Comment on Masood et al, page 1310
The role of the HHV-8 vGPCR gene in KS pathogenesis is further explained by the finding that it induces ephrin B2 expression. That KS cell survival is dependent upon ephrin B2 raises broader questions about the biology and therapy of this tumor.
Human herpesvirus, type 8 (HHV-8) is the etiologic agent for all forms of Kaposi sarcoma (KS). The preponderance of evidence indicates that the viral G-protein–coupled receptor (vGPCR), a homologue of the CXC α-chemokine receptor family and related to the interleukin 8 (IL-8) receptor, is key to KS development. Experiments have demonstrated that vGPCR causes endothelial cell transformation and dermal lesions that have striking similarity to natural KS.1 vGPCR is constitutively active, inducing expression of phosphatidylinositol 3–kinase (PI3K)/Akt and a variety of transcription factors thought to be integral to cell transformation and to perpetuation of the neoplastic state via autocrine/paracrine signaling.2,3
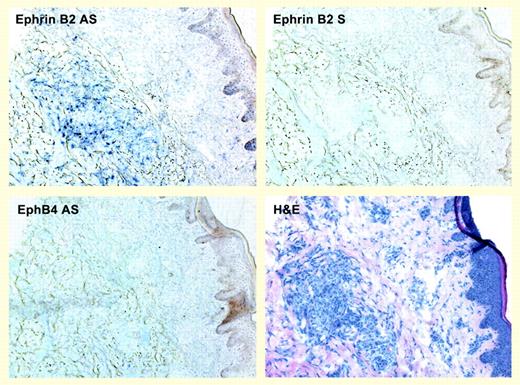
In the current issue, Masood and colleagues show that ephrin B2, but not EphB4, is expressed in KS spindle cells infected with HHV-8, thereby establishing an arterial phenotype. This phenotypic switch—uninfected endothelial cells express EphB4—is remarkable in terms of both biology and viral pathogenesis. They also demonstrate that vGPCR induces expression of ephrin B2 in endothelial cells, and they show that this action is mediated by several factors, including vascular endothelial growth factor (VEGF), IL-8, IL-6, and VEGF-C. These results suggest that ephrin B2 is the most distal element on a multipronged pathway whose activity is driven by vGPCR. Finally, this investigation reveals that KS cell viability in vitro is markedly diminished when ephrin B2 expression is decreased, likely at the hands of death receptor–mediated apoptosis. Thus, ephrin B2 is critical to establishing the phenotype and neoplastic properties of KS.FIG1
Ephrin B2 but not EphB4 is expressed in KS biopsy tissue. See the complete figure in the article beginning on page 1310.
Ephrin B2 but not EphB4 is expressed in KS biopsy tissue. See the complete figure in the article beginning on page 1310.
These observations suggest that therapeutic targeting of vGPCR or factors downstream on the network through to and including ephrin B2 could result in tumor regression. But as in Saturday Night Live's “Coffee Talk with Linda Richman,” this entertaining idea still requires much more to bridge the chasm in understanding.
Like other herpesviruses, HHV-8 exhibits a lytic phase following primary infection, enabling further infection of host cells, followed by viral latency that is thought to provide a means for surviving immune-mediated destruction. If vGPCR is expressed in only a small percentage of HHV-8–infected cells, how is this signaling network sustained? Is vGPCR expressed chronically at low levels or periodically with burst-type elaboration? Because so few KS cells express vGPCR, this factor would be a poor choice as a therapeutic target. HIV Tat induces both vGPCR activity and expression of cytokines involved in development of KS.4 Although use of combined antiretroviral therapy has reduced the incidence and severity of KS, durable complete remissions of aggressive disease are rarely observed.
With activation of many different pathways by vGPCR, can inhibition of VEGF expression result in KS remission? It is hoped that a planned National Cancer Institute (NCI)–supported AIDS Malignancy Consortium (AMC) trial of veglin, a novel antisense oligonucleotide reagent that targets all forms of VEGF, will answer this question. Use of bortezomib to target nuclear factor–κB (NF-κB), which is involved in HHV-8 and HIV pathogenesis, could treat both KS and AIDS. Because c-Kit and platelet-derived growth factor (PDGF) expression is increased in KS, a pilot study of imatinib was undertaken.5 Based on the success of this study, an AMC phase 2 trial of imatinib for KS will begin soon.
Members of the ephrin B/EphB family have been shown to play a role in growth and invasiveness of a variety of tumors.6 Ephrin B2 is a ligand that reverse-signals inside of the cell on which it is expressed, after binding with any of the 3 EphB receptors. Presently, we know nothing about the EphB receptor (or its cell source) that signals through ephrin B2 to maintain KS cell survival. Does ephrin B2 have any other role in KS other than antiapoptosis? Given the burgeoning importance of ephrin B/EphB in cancer, it will be only a matter of time before testing of their antagonists begins. With these and other options to molecularly target KS, it is very possible that we will be able to outsmart KSHV/HHV-8. If so, this would be a first for any of the known human tumor viruses. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal