Abstract
Limited umbilical cord blood (UCB) cell dose compromises the outcome of adult UCB transplantation. Therefore, to augment graft cell dose, we evaluated the safety of the combined transplantation of 2 partially human leukocyte antigen (HLA)–matched UCB units. Twenty-three patients with high-risk hematologic malignancy (median age, 24 years; range, 13-53 years) received 2 UCB units (median infused dose, 3.5 × 107 nucleated cell [NC]/kg; range, 1.1-6.3 × 107 NC/kg) after myeloablative conditioning. All evaluable patients (n = 21) engrafted at a median of 23 days (range, 15-41 days). At day 21, engraftment was derived from both donors in 24% of patients and a single donor in 76% of patients, with 1 unit predominating in all patients by day 100. Although neither nucleated or CD34+ cell doses nor HLA-match predicted which unit would predominate, the predominating unit had a significantly higher CD3+ dose (P < .01). Incidences of grades II-IV and III-IV acute GVHD were 65% (95% confidence interval [CI], 42%-88%) and 13% (95% CI, 0%-26%), respectively. Disease-free survival was 57% (95% CI, 35%-79%) at 1 year, with 72% (95% CI, 49%-95%) of patients alive if they received transplants while in remission. Therefore, transplantation of 2 partially HLA-matched UCB units is safe, and may overcome the cell-dose barrier that limits the use of UCB in many adults and adolescents.
Introduction
Unrelated donor umbilical cord blood transplantation (UCBT) has become a standard therapeutic option for pediatric patients with hematologic malignancies.1-5 When compared with unrelated donor bone marrow (BM), UCB has the advantages of rapid availability6 and low risk of severe, acute graft-versus-host disease (GVHD) despite donor-recipient human leukocyte antigen (HLA) disparity.1-5,7,8 However, UCBT in adults is severely limited by graft cell dose.5,9 For example, Laughlin et al9 found that cryopreserved nucleated cell (NC) dose is a major determinant of neutrophil recovery and that higher CD34+ cell dose is associated with improved survival in adult UCB recipients. Further, Wagner et al5 have observed that recipients of UCB with infused cell doses of less than 1.8 × 107 NC/kg or less than 1.7 × 105 CD34+ cells/kg recipient body weight have a markedly inferior engraftment and survival.
Therefore, we investigated the safety and efficacy of UCBT using UCB units from 2 partially HLA-matched donors as a method of augmenting cell dose in high-risk adults and adolescents with hematologic malignancies. Although bidirectional immune rejection could potentially lead to graft failure from either donor unit, we hypothesized that double-unit UCBT may enhance the availability of UCBT for adults and may be associated with improved incidence of engraftment and more rapid neutrophil recovery. The primary endpoint of this phase 1/2 study was safety as measured by donor-derived neutrophil engraftment.
Patients, materials, and methods
Patients were eligible for UCBT if they had no 5-6/6 HLA-A–, HLA-B–, or HLA-DRB1–matched related donor, and no suitable volunteer donor available. UCB was given priority over volunteer donors for patients requiring transplantation within 3 months of referral. Patients were eligible for double-unit UCBT if no single 4-6/6 HLA-A–, HLA-B–, or HLA-DRB1–matched UCB unit of adequate cell dose was available. From January 2000 to December 2001, all adult patients without a single unit containing a cryopreserved dose of at least 2.5 × 107 NC/kg were eligible for double-unit UCBT in a phase 1/2 prospective study. In January 2002, because safety with double-unit UCBT had been demonstrated, this cell dose threshold was raised in an effort to increase graft cell dose for all UCBT recipients so that all adults without a single unit containing a cryopreserved dose of at least 3.5 × 107 NC/kg were eligible for double-unit UCBT. The treatment plan was reviewed and approved by the University of Minnesota institutional review board. Written informed consent was obtained from all patients prior to transplantation.
UCB unit selection
UCB units were obtained from the New York Blood Center (n = 13), the St Louis Cord Blood Bank (n = 19), the American Red Cross and other UCB banks of the National Marrow Donor Program (NMDP; n = 8), and Netcord (n = 6). Because the goal in graft selection was to maximize the total NC dose of the graft, the largest available UCB unit that was 4-6/6 HLA-A, B, DRB1 matched to the recipient was selected (UCB no. 1). UCB no. 2 had to be both 4-6/6 HLA-A, B, DRB1 matched to the recipient and to UCB no. 1. The minimum allowed cryopreserved graft cell dose was 1.5 × 107 NC/kg (UCB no. 1 ≥ 1.0 × 107 NC/kg; UCB no. 2 ≥ 0.5 × 107 NC/kg). HLA disparity between each unit and the recipient, and between the 2 units, were not necessarily at the same loci.
HLA typing
Prior to August 2003, HLA-A and HLA-B typing was performed using the standard 2-stage complement-dependent microcytotoxicity assay, and antigens were assigned as defined by the World Health Organization HLA nomenclature committee. In August 2003, HLA-A and HLA-B antigen typing methodology was changed to one using a reverse polymerase chain reaction (PCR)/sequence-specific oligonucleotide method using a Luminex (Luminex Corporation, Austin, TX) flow cytometer and bead technology. HLA-DRB1 allele typing was determined using PCR and sequence-specific primer technology (One Lambda, Canoga Park, CA).
Treatment plan
All patients received myeloablative conditioning using cyclophosphamide 120 mg/kg (60 mg/kg/d at 10 am on days -7 and -6 for 2 doses), and total body irradiation (1320 cGy in 8 fractions) and immunosuppression with cyclosporine A (CSA) from day -3 for at least 6 months. In addition, the first 2 patients received antithymocyte globulin (ATGAM; Pharmacia, Uppsala, Sweden) 15 mg/kg every 12 hours on days -3 to -1 and methylprednisone (MP) 1 mg/kg every 12 hours from days 5 to 19. As part of an institutional practice change for all adult UCB recipients, all subsequent patients (n = 21) received low-dose fludarabine (75 mg/m2; 25 mg/m2/d at 9 am on days -8 to -6 for 3 doses) and mycophenolate mofetil, 1 g twice daily from days -3 to +30 as a substitute for the ATG and MP. UCB units were thawed using the method of Rubinstein et al,10 and infused in series after hydration and premedication of acetaminophen and diphenhydramine. Granulocyte–colony stimulating factor (5 μg/kg/d) was administered to all patients from day 1 until an absolute neutrophil count (ANC) greater than or equal to 2.5 × 109/L was achieved for 2 consecutive days.
Donor chimerism analysis
Donor chimerism was determined serially on BM and/or blood at days 21, 60, 100, 180, 360, and 720 after transplantation, with additional time points as needed. Method of analysis was quantitative PCR of informative polymorphic variable number tandem repeat (VNTR) or short tandem repeat (STR) regions in the recipient and donor.11,12 Blood was separated into neutrophil and mononuclear fractions provided the total white blood cells (WBCs) was more than 1.0 × 109/L. DNA was amplified with fluorescent PCR primers for markers found to distinguish the 2 donor and recipient alleles. Fluorescent PCR products were separated using an Applied Biosystems 373 Sequencer or an Applied Biosystems 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA), and GeneScan software (Applied Biosystems) was used to correlate allele peak areas to the percentage of donor or recipient DNA (accuracy ± 5%). One VNTR/STR marker could distinguish the recipient and donors in 90% of cases. For remaining patients, analysis of 2 different VNTR regions was required.
Statistical analysis
The primary endpoint was safety as measured by the incidence of donor-derived neutrophil engraftment (by one or both donor units). Secondary endpoints included the incidences of platelet engraftment, acute and chronic GVHD, relapse and transplantation-related mortality, and the probability of survival as well as a description of the contribution of each unit to engraftment over time. Time of neutrophil recovery was designated as the first of 3 consecutive days with an ANC greater than 0.5 × 109/L. Sustained donor engraftment was defined as neutrophil recovery with both myeloid and lymphoid donor hematopoiesis beyond day 42 without secondary graft failure. Complete chimerism was defined as marrow reconstitution of donor origin of 90% or more. Two patients with refractory acute myeloid leukemia (AML) were censored from the engraftment analysis because of death from infection prior to day 28 after transplantation. The first patient died on day 13 without neutrophil recovery. The second patient, who died on day 25, had neutrophil recovery but the assessment of engraftment status was complicated by the fact that granulocyte transfusions were administered prior to death and a day-21 BM biopsy was not performed to assess donor chimerism because of the severity of the patient's illness.
Diagnosis of GVHD was based on standard clinical criteria with histopathologic confirmation when possible.13 Patients were evaluated for acute GVHD daily during hospitalization, at least weekly after discharge to 100 days, and at least every 3 months thereafter. GVHD grading was assigned retrospectively by independent review. The cumulative incidence of engraftment and GVHD was calculated by treating deaths from other causes as competing risks.14 In order to compare the effect of cell dose and viability of each UCB unit on unit predominance, a matched paired t test was performed, whereas z-score test statistics for normalized data were used to test the association between ABO blood group, sex match, and order of unit infusion and unit predominance.15 The statistical endpoints of survival and disease-free survival were estimated by the Kaplan-Meier method.16 Event times for survival were measured from transplantation day to date of death or last contact. Median follow-up of survivors is 10 months (range, 3.5 months-2.5 years). The analysis was performed as of March 15, 2004.
Results
UCB graft availability
During the study period 26 patients met eligibility criteria for double UCBT using myeloablative conditioning. Three (12%) patients received a single-unit graft because of the inability to identify a suitable second unit.
Patient and graft characteristics
Twenty-three consecutive patients (median age, 24 years [range, 13-53 years]; median weight, 73 kg [range, 48-120 kg]; 57% male; 61% cytomegalovirus antibody positive) were given transplants of 2 UCB units between January 2000 and October 2003. All patients were considered at high risk for relapse except 1 patient with chronic myelogenous leukemia in chronic phase who was refractory to high-dose imatinib and cytarabine. Diagnoses are detailed in Table 1. The median total cryopreserved graft cell dose was 4.8 × 107 NC/kg (range, 1.6-7.0 × 107 NC/kg). Infused graft cell doses are summarized in Table 1. Only 2 (9%) patients received a graft that contained at least one 6/6 HLA-A–, HLA-B–, or HLA-DRB1–matched unit to the recipient; 11 (48%) patients received a graft with at least one 5/6 HLA–matched unit; and 10 (43%) patients received a graft with both units 4/6 HLA–matched to the recipient. The units were 6/6 HLA–antigen matched to each other in 2 patients, 5/6 in 5 patients, and 4/6 in 16 patients.
Diagnosis and graft characteristics
. | No. . |
|---|---|
| N | 23 |
| Diagnosis, no.(%) | |
| ALL | |
| CR1 | 4 (17) |
| CR2 | 1 (4) |
| Relapse | 3 (13) |
| AML* | |
| CR1 | 4 (17) |
| CR2 | 3 (13) |
| CR3 | 1 (4) |
| MDS | 1 (4) |
| Relapse | 4 (17) |
| CML | |
| CP1 | 1 (4) |
| AP1 | 1 (4) |
| Infused NC × 107/kg, median (range) | |
| Total | 3.5 (1.1-6.3) |
| Larger unit | 1.9 (0.6-3.6) |
| Smaller unit | 1.5 (0.5-2.7) |
| Infused CD34+ × 105/kg, median (range) | |
| Total | 4.9 (1.2-14.5) |
| Larger unit | 2.9 (0.7-10.4) |
| Smaller unit | 1.4 (0.5-4.7) |
| Infused CD3+ × 107/kg, median (range) | |
| Total | 1.0 (0.5-2.2) |
| Larger unit | 0.6 (0.3-1.3) |
| Smaller unit | 0.4 (0.1-0.9) |
. | No. . |
|---|---|
| N | 23 |
| Diagnosis, no.(%) | |
| ALL | |
| CR1 | 4 (17) |
| CR2 | 1 (4) |
| Relapse | 3 (13) |
| AML* | |
| CR1 | 4 (17) |
| CR2 | 3 (13) |
| CR3 | 1 (4) |
| MDS | 1 (4) |
| Relapse | 4 (17) |
| CML | |
| CP1 | 1 (4) |
| AP1 | 1 (4) |
| Infused NC × 107/kg, median (range) | |
| Total | 3.5 (1.1-6.3) |
| Larger unit | 1.9 (0.6-3.6) |
| Smaller unit | 1.5 (0.5-2.7) |
| Infused CD34+ × 105/kg, median (range) | |
| Total | 4.9 (1.2-14.5) |
| Larger unit | 2.9 (0.7-10.4) |
| Smaller unit | 1.4 (0.5-4.7) |
| Infused CD3+ × 107/kg, median (range) | |
| Total | 1.0 (0.5-2.2) |
| Larger unit | 0.6 (0.3-1.3) |
| Smaller unit | 0.4 (0.1-0.9) |
ALL indicates acute lymphoblastic leukemia; CR, complete remission; AML, acute myelogenous leukemia; MDS, myelodysplasia; CML, chronic myelogenous leukemia; CP, chronic phase; AP, accelerated phase; and NC, nucleated cell.
Patients with AML in CR1 had disease secondary to prior myelodysplasia. MDS refers to recurrent MDS after AML induction
Double-unit infusion
No major adverse events resulted from double-unit infusion. Fourteen patients had no symptoms, 3 had nausea and/or emesis, 5 had mild to moderate hypertension, 1 had dizziness, and 1 had lip tingling within 2 hours of infusion.
Hematopoietic recovery and chimerism
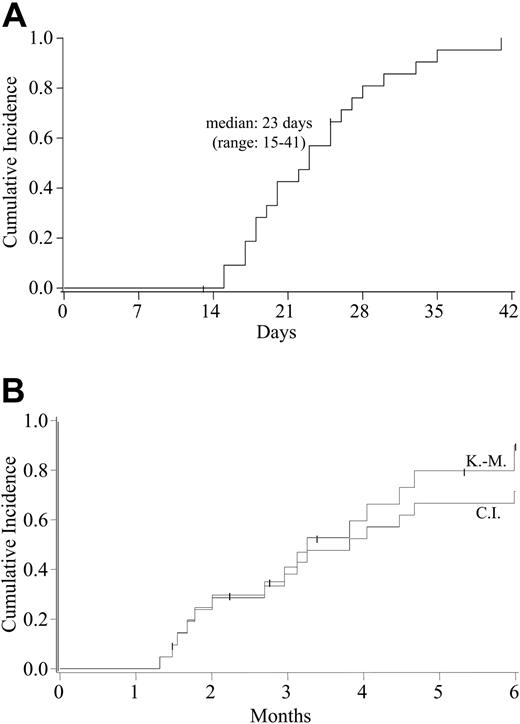
All of the 21 evaluable patients had sustained donor neutrophil engraftment at a median of 23 days (range, 15-41 days; Figure 1A). All of these 21 patients demonstrated complete donor chimerism, with no patient having secondary graft failure. By day 180 after transplantation, the cumulative incidence of platelet engraftment to 50 × 109/L or greater was 71% (95% CI, 47%-95%) with a Kaplan-Meier estimate of 90% (95% CI, 73-100; Figure 1B).
Hematopoietic recovery after double-unit UCBT. (A) Cumulative incidence of sustained donor neutrophil engraftment (ANC > 0.5 × 109/L) after double-unit UCBT. The Kaplan-Meier estimate of neutrophil engraftment was identical to the cumulative incidence. (B) Cumulative incidence and Kaplan-Meier estimates of platelet engraftment (≥ 50 × 109/L) after double-unit UCBT.
Hematopoietic recovery after double-unit UCBT. (A) Cumulative incidence of sustained donor neutrophil engraftment (ANC > 0.5 × 109/L) after double-unit UCBT. The Kaplan-Meier estimate of neutrophil engraftment was identical to the cumulative incidence. (B) Cumulative incidence and Kaplan-Meier estimates of platelet engraftment (≥ 50 × 109/L) after double-unit UCBT.
Serial assessments of the contribution of each unit to hematopoiesis were made beginning at day 21. Of the 21 evaluable patients, hematopoiesis in day 21 BM was accounted for by a single donor in 16 (76%) (median donor chimerism, 100%; range, 73%-100%). Although the remaining 5 (24%) patients had engraftment of both donors (median total donor chimerism, 91%; range, 64%-100%), 1 unit predominated (median, 74% [range, 42%-85%] vs 20% [range, 15%-40%] for the nonsustained unit). Skewed engraftment progressed so that evidence of double-unit hematopoiesis was observed in only 2 patients at day 60, and in none of the evaluable patients by day 100 (n = 17, with 2 of the 21 engrafting patients having died from relapse, 1 from pulmonary hemorrhage, and 1 from Aspergillus infection). Once engraftment was derived from 1 unit, the other unit never contributed to hematopoiesis at subsequent time points.
Influence of cell dose and HLA disparity on engraftment
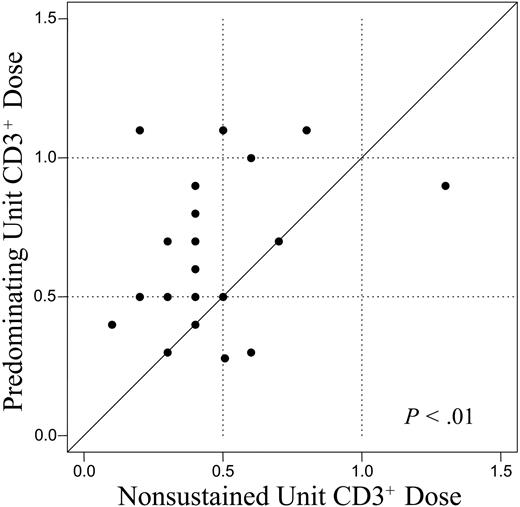
The relative percent viability, infused cell dose, and donor-recipient HLA disparity of the UCB units were examined as potential factors influencing which unit would predominate. The median percent viability (as measured by acridine orange and propidium iodide fluorescent dyes) of the predominating unit was 60% (range, 51%-88%) compared with 61% (range, 44%-90%) in the nonsustained unit (P = .39). The median infused cell doses of the predominating unit were 1.8 × 107 NC/kg (range, 0.7-3.6 × 107 NC/kg), 1.5 × 105 CD34+/kg (range, 0.6-10.4 × 105 CD34+/kg) and 1.9 × 104 granulocyte macrophage colony-forming unit (CFU-GM)/kg (range, 0.2-4.7 × 104 CFU-GM/kg). This compared with 1.9 × 107 NC/kg (range, 0.6-2.9 × 107 NC/kg; P = .32), 2.5 × 105 CD34+/kg (range, 0.5-9.1 × 105 CD34+/kg; P = .93), and 2.0 × 104 CFU-GM/kg (range, 0.08-5.7 × 104 CFU-GM/kg; P = .64) in the nonsustained unit. Notably, in 6 patients the infused CD34+ cell dose of the predominating unit was very low at 1.0 × 105/kg or less (range, 0.6-1.0 × 105/kg). In contrast to these nonpredictive graft parameters, a higher CD3+ cell dose was associated with the UCB unit that would predominate. The median infused CD3+ cell dose of the predominating unit was 0.6 × 107 CD3+/kg (range, 0.3-1.1 × 107 CD3+/kg) compared with 0.4 × 107 CD3+/kg (range, 0.1-1.3 × 107 CD3+/kg; P < .01) in the nonsustained unit (Figure 2).
Comparison of the infused CD3+ dose of the unit predominating in donor engraftment (y-axis) versus the nonsustained unit (x-axis). The solid line is a 1:1 reference. The CD3+ cell dose of the predominating unit was significantly greater than that of the nonsustained unit.
Comparison of the infused CD3+ dose of the unit predominating in donor engraftment (y-axis) versus the nonsustained unit (x-axis). The solid line is a 1:1 reference. The CD3+ cell dose of the predominating unit was significantly greater than that of the nonsustained unit.
Only 8 of the 21 patients with donor engraftment received 2 UCB units with different degrees of HLA disparity. Of these, the better HLA-matched unit to the recipient predominated in 4 patients, while lesser matched units engrafted over the better-matched units in 4 patients. The location of the HLA mismatch (HLA-A and HLA-B vs HLA-DRB1 disparity) was not associated with engraftment. Other factors such as ABO blood group (P = .33) and sex match (P = .39) failed to demonstrate any relationship with unit predominance. In 6 patients the unit infused first predominated in engraftment, whereas in 15 patients the second unit predominated (P = .09).
GVHD and survival
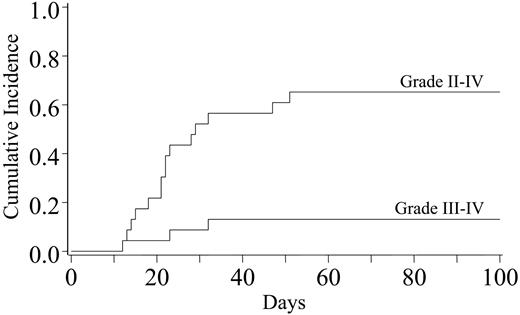
Incidences of grades II-IV and III-IV acute GVHD were 65% (95% CI, 42%-88%) and 13% (95% CI, 0%-26%), respectively (Figure 3). Two patients had grade III acute GVHD, 1 involving skin and gut, and 1 involving skin and liver. One patient had grade IV acute GVHD affecting the skin only. The 3 patients with grades III-IV acute GVHD were treated with topical corticosteroids and CSA. In addition, 1 patient received 15 mg/kg ATG every 12 hours for 10 doses and 60 mg/m2 oral prednisone for 2 weeks with a subsequent taper, whereas 2 patients were enrolled in a blinded randomized study of corticosteroids (2 mg/kg methylprednisolone on days 0-6 with subsequent taper) and daclizumab versus corticosteroids and placebo. All responded to immunosuppression. Five patients have had chronic GVHD (all extensive) for a cumulative incidence of 23% (95% CI, 6%-40%). Six-month transplantation-related mortality was 22% (95% CI, 5%-39%).
Cumulative incidence of grades II-IV and III-IV acute GVHD after double-unit UCBT.
Cumulative incidence of grades II-IV and III-IV acute GVHD after double-unit UCBT.
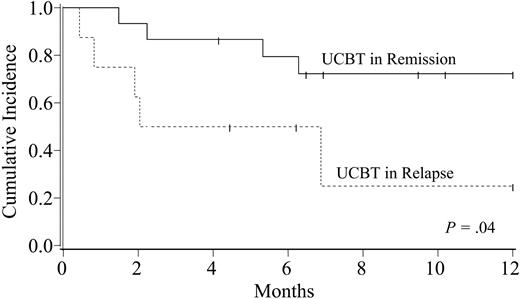
With a median follow-up of 10 months (range, 3.5 months-2.5 years), the probability of disease-free survival at 1 year is 57% (95% CI, 35%-79%). One year disease-free survival for patients who received transplants with acute leukemia in remission or with chronic myeloid leukemia (CML; n = 15) versus that for patients with acute leukemia who received transplants in relapse or with recurrent myelodysplasia (MDS) (n = 8) is 72% (95% CI, 49%-95%) versus 25% (95% CI, 0%-64%), respectively (P = .04; Figure 4). Causes of death were infection (n = 3), organ failure (n = 2), hemorrhage (n = 1), and relapse (n = 3).
Kaplan-Meier probability of disease-free survival after double-unit UCBT according to disease status. Patients with acute leukemia in remission or with chronic myelogenous leukemia had a significantly better survival than those patients with acute leukemia who were given transplants while in relapse, or secondary AML patients with recurrent myelodysplasia.
Kaplan-Meier probability of disease-free survival after double-unit UCBT according to disease status. Patients with acute leukemia in remission or with chronic myelogenous leukemia had a significantly better survival than those patients with acute leukemia who were given transplants while in relapse, or secondary AML patients with recurrent myelodysplasia.
Discussion
As early as 1969, Mathé et al, reported the combined transplantation of BM from different donors as a method of increasing graft cell dose. Since the introduction of UCB, and the demonstration of the critical importance of cell dose on UCBT outcome, there has been renewed interest in the augmentation of graft cell dose.18,19 Zanjani et al18 have investigated the transplantation of human UCB from multiple donors in a fetal sheep model. Enhanced short-term donor engraftment derived from both donors was demonstrated, although long-term hematopoiesis was derived from a single donor. Further, Chen et al20 have demonstrated that combining hematopoietic progenitors from 2 different HLA-mismatched T-cell–depleted murine BM donors had beneficial effects on myeloid engraftment in murine recipients.
Case reports of multidonor UCBT using up to 12 units have been published.21-24 The study by Weinreb et al23 suggested that the unit that was partially HLA-matched would predominate, at least in the short term. More recently, De Lima et al24 demonstrated successful engraftment of both donors in a double-unit transplant recipient with advanced acute leukemia. In this study, we have systematically assessed the safety of myeloablative UCBT using 2 partially matched units with a minimum total cryopreserved graft cell dose of 1.5 × 107 NC/kg, and evaluated posttransplantation donor chimerism by molecular techniques to document the contribution of each unit to hematopoiesis.
These data demonstrate the feasibility and safety of double-unit UCBT. An acceptable double-unit graft can be identified for the majority of patients. All evaluable patients achieved sustained donor engraftment with a low incidence of severe acute GVHD despite HLA disparity. Further, there was no apparent increase in acute GVHD incidence compared with single-unit UCBT.5,9 Interestingly, sustained hematopoiesis was derived from a single donor which predominated as early as 3 weeks after transplantation. Although the mechanisms that determine the fate of each donor are not known, it is notable that of the factors analyzed, only a larger CD3+ cell dose was associated with which unit predominated. Recently, Kim et al19 have reported on the outcome of double-unit UCBT in immunodeficient mice. These investigators found 1 unit predominated when using unfractionated grafts, whereas depletion of lineage-committed cells resulted in sustained double-unit engraftment. These data suggest a graft-versus-graft reaction was responsible for single-donor predominance. The results of our study would support the hypothesis that donor predominance is immune mediated.
Whether the transplantation of 2 UCB units improves engraftment compared with single-unit UCBT is not yet known. However, in this study, all evaluable patients engrafted at a median of 23 days which is a higher engraftment rate than previously observed by our group5 and other studies in the literature.9,25-27 Prior to double-unit UCBT only 30% of adult referrals to the University of Minnesota were eligible for UCBT on the basis of having a single unit with an adequate cell dose. Further, of those patients who proceeded to single-unit UCBT, only 72% (95% CI, 51%-93%) engrafted at a median of 34 days (range, 17-54 days) if the infused cell dose was less than 1.7 × 105 CD34/kg.5 In contrast, a double-unit graft may be identified for the majority of patients, and this approach has been associated with all evaluable patients engrafting despite the low infused CD34+ cell dose (median 1.5 × 105/kg) in the predominating unit. Although further study is required to elucidate the biology of double-unit UCBT, we speculate that the nonsustained unit may facilitate the engraftment of the predominating unit by immunologic mechanisms.
Double-unit UCBT extends access to transplantation to many patients who were previously disqualified on the basis of the available cell dose in a single unit. The high engraftment rate and low incidence of severe acute GVHD has resulted in a relatively low transplantation-related mortality. Therefore, further investigation of this approach in the context of larger clinical trials is indicated to determine the full impact of double-unit UCBT on transplantation outcome in adults and larger adolescents.
Prepublished online as Blood First Edition Paper, October 5, 2004; DOI 10.1182/blood-2004-07-2717.
Supported in part by National Cancer Institute grant PO1-CA65493 (J.E.W., T.E.D., C.M.V., P.B.M., J.S.M.), and a grant from the Children's Cancer Research Fund (J.E.W., J.N.B., T.E.D.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal