Abstract
Nonmyeloablative stem cell transplantation (NST) is increasingly used in older patients. The impact of the shift from myeloablative transplantation to NST on relapse, transplant complications, and outcome has yet to be fully examined. We performed a retrospective analysis of 152 patients older than 50 years undergoing NST or myeloablative transplantation. Seventy-one patients received nonmyeloablative conditioning, fludarabine (30 mg/m2/d × 4) and intravenous busulfan (0.8 mg/kg/d × 4); 81 patients received myeloablative conditioning, primarily cyclophosphamide and total body irradiation. NST patients were more likely to have unrelated donors (58% versus 36%; P = .009), a prior transplant (25% versus 4%; P = < .0001), and active disease at transplantation (85% versus 59%; P = < .001). Despite the adverse characteristics, overall survival was improved in the NST group at 1 year (51% versus 39%) and 2 years (39% versus 29%; P = .056). There was no difference in progression-free survival (2 years, 27% versus 25%; P = .24). The incidence of grade 2 to 4 graft-versus-host disease was similar (28% versus 27%). The nonrelapse mortality rate was lower for NST patients (32% versus 50%; P = .01), but the relapse rate was higher (46% versus 30%; P = .052). Our experience suggests that, in patients over age 50, NST with fludarabine and low-dose busulfan leads to an overall outcome at least as good as that following myeloablative therapy. (Blood. 2005;105:1810-1814)
Introduction
Over the past few years, nonmyeloablative or reduced-intensity conditioning regimens have been offered as alternatives to conventional high-dose chemo/radiotherapy for older patients undergoing allogeneic hematopoietic stem cell transplantation.1-5 It is argued that a less intense preparative regimen is likely to produce considerably less organ toxicity and, therefore, would be better tolerated by older patients. If indeed regimen-related toxicity could be substantially reduced, it might then be possible to extend access to allogeneic transplantation to patients who, because of their age, would not have previously been considered reasonable candidates. This holds particular appeal because the median age of patients with diseases such as acute myelogenous leukemia, myelodysplasia, multiple myeloma, and low-grade lymphoma is considerably higher than the average age of the traditional transplant recipient.
It is uncertain, however, whether the growing trend to use nonmyeloablative conditioning in older patients is justified. Assuming that the graft-versus-malignancy effect is similar between the 2 procedures, the success of nonmyeloablative transplantation depends on its ability to decrease treatment-related mortality sufficiently to compensate for the degree of antitumor activity lost as a consequence of less intensive chemotherapy or radiotherapy. We examined our experience with nonmyeloablative transplantation using low-dose intravenous busulfan and fludarabine as conditioning in patients over the age of 50 years and compared outcome and reasons for treatment failure with patients of a similar age receiving conventional high-dose preparative regimens.
Patients, materials, and methods
Patient population
The clinical outcome of patients with hematologic malignancies over age 50 undergoing either nonmyeloablative or myeloablative allogeneic transplantation were assessed. All patients over age 50 years of age undergoing T cell-replete HLA-matched allogeneic transplantation from 1997 through 2002 at our institution were included in the analysis. Myeloablative transplantation eligibility requirements included an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2, absence of active infection at the time of study entry, and normal or near-normal parameters of organ function.
Nonmyeloablative transplantation eligibility requirements included ECOG performance status of 0 to 2, ejection fraction more than 30%, and no uncontrolled infection. All patients were evaluated for myeloablative transplantation and considered to have contraindications to that approach. Contraindications to myeloablative transplantation included prior myeloablative transplantation, advanced age (> 50 years), or significant organ dysfunction. From September 2000 to December 2002, 71 patients over age 50 were treated using a nonmyeloablative approach. The decision to pursue nonmyeloablative as opposed to myeloablative conditioning during this period was based on patient and physician preference.
The Human Subjects Protection Committee of Dana-Farber Cancer Institute approved all investigational protocols. Written informed consent was obtained in all cases.
Donors
All donors included in this analysis were HLA matched at A, B, and DR loci. Unrelated donors were required to match recipients at HLA-DR loci by molecular analysis. Class II typing was performed with sequence-specific oligonucleotide probes. The majority of donors in the nonmyeloablative cohort donated granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood stem cells (PBSCs). In the myeloablative group, 16 (20%) donated G-CSF-mobilized PBSCs and 65 (80%) donated bone marrow. PBSC donors in both groups were mobilized with filgrastim at 10 μg/kg/d for 5 days. Stem cell collection was initiated on the fifth day of filgrastim treatment and continued until sufficient numbers of CD34+ stem cells were obtained. The targeted cell number for nonmyeloablative transplantation was at least 1 × 107 CD34+ cells/kg. The targeted PBSC number for myeloablative transplantation was at least 5 × 106 CD34+ cells/kg. Bone marrow was obtained in the operating room under general or epidural anesthesia and the targeted cell count was more than 2 × 108 nucleated cells/kg.
Conditioning regimens
All 71 patients undergoing nonmyeloablative transplantation received fludarabine (30 mg/m2/d for 4 days) and intravenous busulfan (0.8 mg/kg/d for 4 days) on days -6, -5, -4, and -3. Patients receiving myeloablative transplants were treated with either high-dose cyclophosphamide (1800 mg/m2 for 2 days) and fractionated total body irradiation (TBI; 1400 cGy in 7 fractions over 4 days) in 94% of patients or oral busulfan (16 mg/kg divided over 4 days) and cyclophosphamide (6%).
GVHD prophylaxis
All patients received immune suppressive therapy as graft-versus-host disease (GVHD) prophylaxis. The recipients of nonmyeloablative transplants were treated on sequential protocols with defined GVHD prophylaxis. The initial 16 patients (22%) received GVHD prophylaxis consisting of cyclosporine plus corticosteroids. The subsequent 55 (78%) patients received tacrolimus combined with methotrexate. Among the 81 patients who underwent myeloablative conditioning, 77 (95%) received GVHD prophylaxis consisting of tacrolimus or cyclosporine and methotrexate. Four (5%) patients with a contraindication to methotrexate administration were given corticosteroids.
Statistical considerations
Descriptive statistical analysis was performed to assess patient baseline characteristics, disease, disease status at conditioning, GVHD prophylaxis, and source of progenitor cells. The 2-sided Fisher exact test was used for 2 × 2 table analysis, and the 2-sided Wilcoxon rank sum test was used for 2-sample comparison of continuous variables.
Cumulative incidence curves for nonrelapse death and relapse with or without death were constructed reflecting time to relapse and time to nonrelapse death as competing risks.6 Time to relapse and time to nonrelapse death were measured from the date of stem cell infusion. Patients who were alive without relapse were censored at the time last seen alive and relapse free. Overall survival (OS) and progression-free survival (PFS) were calculated using the Kaplan-Meier method. The log-rank test was used for group comparisons. PFS was defined as the time between stem cell infusion to relapse or death from any cause. OS was defined as the time between marrow infusion to death from any cause. Potential prognostic factors for survival and relapse were examined in the Cox proportional hazards model.
Results
Patient characteristics
The characteristics of all patients are detailed in Table 1. The median ages of patients receiving a nonmyeloablative transplant or myeloablative transplant were 58 and 54 years, respectively. A large number of patients in both groups had acute leukemia or myelodysplasia, 51% receiving a nonmyeloablative transplant and 41% receiving a myeloablative transplant. All patients received T cell-replete marrow or stem cells with immunosuppressive medication as GVHD prophylaxis. The median cell dose infused for patients having nonmyeloablative PBSC transplantation was 6.4 × 106 CD34+ cells/kg (range, 1.0-31.0 × 106 CD34+ cells/kg). Patients receiving nonmyeloablative conditioning were more likely to have unrelated donors (58% versus 36%, P = .009), to have received a prior transplant (25% versus 4%, P =< .0001), and to have active disease at the time of transplantation (85% versus 59%, P =< .001). Ten percent of patients in the nonmyeloablative transplantation group were in complete remission 1 (CR1) or had early-stage disease at transplantation compared with 40% of those in the myeloablative transplantation group. The primary indication for patients receiving nonmyeloablative transplants is outlined in Table 2. The median follow-up is 18 months (range, 6-34 months) for patients receiving nonmyeloablative transplants and 46 months (range, 3-73 months) for patients receiving myeloablative transplants.
Patient characteristics
. | Nonmyeloablative . | Myeloablative . | P . |
|---|---|---|---|
| No. of patients | 71 | 81 | — |
| Age, y | 58 (51-70) | 54 (51-66) | < .001 |
| Disease type with disease status at conditioning, no. (%) | .03* | ||
| AML | 21 (30) | 13 (16) | — |
| CR1 | 5 | 3 | — |
| CR2 | 4 | 1 | — |
| Relapse | 10 | 2 | — |
| Induction failure | 2 | 7 | — |
| ALL | 1 (1) | 3 (4) | — |
| CR1 | — | 1 | — |
| Relapse | 1 | 2 | — |
| CML | 9 (13) | 33 (41) | — |
| Early phase | 2 | 27 | — |
| Advanced phase | 7 | 6 | — |
| CLL | 13 (18) | 2 (2) | — |
| CR1 | — | 1 | — |
| Relapse | 13 | 1 | — |
| MDS | 15 (21) | 17 (21) | — |
| RA | 1 | — | — |
| RAEB | 14 | 17 | — |
| NHL | 9 (13) | 10 (12) | — |
| Low grade | 5 | 2 | — |
| Large cell | 4 | 8 | — |
| CMML | 3 (4) | — | — |
| Untreated | 3 | — | — |
| Other, no. (%) | — | 3 (4) | — |
| Type of transplant, no. (%) | — | — | .009 |
| MRD | 30 (42) | 52 (64) | — |
| URD | 41 (58) | 29 (36) | — |
| Transplant conditioning regimen, no. (%) | |||
| Flu/Bu | 71 (100) | 0 | — |
| CTX/TBI | 0 | 76 (94) | — |
| Bu/CTX | 0 | 5 (6) | — |
| GVHD prophylaxis, no. (%) | — | — | < .001 |
| Cyclosporine/prednisone | 16 (23) | 3 (4) | — |
| Tacrolimus/MTX | 55 (78) | 78 (96) | — |
| Stem cell source, no. (%) | < .0001 | ||
| PBSC | 66 (93) | 16 (20) | — |
| BM | 5 (7) | 65 (80) | — |
| Prior myeloablative transplant, no. (%) | 18 (25) | 3 (4) | < .0001 |
. | Nonmyeloablative . | Myeloablative . | P . |
|---|---|---|---|
| No. of patients | 71 | 81 | — |
| Age, y | 58 (51-70) | 54 (51-66) | < .001 |
| Disease type with disease status at conditioning, no. (%) | .03* | ||
| AML | 21 (30) | 13 (16) | — |
| CR1 | 5 | 3 | — |
| CR2 | 4 | 1 | — |
| Relapse | 10 | 2 | — |
| Induction failure | 2 | 7 | — |
| ALL | 1 (1) | 3 (4) | — |
| CR1 | — | 1 | — |
| Relapse | 1 | 2 | — |
| CML | 9 (13) | 33 (41) | — |
| Early phase | 2 | 27 | — |
| Advanced phase | 7 | 6 | — |
| CLL | 13 (18) | 2 (2) | — |
| CR1 | — | 1 | — |
| Relapse | 13 | 1 | — |
| MDS | 15 (21) | 17 (21) | — |
| RA | 1 | — | — |
| RAEB | 14 | 17 | — |
| NHL | 9 (13) | 10 (12) | — |
| Low grade | 5 | 2 | — |
| Large cell | 4 | 8 | — |
| CMML | 3 (4) | — | — |
| Untreated | 3 | — | — |
| Other, no. (%) | — | 3 (4) | — |
| Type of transplant, no. (%) | — | — | .009 |
| MRD | 30 (42) | 52 (64) | — |
| URD | 41 (58) | 29 (36) | — |
| Transplant conditioning regimen, no. (%) | |||
| Flu/Bu | 71 (100) | 0 | — |
| CTX/TBI | 0 | 76 (94) | — |
| Bu/CTX | 0 | 5 (6) | — |
| GVHD prophylaxis, no. (%) | — | — | < .001 |
| Cyclosporine/prednisone | 16 (23) | 3 (4) | — |
| Tacrolimus/MTX | 55 (78) | 78 (96) | — |
| Stem cell source, no. (%) | < .0001 | ||
| PBSC | 66 (93) | 16 (20) | — |
| BM | 5 (7) | 65 (80) | — |
| Prior myeloablative transplant, no. (%) | 18 (25) | 3 (4) | < .0001 |
— indicates not applicable; AML, acute myelogenous leukemia; ALL, acute lymphocytic leukemia; CML, chronic myelogenous leukemia; MDS, myelodysplastic syndrome; RA, refractory anemia; RAEB, refractory anemia with excess blasts; NHL, non-Hodgkin lymphoma; CMML, chronic myelomonocytic leukemia; MRD, matched related donor; URD, unrelated donor; Flu, fludarabine; Bu, busulfan; CTX, corticoste-roids; TBI, total body irradiation; MTX, methotrexate; BM, bone marrow.
AML/MDS/CML versus other disease.
Primary indication for nonmyeloablative transplantation
Indication . | No. of patients (%) . |
|---|---|
| Older than 50 y | 40 (56) |
| Prior myeloablative transplant | 17 (24) |
| Disease type | 9 (13) |
| Other medical condition/organ dysfunction | 5 (7) |
Indication . | No. of patients (%) . |
|---|---|
| Older than 50 y | 40 (56) |
| Prior myeloablative transplant | 17 (24) |
| Disease type | 9 (13) |
| Other medical condition/organ dysfunction | 5 (7) |
GVHD
The incidence of grades 2 to 4 acute GVHD was similar in patients undergoing nonmyeloablative and myeloablative transplantation, 29% versus 27%, respectively (Table 3). The incidence of grade 2 to 4 acute GVHD was higher in patients receiving transplants from unrelated donors compared with related donors: nonmyeloablative 34% versus 20%, respectively, and myeloablative 31% versus 25%, respectively. The incidence of grade 3 to 4 acute GVHD was also similar, 20% versus 17%.
Incidence of acute GVHD
Grade GVHD . | Nonmyeloablative, no. (%) . | Myeloablative, no. (%) . |
|---|---|---|
| 0 | 45 (63) | 38 (47) |
| 1 | 6 (9) | 21 (26) |
| 2 | 6 (9) | 8 (10) |
| 3-4 | 14 (20) | 14 (17) |
Grade GVHD . | Nonmyeloablative, no. (%) . | Myeloablative, no. (%) . |
|---|---|---|
| 0 | 45 (63) | 38 (47) |
| 1 | 6 (9) | 21 (26) |
| 2 | 6 (9) | 8 (10) |
| 3-4 | 14 (20) | 14 (17) |
Transplant-related mortality
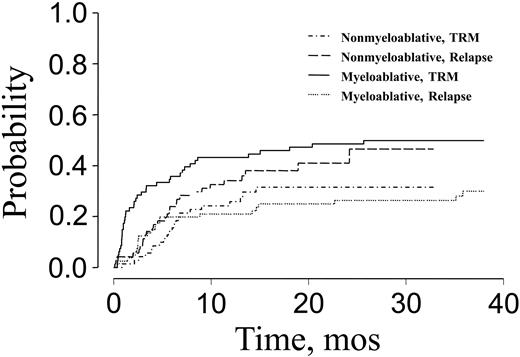
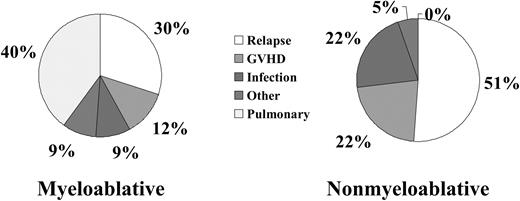
The 100-day treatment-related mortality (TRM) was significantly lower for patients receiving nonmyeloablative transplants than for recipients of myeloablative conditioning (6% versus 30%). The cumulative incidence of nonrelapse mortality was 32% for patients receiving nonmyeloablative conditioning compared with 50% for patients receiving myeloablative conditioning (P = .01; Figure 1). Nonrelapse death and disease relapse were considered competing risks in this analysis. Major causes of nonrelapse mortality for patients receiving nonmyeloablative transplants were GVHD and infection. Pulmonary complications, in addition to GVHD and infection, were the major causes of treatment failure after myeloablative transplantation (Figure 2). There was a significantly higher incidence of fatal pulmonary complications after myeloablative transplantation, 28%, compared with 0% after nonmyeloablative transplantation (P < .0001).
Cumulative incidence of TRM and risk of relapse after nonmyeloablative or myeloablative transplantation for patients over the age of 50.
Cumulative incidence of TRM and risk of relapse after nonmyeloablative or myeloablative transplantation for patients over the age of 50.
Comparison of the causes of treatment failure for patients over the age of 50 receiving either nonmyeloablative or myeloablative transplants.
Comparison of the causes of treatment failure for patients over the age of 50 receiving either nonmyeloablative or myeloablative transplants.
Relapse
Disease relapse was the primary cause of treatment failure for patients receiving nonmyeloablative transplants. The cumulative incidence of relapse was 46% for patients receiving nonmyeloablative conditioning and 30% for patients receiving myeloablative conditioning (P = .052; Figure 1).
OS and PFS
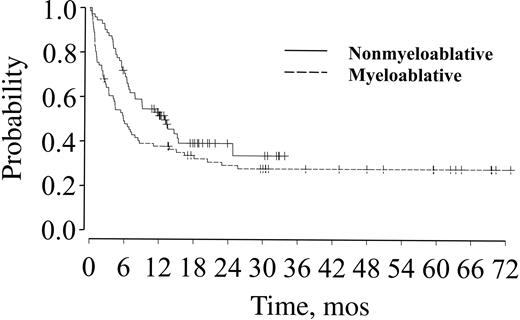
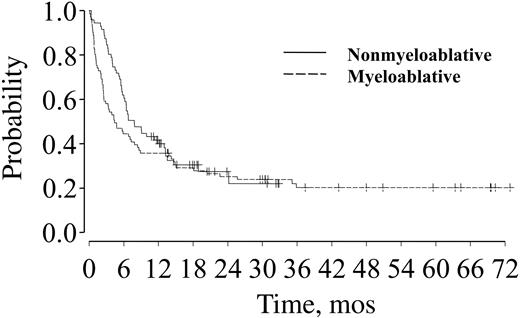
Estimates of OS for nonmyeloablative and myeloablative transplant recipients are 51% and 38% at 1 year and 39% and 29% at 2 years (P = .06), respectively (Figure 3; Table 4). PFS rates at 1 and 2 years after transplantation were 40% and 36% at 1 year and 27% and 25% at 2 years (P = .24) for patients receiving nonmyeloablative and myeloablative conditioning, respectively (Figure 4; Table 4). Donor source did not influence OS or PFS for either group.
OS for patients over age 50 after nonmyeloablative or myeloablative transplantation.
OS for patients over age 50 after nonmyeloablative or myeloablative transplantation.
OS and PFS
. | Median (95% CI), mo . | 1 y, % . | 2 y, % . | P . |
|---|---|---|---|---|
| OS | ||||
| Nonmyeloablative | 13.0 (7.8, 24.8) | 51 | 39 | .06 |
| Myeloablative | 5.8 (3.4, 8.6) | 38 | 29 | — |
| PFS | ||||
| Nonmyeloablative | 7.8 (6.0, 13.0) | 40 | 27 | .24 |
| Myeloablative | 4.4 (2.5, 8.4) | 36 | 25 | — |
. | Median (95% CI), mo . | 1 y, % . | 2 y, % . | P . |
|---|---|---|---|---|
| OS | ||||
| Nonmyeloablative | 13.0 (7.8, 24.8) | 51 | 39 | .06 |
| Myeloablative | 5.8 (3.4, 8.6) | 38 | 29 | — |
| PFS | ||||
| Nonmyeloablative | 7.8 (6.0, 13.0) | 40 | 27 | .24 |
| Myeloablative | 4.4 (2.5, 8.4) | 36 | 25 | — |
— indicates not applicable.
PFS for patients over age 50 after nonmyeloablative (solid line) or myeloablative (dashed line) transplantation.
PFS for patients over age 50 after nonmyeloablative (solid line) or myeloablative (dashed line) transplantation.
A subset analysis was performed assessing OS and PFS in patients with advanced leukemia (beyond CR1) and advanced myelodysplastic syndrome (MDS). Thirty-one patients, 17 with advanced leukemia and 14 with advanced MDS, who received nonmyeloablative transplants were compared with 29 patients, 12 with advanced leukemia and 17 with advanced MDS, receiving myeloablative transplants. OS at 2 years was 28% for patients receiving nonmyeloablative transplants and 16% for patients receiving myeloablative transplants (P = .08). PFS was not significantly different for patients receiving either nonmyeloablative transplants or myeloablative transplants (P = .09).
Factors associated with outcome and toxicity
Cox regression analysis was performed to identify factors associated with OS, PFS, and TRM. Factors analyzed included age, transplantation conditioning regimen (nonmyeloablative versus myeloablative), patient-donor sex mismatch, donor type (related versus unrelated), stem cell source (bone marrow versus peripheral blood), development of acute GVHD, prior transplantation, and remission status at time of transplantation. No factor, including conditioning regimen, influenced OS in patients older than 50 years. Analysis of factors having an impact on PFS identified both patient-donor sex mismatch and remission status at the time of transplantation as important variables (Table 5). Patients with a donor of the opposite sex had an improved PFS (hazard ratio, 0.67; P = .03). Consistent with prior studies, patients in remission at the time of transplantation had an improved PFS compared with patients with active disease at the time of transplantation.
Factors having an impact on PFS
Factor . | Hazard ratio . | P . |
|---|---|---|
| Conditioning regimen | 0.7 | .27 |
| Sex mismatch | 0.6 | .03 |
| Donor type | 1.0 | .98 |
| Disease type* | 1.3 | .28 |
| Acute GVHD | 0.9 | .64 |
| Prior transplant | 1.2 | .48 |
| Remission status | 0.6 | .03 |
| Stem cell source | 0.9 | .81 |
| GVHD prophylaxis | 0.9 | .82 |
Factor . | Hazard ratio . | P . |
|---|---|---|
| Conditioning regimen | 0.7 | .27 |
| Sex mismatch | 0.6 | .03 |
| Donor type | 1.0 | .98 |
| Disease type* | 1.3 | .28 |
| Acute GVHD | 0.9 | .64 |
| Prior transplant | 1.2 | .48 |
| Remission status | 0.6 | .03 |
| Stem cell source | 0.9 | .81 |
| GVHD prophylaxis | 0.9 | .82 |
AML/MDS/CML versus all others.
Discussion
Our data suggest that nonmyeloablative conditioning with fludarabine and intravenous busulfan is a reasonable alternative to traditional myeloablative transplantation in patients older than 50 years with hematologic malignancies undergoing allogeneic transplantation. OS and PFS were not diminished by reduction in the intensity of the preparative regimen. The similarity in PFS between the 2 cohorts of patients is even more impressive given that patients receiving nonmyeloablative conditioning were more likely to have undergone previous transplantation and were more likely to have active disease at the time of transplantation.
Several reports have demonstrated the feasibility of nonmyeloablative allogeneic transplantation in older adults.1-3 With a variety of conditioning regimens, 1-year nonrelapse mortality rates ranged from 7% to 55% and 1-year OS from 44% to 68%. With improvements in transplantation, such as better supportive care and GVHD prophylaxis, results after myeloablative transplantation for older individuals has improved. Nonetheless, TRM and risk of relapse remain significant.7-12
Our data suggest that the intensity of conditioning does affect the rate of disease recurrence after transplantation in older individuals with an increased risk of relapse noted after nonmyeloablative transplantation. Nonetheless, given that the majority of patients undergoing nonmyeloablative transplantation had active disease at the time of transplantation, our results confirm that the potent allogeneic graft-versus-leukemia (GVL) reactions after nonmyeloablative transplantation play an important role in controlling disease recurrence and suggest that these GVL responses are more critical than the contribution of the conditioning regimen. This finding supports prior clinical observations that older patients with leukemia are unlikely to be cured by intensive chemotherapy alone.13 Factors contributing to the lower cure rate in older patients with leukemia include the increased incidence of antecedent hematologic disorders, as well as the higher prevalence of adverse cytogenetics and increased expression of multidrug resistance genes in older individuals.14-16
As anticipated, nonmyeloablative conditioning with fludarabine and intravenous busulfan was associated with a lower nonrelapse mortality than that observed after myeloablative conditioning. Nonetheless, GVHD and infectious complications remain significant complications for patients receiving nonmyeloablative conditioning. The time to onset of GVHD is often delayed in onset and has a distinct clinical appearance when compared with acute GVHD after myeloablative transplantation.17 Most, but not all, reports of infectious complications after nonmyeloablative transplantation have demonstrated a lower incidence of bacterial infections after transplantation but a persistent risk of invasive fungal and cytomegalovirus (CMV) infections.18-23 Pulmonary complications and veno-occlusive disease were much reduced following nonmyeloablative transplantation compared with myeloablative transplantation. Similar reductions in pulmonary complications after nonmyeloablative transplantation have been reported.24 In total, these findings suggest further reduction in treatmentrelated morbidity and mortality after nonmyeloablative transplantation efforts should focus on reducing the incidence of GVHD and improving immune reconstitution after transplantation.
It is premature to conclude that all patients over 50 years of age be preferentially offered nonmyeloablative conditioning. There may be specific disease circumstances and patient characteristics where such reduced conditioning will be inferior to conventional ablation with regard to long-term disease control. Nonetheless, it does seem that, in general, overall success is not hampered by our reduced-intensity preparative regimen. If, indeed, that is the case, then economic and quality of life issues must be evaluated to determine what approach is best to adopt in this patient population.
Prepublished online as Blood First Edition Paper, September 30, 2004; DOI 10.1182/blood-2004-05-1947.
Supported in part by grant PO1 HL070149 from the National Heart, Lung, and Blood Institute and the Ted and Eileen Pasquerello Fund. R.J.S. is a Clinical Research Scholar of the Leukemia and Lymphoma Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal