602868 strongly decreased the amount of IκB-α mRNA. No correlations were found with AML subtype or with intensity of the response to AS602868. Interestingly, there was some correlation with the percentage of AML blasts and, to a lesser extent, with the number of leukocytes in the periphery (Table 5).
NF-κB activation in normal PBL and 2 transformed leukemic cell lines
Cells . | IκB-α mRNA, molecules per cell . | White blood cells, 103/μL . |
|---|---|---|
| PBLs | 11 | 5-8 |
| Jurkat (ALL) | 19 | — |
| HDLM-2 (Hodgkin) | 558 × 103 | — |
Cells . | IκB-α mRNA, molecules per cell . | White blood cells, 103/μL . |
|---|---|---|
| PBLs | 11 | 5-8 |
| Jurkat (ALL) | 19 | — |
| HDLM-2 (Hodgkin) | 558 × 103 | — |
PBLs indicates peripheral blood leukocytes;—, not applicable.
Analysis of NF-κB activation, WBC counts, and blast percentage in 8 patients with AML
Patient no. . | IκB-α mRNA, molecules per cell . | . | White blood cells, 103/μL . | Blasts, % . | |
|---|---|---|---|---|---|
| . | Medium . | AS602868, 10 μM . | . | . | |
| 9 | 400 × 103 | 10 | 79.2 | > 90 | |
| 1 | 230 × 103 | 0.3 | 4.3 | 56* | |
| 13 | 158 × 103 | 55 | 129 | > 90 | |
| 4 | 1434 | 168 | 161.8 | > 90 | |
| 8 | 813 | 0.3 | 25.6 | 12 | |
| 2 | 238 | 0.1 | 19.9 | 64* | |
| 12 | 7.3 | 3 | 22.6 | 38* | |
| 3 | 6.8 | 0.1 | 15.2 | 17* | |
Patient no. . | IκB-α mRNA, molecules per cell . | . | White blood cells, 103/μL . | Blasts, % . | |
|---|---|---|---|---|---|
| . | Medium . | AS602868, 10 μM . | . | . | |
| 9 | 400 × 103 | 10 | 79.2 | > 90 | |
| 1 | 230 × 103 | 0.3 | 4.3 | 56* | |
| 13 | 158 × 103 | 55 | 129 | > 90 | |
| 4 | 1434 | 168 | 161.8 | > 90 | |
| 8 | 813 | 0.3 | 25.6 | 12 | |
| 2 | 238 | 0.1 | 19.9 | 64* | |
| 12 | 7.3 | 3 | 22.6 | 38* | |
| 3 | 6.8 | 0.1 | 15.2 | 17* | |
Patients are listed according to the level of IKB-α mRNA expression, and therefore do not appear in numerical order.
CD34+ cells were purified by positive selection on a column using magnetic field (MACS cell isolation kit)
These results should, however, be confirmed in a larger panel of patients, especially at diagnosis. We cannot rule out that the treatments affected NF-κB activation despite that measurements were made at distance from therapy.
Mechanisms of apoptosis after NF-κB inhibition in AML cells
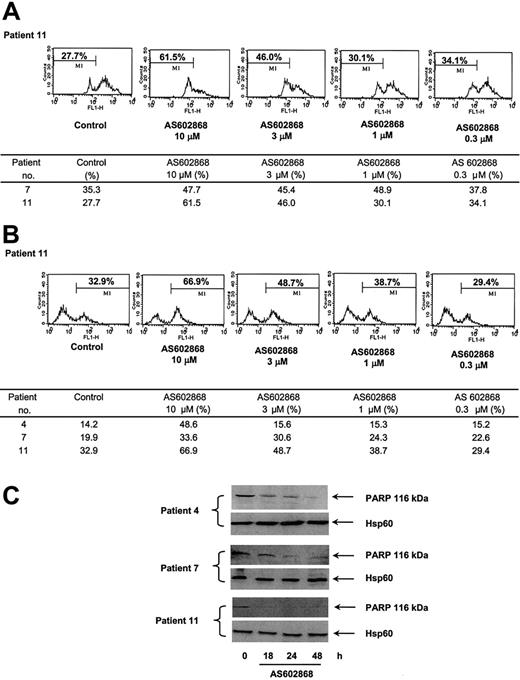
Apoptosis can be initiated at the membrane level by engagement of death receptors or by the release from mitochondria of proapoptotic proteins. This latter event is preceded by changes in the mitochondrial transmembrane potential. To define the molecular apoptotic mechanisms triggered by AS602868, AML cells were labeled with DiOC6(3) and analyzed by flow cytometry. In patient 11, 27.7% of cells had a spontaneous decreased mitochondrial potential after a 48-hour period in culture (Figure 3A). This percentage increased to 61.5% using 10 μM AS602868 and gradually decreased with lower doses of the drug. A similar result was observed in cells from patient 7 (Figure 3). These results suggest that inhibition of NF-κB can activate the mitochondrial cell death pathway. Whether this event is sufficient by itself to induce the apoptotic response observed in AML cells will require complementary experiments.
Effect of AS602868 on mitochondrial membrane depolarization and caspase activation in AML blasts. (A) Leukemic blasts from patients 7 and 11 were incubated with indicated doses of AS602868 for 48 hours. Mitochondrial membrane depolarization was assessed by loss of DiOC6(3) staining by flow cytometry. (B) AS602868 induced caspase-3 activation. AML blasts from patients 4, 7, and 11 were incubated with indicated doses of AS602868 for 48 hours before permeabilization and labeling with an antibody against active caspase-3 and analyzed by flow cytometry. (C) AS602868 induces cleavage of PARP. Blasts from patients 4, 7, and 11 were treated with AS602868 (10 μM) for indicated times before analysis of PARP cleavage by Western blotting. Hsp60 levels represent loading controls.
Effect of AS602868 on mitochondrial membrane depolarization and caspase activation in AML blasts. (A) Leukemic blasts from patients 7 and 11 were incubated with indicated doses of AS602868 for 48 hours. Mitochondrial membrane depolarization was assessed by loss of DiOC6(3) staining by flow cytometry. (B) AS602868 induced caspase-3 activation. AML blasts from patients 4, 7, and 11 were incubated with indicated doses of AS602868 for 48 hours before permeabilization and labeling with an antibody against active caspase-3 and analyzed by flow cytometry. (C) AS602868 induces cleavage of PARP. Blasts from patients 4, 7, and 11 were treated with AS602868 (10 μM) for indicated times before analysis of PARP cleavage by Western blotting. Hsp60 levels represent loading controls.
AS602868-induced caspase activation
We next analyzed the possible implication of cellular caspases in the effect of AS602868. This was investigated by flow cytometry using an antibody against active caspase-3. Inhibition of IKK2 with 10 μM AS602868 for 48 hours in blasts from patient 11 induced a 2-fold increase in the number of cells with active caspase-3 (66.9% versus 32.9%). The effect of AS602868 was dose dependent. Comparable results were observed in patients 4 and 7 (Figure 3). The cleavage of the PARP polymerase, a major target for caspases, was analyzed by Western blotting with specific antibodies in cells from patients 4 (M2), 7 (M1), and 11 (M4) treated in vitro with 10 μM AS602868 for various times (Figure 3C). PARP appeared to be cleaved within 18 hours. This was particularly evident for patient 11, in which the PARP signal completely disappeared after 18 hours. Interestingly, the results obtained with PARP cleavage match the intensity of caspase-3 activation in these patients (Figure 3B).
These results demonstrate that AML death induced by inhibition of IKK2 involves, at least in part, activation of cellular caspases.
Blocking NF-κB potentiates chemotherapeutic drug-induced apoptosis
The concept of blocking NF-κB for an adjuvant cancer therapy was tested in vitro using the anthracycline doxorubicin, which is used to treat patients with AML. Suboptimal concentrations of AS602868 (1 and 0.3 μM) were used in these experiments.
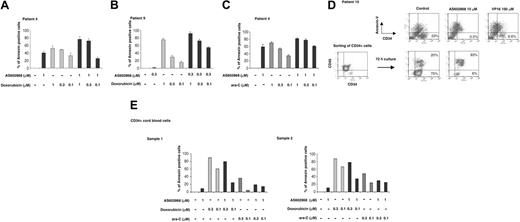
Cells from patient 4 (Figure 4A) appeared sensitive to AS602868 (40.8% of apoptotic cells after 72 hours in the presence of 1 μM AS602868) and to doxorubicin (52.8%, 49.4%, and 33.4%, respectively, for 1, 0.3, and 0.1 μM). Combining 1 μM AS602868 with doxorubicin increased cell apoptosis (77.1% and 73.2%), except for 0.1 μM doxorubicin (25.7%).
AS602868 potentiates the cytotoxic effect of doxorubicin and AraC on leukemic blasts but not on normal CD34+ hematopoietic precursor cells. Cells from patients 4 (A) and 9 (B) were incubated with indicated doses of AS602868, doxorubicin, or a combination of both for 72 hours. Apoptosis was measured with annexin V/propidium iodide staining and quantified as described in “Materials and methods.” Histograms represent the percentages of apoptotic blasts measured in duplicate samples. (C) Leukemic cells from patient 4 were treated with AS602868, cytarabine, or a combination of both for 72 hours. Apoptosis was measured as in panel A. (D; top row) Cells from patient 10 were treated with the indicated doses of AS602868 and VP16 for 72 hours before analysis of apoptosis as described (panel D, bottom). CD34+ blasts from patient 10 were purified with magnetic CD34 microbeads and incubated with indicated doses of AS602868 for 72 hours. Cells were stained with anti-CD34-FITC and annexin V-PE antibody. (E) CD34+ cells were isolated from normal cord blood as described in “Materials and methods” and incubated with indicated doses of AS602868 alone or in combination with doxorubicin and cytarabine. Flow cytometry analysis was performed after double staining with anti-CD34-FITC antibody and annexin V-PE. Results represent percentages of apoptosis quantified as described in “Materials and methods.”
AS602868 potentiates the cytotoxic effect of doxorubicin and AraC on leukemic blasts but not on normal CD34+ hematopoietic precursor cells. Cells from patients 4 (A) and 9 (B) were incubated with indicated doses of AS602868, doxorubicin, or a combination of both for 72 hours. Apoptosis was measured with annexin V/propidium iodide staining and quantified as described in “Materials and methods.” Histograms represent the percentages of apoptotic blasts measured in duplicate samples. (C) Leukemic cells from patient 4 were treated with AS602868, cytarabine, or a combination of both for 72 hours. Apoptosis was measured as in panel A. (D; top row) Cells from patient 10 were treated with the indicated doses of AS602868 and VP16 for 72 hours before analysis of apoptosis as described (panel D, bottom). CD34+ blasts from patient 10 were purified with magnetic CD34 microbeads and incubated with indicated doses of AS602868 for 72 hours. Cells were stained with anti-CD34-FITC and annexin V-PE antibody. (E) CD34+ cells were isolated from normal cord blood as described in “Materials and methods” and incubated with indicated doses of AS602868 alone or in combination with doxorubicin and cytarabine. Flow cytometry analysis was performed after double staining with anti-CD34-FITC antibody and annexin V-PE. Results represent percentages of apoptosis quantified as described in “Materials and methods.”
Similar results were obtained with patient 9 (Figure 4B). After 72 hours these cells had a minimal response to 0.3 μM AS602868 (1%) while they underwent dose-dependent apoptosis with doxorubicin (75.1%, 29.5%, and 15.4%, respectively, for 1, 0.3, and 0.1 μM). The combination of the 2 drugs enhanced the apoptotic response (92.2%, 72.7%, and 54.6%). In this example, despite no answer to AS602868 and a low effect of 0.1 μM doxorubicin, combination of the 2 drugs induced significant apoptosis (54.6%).
Cytarabine (AraC), a pyrimidine analog that blocks DNA synthesis, is also used to treat patients with AML. Cells from patient 4 responded similarly to 1 μM AraC (70.3% apoptotic cells) and to 1 μM AS602868 (58.9%) (Figure 4C). The effect of AraC appeared dose dependent (53.3% and 34.2%, respectively, for 0.3 μM and 0.1 μM). Simultaneous addition of 1 μM AS602868 to AraC enhanced the apoptotic index (81.3%, 77.4%, and 61%).
These results clearly show that addition of AS602868 to the conventional chemotherapeutic drugs doxorubicin and AraC enhanced the apoptotic in vitro responses of primary AML cells.
Effect of NF-κB inhibition on leukemic cells from a chemoresistant patient
Patient 10 was under therapy at the time of analysis and appeared resistant to anthracycline and AraC treatments. No remission could be obtained, and the disease was only contained by repeated VP16 injections. Fifteen days after the last VP16 injection, the percentage of blasts had reached 23% in the sample displayed in Figure 4D. Apoptosis was analyzed after CD34 and annexin V double staining. Addition of a high dose of VP16 (100 μM) decreased the number of leukemic blasts from 23% to 6.6%. Strikingly, upon incubation with 10 μM AS602868, very few viable CD34+ leukemic cells could be detected (0.5%). Similar results were obtained when affinity-purified CD34 blasts were sorted out (Figure 4D, lower panels). Incubation with 10 μM AS602868 for 72 hours induced a strong apoptosic response (93%) compared with unstimulated cells (20%).
Therefore, targeting NF-κB appeared efficient in vitro in this patient, despite cell resistance to conventional chemotherapy.
Effect of NF-κB inhibition on the survival of normal CD34 hematopoietic precursors
We next compared the sensitivity of normal CD34+ cells to AS602868 combined to chemotherapy in 2 healthy donors (Figure 4E).
After 72 hours of treatment, purified CD34+ cells showed low sensitivity to 1 μM AS602868 (9.3% and 10.9% for samples 1 and 2, respectively). Doxorubicin induced dose-dependent cell death (sample 1: 90% and 60.7% for 0.3 and 0.1 μM, respectively; sample 2: 87.9% and 66.8% for 0.3 and 0.1 μM, respectively). In contrast to leukemic blasts, combination of AS602868 with doxorubicin clearly did not improve the responses that were reproductively lower (sample 1: 80.1% and 24.7% for 0.3 and 0.1 μM, respectively; sample 2: 77.6% and 34.4% for 0.3 and 0.1 μM, respectively).
Treatment of both samples with AraC for 72 hours led to lower apoptosis index (sample 1: 35.9% and 4.7% for 0.3 and 0.1 μM, respectively; sample 2: 48.5% and 24.5% for 0.3 and 0.1 μM, respectively). Combination with 1 μM AS602868 did not further increase the responses (sample 1: 19.3% and 14.6% for 0.3 and 0.1 μM, respectively; sample 2: 29.6% and 25.2% for 0.3 and 0.1 μM, respectively).
These results demonstrate a fundamental difference in the sensitivity of leukemic versus normal CD34 precursors to inhibition of NF-κB.
Discussion
Spontaneous and abnormal activation of NF-κB has been described in various hematopoietic malignancies.7 The underlying mechanisms could be quite diverse: amplifications or rearrangements of genes coding for some NF-κB family members (various leukemias and lymphomas), mutations/deletions that invalidate the inhibitory function of IκB (Hodgkin lymphoma), oncogenic activation of the IKK complex (leukemias, lymphomas), and paracrine or autocrine secretion of NF-κB activators (Hodgkin lymphoma) (reviewed by Rayet and Gélinas21 ). In addition, v-rel, an oncogenic version of c-rel, induces avian lymphomas and leukemias.22 AML displays a constitutive activation of NF-κB because of a sustained stimulation of the IKK complex through still unknown mechanisms.23 We show here that cells from 18 patients with AML undergo apoptosis in vitro after NF-κB inhibition by AS602868, a pharmacologic blocker of the IKK2 kinase. The effect of AS602868 was dose dependent and affected both patients at diagnosis or under treatment, demonstrating a strong prosurvival potential for NF-κB in AML cells.
We observed that the apoptotic response of AML cells to AS602868 involved disruption of the mitochondrial transmembrane potential, an event that precedes and triggers the release of proapoptotic molecules from mitochondria to promote caspase-9 activation.24 Indeed, NF-κB controls genes that code for antiapoptotic proteins, some acting at the mitochondrial level (bcl-xl, bfl-1/A1)7 or directly blocking caspase activation (c-IAP1, c-IAP2, X-linked IAP [XIAP]).25 In a human lung carcinoma cell line model, bcl-xl and bfl-1 mediate the survival effect of NF-κB toward chemotherapeutic drugs.26 Down-regulation of NF-κB could therefore result in a decrease in crucial antiapoptotic influences both at the mitochondrial and the membrane death receptors levels. We have quantified the transactivation potential of NF-κB through the real-time PCR analysis of IκB gene expression. Although it has been reported that the level of constitutive IKK activation was higher in M4/M5 AML subtypes compared with M1/M2 specimens,23 no correlations were found with AML subtypes. However and interestingly, NF-κB activation levels had a tendency to evoluate similarly to the percentage of leukemic blasts in blood, either in naive or treated patients. This observation will need further confirmation on a larger panel of patients, especially naive ones, to avoid possible effects of treatments on NF-κB activation levels. This result suggests a link between NF-κB and the proliferation status of the blasts. Cyclin D1, whose gene is regulated by NF-κB 27 and overexpressed in several human neoplasias,28 could be a candidate to mediate the NF-κB effect on blast proliferation. Alternatively, NF-κB action could also be due to induction of genes coding for AML growth factor(s) such as G-CSF or GM-CSF.12 Transcriptome studies are now required to identify the key NF-κB target genes in AML cells.
The AS602868 molecule appears to have an important apoptotic action on AML, with similar or even higher efficiency than the anthracycline doxorubicin, the nucleoside analog AraC, or the topoisomerase poison VP16, which are currently used to treat patients with AML.2 Importantly and in most cases, stronger and statistically significant apoptotic responses could be obtained when doxorubicin or AraC was combined with the IKK2 inhibitor. It was therefore possible to decrease the concentration of drugs and AS602868 without significant loss of apoptotic efficiency. This observation could be particularly important in future clinical trials for such adjuvant therapy.
Inhibition of NF-κB could be expected to give unwanted side effects, especially by decreasing immune responses. However, this adjuvant therapy should be considered as transient, allowing recovery of immune functions after cessation of the treatment. Moreover, one must remember that current chemotherapy regimens are unable to cure AML and have strong deleterious side effects. Doxorubicin exhibits strong cardiac toxicity that limits its use, especially in children, whereas araC is broadly toxic and VP16 is harmful to hematopoietic cells.
It is therefore crucial to compare in animal models the benefits and the drawbacks of NF-κB inhibition during the course of cancer treatment. Proteasome inhibitors that prevent NF-κB activation appear promising in preclinical trials.16,29 Besides NF-κB, these molecules also block important cancer-deregulated pathways such as p53. It would be interesting to compare the effects of AS602868, a specific NF-κB inhibitor, with the action of proteasome blockers; one might expect lower side effects for AS602868.
Our group of patients included individuals older than 60 years, who usually represent 75% of all AML cases and are characterized by a lower tolerance to chemotherapy compared with younger patients. Our results suggest that combining AS602868 with classical chemotherapy could not only improve the therapeutic actions but also lower the toxic effects of the treatment in this class of patients.
Moreover, we observed a strong apoptotic response in an AML patient who was proven resistant to anthracycline and cytarabine therapies. Although this result needs to be confirmed in a larger panel of resistant patients, it suggests that targeting an original survival pathway could be an alternative therapy.
We also show here that blocking NF-κB induced apoptosis of leukemic blasts without dramatic damage to normal CD34 precursors. LSCs are responsible for the continuous production of pathological blasts.1 LSCs appear to escape chemotherapy because of a lower cell cycling activity4,30 and could be responsible for disease relapse.3 LSCs are therefore the major targets in a therapy to cure AML reviewed by Jordan.3 Interestingly, a proteasome inhibitor that inhibits NF-κB but also other signaling pathways has shown selective toxicity for LSCs but not HSCs in vitro,4,31 suggesting that the constitutive activity of NF-κB in LSCs could be their Achilles heel in a targeted therapy. The apoptotic response to IKK2 inhibition we observed is mainly due to the death of mature blasts. Whether this regimen will also affect LSCs will need further experiments of AML transplantation in nonobese diabetic/severe combined immunodeficiency (NOD/SCID) irradiated mice.32
New therapeutic opportunities are emerging using small inhibitory molecules to target precise proteins of deregulated functions in cancer cells. For instance, inhibition of mammalian target of rapamycin (mTOR) with rapamycin reverses chemoresistance of activated Akt-driven B-cell lymphoma in an Eμ-Myc mouse model.33 We demonstrate here for the first time that specifically targeting NF-κB activation can eliminate crucial survival signals in primary AML blasts. It will be important to extend these observations to other hematopoietic malignancies where NF-κB is suspected to play a role. In particular, Hodgkin lymphomas5 and a subgroup of diffuse large B-cell lymphoma (DLBCL) with poor prognostic outcome characterized by constitutive NF-κB activation34 could be candidate malignancies for a treatment with AS602868. Moreover, our results suggest an important benefit to associate NF-κB inhibition to conventional therapeutic approaches and urge testing of the action of AS602868, a pharmacologic blocker of NF-κB, as a future treatment of human acute myeloid leukemia.
Prepublished online as Blood First Edition Paper, September 28, 2004; DOI 10.1182/blood-2004-04-1463.
Supported by INSERM, Serono, Association pour la Recherche sur le Cancer (ARC) (3392), and la Fondation de France, Comité Leucémies (2002004519).
This study is supported in part by research funding from Serono International to J.-F.P., and one of the authors (M.D.) has declared a financial interest in a company whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Pierre-Alain Witte (Serono, Geneva, Switzerland), Patricia Lagadec (INSERM U526, Nice, France), and Dr Laurence Legros (Service d'Hématologie, CHU, Nice, France) for helpful discussions. We thank Dr Sophie Raynaud (Service de Génétique, CHU, Nice, France) for karyotype analysis and Isabelle Sudaka (Service d'Hématologie, CHU, Nice, France) for patient data. We thank nurses from the Obstetric Department of the CHU-Nice for cord blood collection and laboratory technicians of the Unité d'Exploration Fonctionelle Cellulaire et Tissulaire (UEFCT) (CHU-Nice) for help in sample preparation. We are grateful to Drs Ched Grimshaw and Shripad Bhagwat (Celgene, San Diego, CA) for sharing information on the properties of AS602868.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal