Abstract
Fas ligand (FasL) and perforin pathways not only are the major mechanisms of T cell–mediated cytotoxicity but also are involved in homeostatic regulation of these T cells. In the present study, we tested whether CD8+ donor T cells that are deficient in both perforin and FasL (cytotoxic double deficient [cdd]) could induce graft-versus-host disease (GVHD) in a major histocompatibility complex class I–mismatched lethally irradiated murine model. Interestingly, recipients of cdd CD8+ T cells demonstrated significantly greater serum levels of interferon gamma and tumor necrosis factor alpha and histopathologic damage from GVHD than wild-type (wt) T cells on day 30 after allogeneic bone marrow transplantation (P < .05). Wt and either perforin-deficient or FasL-deficient CD8+ T cells expanded early after transplantation followed by a contraction phase in which the majority of expanded CD8+ T cells were eliminated. In contrast, cdd CD8+ T cells exhibited prolonged expansion and reduced apoptosis to alloantigen stimulation in vivo and in vitro. Together these results suggest that donor cdd CD8+ T cells expand continuously and cause lethal GVHD, and that both perforin and FasL are required for the contraction of allo-reactive CD8+ T cells.
Introduction
Graft-versus-host disease (GVHD) is the major complication of allogeneic bone marrow transplantation (BMT). GVHD occurs when donor T cells recognize major histocompatibility complex (MHC) and their associated peptides on host-derived antigen-presenting cells (APCs).1-3 The target organs of GVHD are the skin, gut, liver, and lymphohematopoietic compartments. We recently demonstrated that alloantigen expression on host target epithelium is not necessary to initiate GVHD in mouse models of BMT, and inflammatory cytokines play a central role in the CD4+-mediated GVHD.4 Interestingly, single cytotoxic-deficient (perforin or Fas ligand [FasL]) donor T cells can induce CD4+-mediated GVHD,5,6 and recently, studies have demonstrated that cytotoxic double-deficient (cdd) CD3+ and CD4+CD8– T cells can also effect GVHD in allogeneic minor histocompatibility antigen (mHA) or MHC-disparate BMT models.5-7 CD8+-dependent GVHD is reportedly induced by both cytokine- and cytolytic T lymphocyte (CTL)–mediated cytotoxicity as several studies using FasL- or perforin/granzyme-deficient mice suggested that CTL-mediated cytotoxicity plays a role in the CD8+-mediated, MHC class I–mismatched GVHD model.5,8,9
Recent studies have demonstrated that FasL and perforin not only are the principal cytotoxic effector molecules, but also are involved in homeostatic regulation of CD8+ T cells. The role of Fas/FasL in lymphocyte homeostasis was clearly established with the recognition that functional null mutations in these proteins were associated with exacerbated autoimmune disease.10,11 Perforindeficient mice have essentially normal immune homeostasis. However, considerable evidence indicates the existence of a perforin-dependent mechanism to regulate the magnitude of CD8+ T cell expansion in models as diverse as GVHD, viral and bacterial infections, and dendritic cell (DC) immunization.12-19 The present studies demonstrate that CD8+ T cells lacking the major cytotoxic pathways can induce GVHD across an MHC class I–only disparity. Moreover, we also found that both perforin and FasL contribute to the regulation of alloreactive CD8+ T cell expansion and contraction during the acute period of GVHD in this model.
Materials and methods
Mice
Female C57BL/6 (B6, H-2b), B6.C-H2bm1/ByJ (bm1), C57BL/6-Prf1tm1Sdz/J (pfp–/–, H-2b), and B6Smn.C3-Tnfsf6gld/J (gld, H-2b) mice were purchased from the Jackson Laboratories (Bar Harbor, ME). The bm1 mice possess a mutant class I allele that differs from B6 mice. C57BL/6 cytotoxic double-deficient (perforin and FasL) mice (cdd, H-2b) were generated from the breeding of pfp+/–gld pairings as described previously.7 The offspring were then screened for homozygous perforin deficiency by polymerase chain reaction (PCR) as previously reported20,21 to select for pfp–/–gld (ie, cdd). The cdd mice (6 to 8 weeks old) were maintained in pathogen-free conditions in the Department of Microbiology and Immunology at the University of Miami School of Medicine. gld and cdd mice were used as donors before the age of 9 weeks after a flow cytometric analysis confirmed that there was no evidence of accumulation of CD3+CD4–CD8–B220+ T cells in their spleens. The age range of mice used as other bone marrow (BM) transplant donors and recipients was between 8 and 12 weeks.
BMT
Mice underwent transplantation according to a standard protocol described previously.4 Briefly, mice were irradiated again with 13 Gy total body irradiation (TBI), split into 2 doses, and injected with 2 × 106 CD8+ splenic T cells with 5 × 106 T-cell–depleted (TCD) BM cells from wild-type (wt) B6 donors after 13-Gy total body irradiation (TBI). CD8+ T cells were negatively isolated by using CD4, DX5, MHC class II, and CD11b Micro Beads and the auto magnetic-activated cell sorter (MACS) following nylon wool purification of T cells from splenocytes.
Systemic and histopathologic analysis of GVHD
Survival after BMT was monitored daily and the degree of clinical GVHD was assessed weekly by a scoring system that sums changes in 5 clinical parameters: weight loss, posture, activity, fur texture, and skin integrity (maximum index = 10) as described.22 This score is a more sensitive index of GVHD severity than weight loss alone in multiple murine models.22 Acute GVHD was also assessed by detailed histopathologic analysis of liver, intestine, and skin, as described.23,24 The degree of cell infiltration in tissue was assessed by a scoring system incorporating 4 parameters: cell infiltration in portal triada, bill ducts/ductules, vascular, and hepatocellular area. Slides were coded without reference to mouse type or prior treatment status and examined systematically by a single pathologist (C.L.) using a semiquantitative scoring system.23,24
Mixed leukocyte reactions
Splenic CD8+ T cells and dendritic cells (DCs) were isolated using CD8 and CD11c Micro Beads, respectively. The purity of the CD8 T cell and DC suspension was more than 90%. CD8+ T cells were used as responders at 2 × 105/well against irradiated (20 Gy) DCs (1 × 104/well) for 2 to 4 days. During the final 12 hours of culture, cells were pulsed with 1 μCi (0.037 MBq) [3H] thymidine (2 Ci/mmol [74.0 GBq]; Perkin Elmer, Billerica, MA) and proliferation was determined on a Top Count NTX (Packard Instrument, Meriden, CT).
Flow cytometric analysis
A flow cytometric analysis was performed using fluorescein isothiocyanate (FITC)–, phycoerythrin (PE)–, or allophycocyanin-conjugated monoclonal antibodies (mAbs) to mouse CD45.1, CD3, CD4, CD8, B220, and CD11c (BD Pharmingen, San Diego, CA). Cells were preincubated with 2.4G2 mAbs to block Fcγ receptor, and were then incubated with the relevant mAbs for 30 minutes at 4°C. Finally, cells were washed twice with 0.2% bovine serum albumin in phosphate-buffered saline (PBS), fixed with 1% paraformaldehyde in PBS and analyzed by EPICS Elite ESP cell sorter (Beckman-Coulter, Miami, FL). Irrelevant immunoglobulin G2a/b mAbs were used as a negative control. For analysis of donor cell apoptosis, spleens from recipient mice were harvested 10 days after transplantation and stained with PE-CD8, then washed with PBS, and then stained with FITC–conjugated annexin (R&D Systems, Minneapolis, MN) in the dark for 15 minutes at room temperature. Donor cell apoptosis was identified based on double staining for CD8 and annexin.
Enzyme-linked immunosorbent assay (ELISA)
ELISA for interferon-gamma (IFN γ) and tumor necrosis factor-alpha (TNF α) (BD Pharmingen) was performed as described.25 Samples and standards were run in duplicate.
Statistical analysis
The Mann-Whitney U test was used for the statistical analysis of in vitro data, while the Wilcoxon rank test was used to analyze survival data. Linear regression and analysis of covariance were used to quantify the relationship between 2 variables. P less than .05 was considered statistically significant.
Results
Cytotoxic double-deficient CD8+ T cells cause acute GVHD
Previous studies have shown that lack of either the perforin (pfp–/–) or FasL (gld) in donor T cells significantly reduced GVHD following nonmyeloablative conditioning in a murine model.26 We evaluated the role of each pathway in donor CD8+ T cells during GVHD following myeloablative conditioning in a donor-recipient strain combination that differs at a single MHC class I antigen.27 bm1 mice received 13 Gy total body irradiation (TBI) on day 0 and then received transplants of 5 × 106 TCD BM from wt B6 and 2 × 106 CD8+ T cells from either wt, gld, or pfp–/– B6 donors; survival at day 30 after BMT was similar in all donor groups (8/10, 8/10, and 9/10, respectively). We analyzed histologic changes of GVHD at day 30 in the liver and intestine using a semiquantitative pathology index (“Materials and methods”). As shown in Figure 1, GVHD damage in target organs did not differ among donor groups, although histologic damage in all groups was relatively high due to the young age of recipients. Therefore, the deficiency of a single cytotoxic effector molecule in CD8+ donor T cells did not reduce GVHD in this BMT model following myeloablative conditioning.
Effects of perforin- or FasL-deficient CD8+T cells on acute GVHD. Lethally irradiated bm1 mice received transplants as described in “Materials and methods” from wt B6 (wt), perforin-deficient B6 (pfp–/–), or Fas ligand–deficient B6 (gld) donors. Syngeneic B6 BM transplant recipients (syn) served as no-GVHD controls. At 30 days after BMT, liver and intestine (bottom column [•], small intestine; upper column, large intestine) were harvested and scored semiquantitatively (n = 4 mice/group). Data represent mean ± SD.
Effects of perforin- or FasL-deficient CD8+T cells on acute GVHD. Lethally irradiated bm1 mice received transplants as described in “Materials and methods” from wt B6 (wt), perforin-deficient B6 (pfp–/–), or Fas ligand–deficient B6 (gld) donors. Syngeneic B6 BM transplant recipients (syn) served as no-GVHD controls. At 30 days after BMT, liver and intestine (bottom column [•], small intestine; upper column, large intestine) were harvested and scored semiquantitatively (n = 4 mice/group). Data represent mean ± SD.
We next evaluated cytotoxic double-deficient (cdd) CD8+ T cells in this GVHD model. The bm1 mice received transplants as before from wt or cdd B6 donors. Surprisingly, cdd CD8+ T cells caused more rapid mortality than wt donor cells (Figure 2A). Histologic GVHD damage in the liver and intestine was also significantly greater on day 30 after BMT in recipients of cdd T cells (Figure 2B). We next evaluated inflammatory cytokines associated with acute GVHD. Compared with wt cells, cdd donor T cells caused dramatic increases in serum levels of IFN γ and TNF α on day 30 even though single cytotoxic deficient donor cells did not (Figure 2C).
Cdd CD8+T cells cause acute GVHD. The bm1 cells were transplanted from wt B6 (•, n = 11) or cdd B6 (▴, n = 6) donors as in Figure 1. B6 syngeneic BM transplant recipients (○,n = 9) served as no-GVHD controls. (A) Survival. (B) Histology. At 30 days after BMT, liver, intestine (bottom column [•], small intestine; upper column, large intestine), and skin were harvested and scored semiquantitatively (n = 4/group). B6 → B6 (syn; □), wt B6 → bm1 (wt; ▦), and cdd B6 → bm1 (cdd; ▩ ). (C) Serum cytokines. Mice received transplants of wt, pfp–/–, gld, or cdd as in Figure 1 and were killed on day 30 (n = 3 mice/group). Serum was harvested and analyzed for TNF α and IFN γ by ELISA (pg/mL). Each graph represents 1 of 3 similar experiments. Data represent mean ± SD. wt versus cdd, *P < .05 by Mann-Whitney U test.
Cdd CD8+T cells cause acute GVHD. The bm1 cells were transplanted from wt B6 (•, n = 11) or cdd B6 (▴, n = 6) donors as in Figure 1. B6 syngeneic BM transplant recipients (○,n = 9) served as no-GVHD controls. (A) Survival. (B) Histology. At 30 days after BMT, liver, intestine (bottom column [•], small intestine; upper column, large intestine), and skin were harvested and scored semiquantitatively (n = 4/group). B6 → B6 (syn; □), wt B6 → bm1 (wt; ▦), and cdd B6 → bm1 (cdd; ▩ ). (C) Serum cytokines. Mice received transplants of wt, pfp–/–, gld, or cdd as in Figure 1 and were killed on day 30 (n = 3 mice/group). Serum was harvested and analyzed for TNF α and IFN γ by ELISA (pg/mL). Each graph represents 1 of 3 similar experiments. Data represent mean ± SD. wt versus cdd, *P < .05 by Mann-Whitney U test.
Cdd T cells exhibit prolonged expansion to alloantigen stimulation in vivo and in vitro
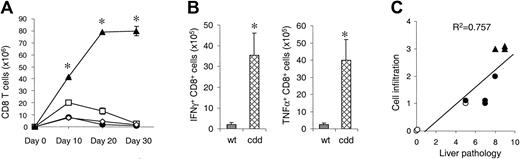
Because recipients of cdd cells showed dramatic increase in serum levels of inflammatory cytokines and recipients of single deficient cells did not, we hypothesized that a mechanism in addition to CTL toxicity might be operative in the induction of GVHD. FasL and perforin not only are important effector molecules of CTL, but they also are involved in homeostatic regulation of CD8+ T cells.17-19 In this light, we hypothesized that cdd T cells proliferated to a greater extent than wt cells, causing increased cytokine production and more severe GVHD. We thus compared in vivo expansion of donor T cells in recipients of allogeneic CD8+ T cells from wt, gld, pfp–/–, and cdd donors. T cells from wt donors expanded in the spleen until day 10 after BMT, followed by a rapid decline (contraction phase) (Figure 3A). gld T cells showed similar kinetics as wt T cells, whereas the pfp–/– T cells showed a significantly greater peak on day 10 but also contracted by day 30. By contrast, cdd CD8+ T cells expanded continuously up to day 30 after BMT, peaking at 100 times the number of wt T cells. Flow cytometric analysis of intracytoplasmic cytokines revealed that a greater proportion of cdd CD8+ T cells produced IFN γ and TNF α compared with wt T cells (P < .05, Figure 3B), consistent with the increased serum levels of these cytokines. We then analyzed whether GVHD damage correlated with the number of infiltrating mononuclear cells. We chose the liver for this evaluation because of the large dynamic range of cellular infiltration in this target organ, incorporating portal triads and bile ducts/ductules as well as vascular and hepatocellular areas. As shown in Figure 3C, the amount of hepatic tissue damage correlated closely with the degree of cellular infiltration (P < .01). Taken together, these data demonstrate that the absence of both cytotoxic pathways led to increased donor CD8+ T-cell expansion and greater cytokine production, causing greater GVHD target organ damage.
Deficiency of both perforin and FasL in donor CD8+T cells cause prolonged expansion after BMT. (A) Mice underwent transplantation as in Figure 1. Splenocytes were harvested 10, 20, and 30 days (n = 3 mice/group) after BMT and analyzed by fluorescence-activated cell sorter (FACS). wt B6 → bm1 (•), pfp–/–→ bm1 (□), gld → bm1 (⋄), and cdd → bm1 (▴). wt B6 → bm1 versus cdd → bm1, *P < .05 by Mann-Whitney U test. (B) TNF α and IFN γ production by donor CD8+ T cells was determined by intracytoplasmic staining. *P < .05 by Mann-Whitney U test. (C) Overall pathologic damage of liver specimens correlated to the intensity of cell infiltration in portal triads, bile ducts/ductules, vascular, and hepatocellular areas. P < .01 by linear regression analysis. Symbols are same as in 3A. Error bars indicate mean ± SD.
Deficiency of both perforin and FasL in donor CD8+T cells cause prolonged expansion after BMT. (A) Mice underwent transplantation as in Figure 1. Splenocytes were harvested 10, 20, and 30 days (n = 3 mice/group) after BMT and analyzed by fluorescence-activated cell sorter (FACS). wt B6 → bm1 (•), pfp–/–→ bm1 (□), gld → bm1 (⋄), and cdd → bm1 (▴). wt B6 → bm1 versus cdd → bm1, *P < .05 by Mann-Whitney U test. (B) TNF α and IFN γ production by donor CD8+ T cells was determined by intracytoplasmic staining. *P < .05 by Mann-Whitney U test. (C) Overall pathologic damage of liver specimens correlated to the intensity of cell infiltration in portal triads, bile ducts/ductules, vascular, and hepatocellular areas. P < .01 by linear regression analysis. Symbols are same as in 3A. Error bars indicate mean ± SD.
Deficiency of both perforin and FasL causes decreased apoptosis of donor CD8+ T cells after BMT
We next evaluated whether the increased expansion of cdd T cells was due to more rapid proliferation or to decreased activation-induced cell death (AICD). CD8+ T cells were isolated from wt and cdd mice and were cultured with DCs isolated from bm1 mice. Proliferation of both cell types was equivalent for the first 48 hours of culture. Proliferation of wt cells peaked at 72 hours after the initiation of culture and then declined; in contrast, proliferation of cdd CD8+ T cells continued to expand at least up to 96 hours (Figure 4A).
Deficiency of both perforin and FasL causes decreased apoptosis of donor CD8+T cells after BMT. (A) Wild-type (•) and cdd (▴) CD8+ T cells at 2 × 105/well were cultured with irradiated bm1 DCs (1 × 104/well) for 2 to 4 days. During the final 12 hours of culture, cells were pulsed with [3H] thymidine and assayed for proliferation. *P < .05 by Mann-Whitney U test. (B) Mice underwent transplantation as in Figure 1 and splenocytes were analyzed on day 10 after BMT by 2-color flow cytometry for expression of annexin and CD8+ cells. Gates were set for CD8+ cells, and the percentage of cells expressing annexin was determined. Data represent mean ± SD. wt versus cdd, *P < .05 by Mann-Whitney U test.
Deficiency of both perforin and FasL causes decreased apoptosis of donor CD8+T cells after BMT. (A) Wild-type (•) and cdd (▴) CD8+ T cells at 2 × 105/well were cultured with irradiated bm1 DCs (1 × 104/well) for 2 to 4 days. During the final 12 hours of culture, cells were pulsed with [3H] thymidine and assayed for proliferation. *P < .05 by Mann-Whitney U test. (B) Mice underwent transplantation as in Figure 1 and splenocytes were analyzed on day 10 after BMT by 2-color flow cytometry for expression of annexin and CD8+ cells. Gates were set for CD8+ cells, and the percentage of cells expressing annexin was determined. Data represent mean ± SD. wt versus cdd, *P < .05 by Mann-Whitney U test.
We next harvested spleens on day 10 after BMT and analyzed donor T cells by 2-color flow cytometry for annexin as a measure of apoptosis. As shown in Figure 4B, percentages of apoptosis of pfp–/– and gld CD8+ T cells were comparable with wt cells (P = .27 and .51, respectively). In contrast, cdd donor CD8+ T cells showed significantly less apoptosis (P < .05), demonstrating that impaired AICD in cdd T cells results in their greater expansion.
Discussion
Little is known regarding the regulation of the contraction phase of CD8+ T cells after alloantigen stimulation. The present study demonstrates that both perforin and FasL are required for the normal AICD-mediated contraction of activated CD8+ T cells during GVHD following myeloablative conditioning. Absence of either of these pathways alone did not affect AICD and did not reduce GVHD. Our findings are consistent with those of Spaner et al12 and others who reported that pfp–/– CD8+ T cells proliferate more than wt CD8+ T cells after transfer to allogeneic mice even though most pfp–/– CD8+ T cells are eliminated in the contraction phase.28 Unlike Fas-mediated AICD in CD4+ T cells, the role of Fas/FasL on the homeostasis of CD8+ T cells appears to be minimal.29-31 The absence of both pathways leads to the unrestrained expansion of CD8+ T cells, causing more severe GVHD. Although the involvement of IFN-γ and/or TNF-α in homeostasis in CD8+ T cells after BMT remains to be determined,32 these effects appear to be overshadowed by the 2 cytotoxic pathways. These findings support the findings of Marks et al, demonstrating that these cytolytic molecules must be present in donor CD8+ T cells to regulate the contraction of alloreactive CD8+ T cells after transplantation (L. Marks, E. R. Podack, R.B.L., Donor T cell contraction following MHC-mismatched allogeneic bone marrow transplantation requires CD8 mediated perforin and FasL dependent regulation, manuscript submitted).
The current results sharply contrast with the study by Braun et al26 demonstrating that the absence of either pathway in donor T cells reduced GVHD and that the absence of both pathways completely abrogated GVHD lethality. That study used a nonmyeloablative conditioning regimen and demonstrated the important role of host immune cells containing perforin to prevent donor T cell expansion critical to the induction of GVHD.6,26 The use of a myeloablative regimen in the current study probably reduced the host-versus-graft response to an insignificant level, magnifying the role of the cytotoxic pathways in AICD.
Perforin deficiency could enhance CD8+ T cell expansion through decreased killing of APCs, resulting in prolonged stimulation of additional naive T cells.33,34 Therefore, we determined whether the absence of cytolytic effector function permitted longer survival of host DCs, resulting in greater stimulation of donor T cells. Host DCs were not detected, however, in either the spleen, BM, or gut of any recipients of CD8+ T cells on day 6 after BMT (data not shown). Recently, Merad et al35 demonstrated that donor T cells eliminated host Langerhans cells (LCs) through a FasL-dependent cytolytic mechanism and that persistent host LCs could stimulate skin GVHD. Although host LCs might persist and play a role in skin GVHD in cdd recipients, our data suggest that the residual skin LCs are not responsible for the unrestrained expansion of only cdd cells because no such expansion was observed in recipients of gld cells (Figure 3A). In addition, we created BM chimeras (bm1 into B6) that would express allogeneic MHC class I only on BM-derived APCs and not on target epithelial cells, as described in a previous study.4 B6 cdd T cells induced lethal GVHD in these chimeras (mean survival time [MST]: day 30) similar to wt bm1 recipients, confirming that persistent alloantigen expression on host cells did not account for unrestrained expansion of cdd cells.
In summary, our data suggest that in the CD8+-mediated GVHD, the lack of both perforin and FasL impaired AICD, causing unrestrained expansion of CD8+ T cells and lethal immunopathology of GVHD.
Prepublished online as Blood First Edition Paper, October 5, 2004; DOI 10.1182/blood-2004-08-3036.
Supported by National Institutes of Health Grants K08 AI052863-01 (P.R.), RR11576, AI46689 (R.B.L.), and CA 49542 (J.L.M.F.)
T.T. and J.L.M.F. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Monica Jones (University of Miami School of Medicine) for maintaining and screening the B6-cdd mice used in these studies.

![Figure 1. Effects of perforin- or FasL-deficient CD8+ T cells on acute GVHD. Lethally irradiated bm1 mice received transplants as described in “Materials and methods” from wt B6 (wt), perforin-deficient B6 (pfp–/–), or Fas ligand–deficient B6 (gld) donors. Syngeneic B6 BM transplant recipients (syn) served as no-GVHD controls. At 30 days after BMT, liver and intestine (bottom column [•], small intestine; upper column, large intestine) were harvested and scored semiquantitatively (n = 4 mice/group). Data represent mean ± SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/5/10.1182_blood-2004-08-3036/6/m_zh80050574970001.jpeg?Expires=1764965742&Signature=n63A5kWwBteoGsLSJIPiQjm05MPqyXF9GydNASs~GJqSGzUP1uK8iu6FwmcYZeSZW-2JmS-RqWbWCLWjk2Eu4SOS5dd0iLp7XxWghk~ETUF9-yhsMeOyKSSAMGdSNbTiRnbmD4uIJc41wz7i1ib6MnQpVF-ZU4UtkIllTGBX0dAx3s9XKeddjC-N0BqwSKqAl9nsYtCITjiK80S9n2UJ3VJ~7ykQNE-ZBI6usiKF8V-nMEADH-Cv7tzurvPAuFBzBSavdIJX5lpr~YbF3nW0~PypwdZYSjRMy9aEIkHvEa9GhVALac4glKlnGbANfjXeDt11JiULim6ewwTwXERacg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Cdd CD8+ T cells cause acute GVHD. The bm1 cells were transplanted from wt B6 (•, n = 11) or cdd B6 (▴, n = 6) donors as in Figure 1. B6 syngeneic BM transplant recipients (○,n = 9) served as no-GVHD controls. (A) Survival. (B) Histology. At 30 days after BMT, liver, intestine (bottom column [•], small intestine; upper column, large intestine), and skin were harvested and scored semiquantitatively (n = 4/group). B6 → B6 (syn; □), wt B6 → bm1 (wt; ▦), and cdd B6 → bm1 (cdd; ▩ ). (C) Serum cytokines. Mice received transplants of wt, pfp–/–, gld, or cdd as in Figure 1 and were killed on day 30 (n = 3 mice/group). Serum was harvested and analyzed for TNF α and IFN γ by ELISA (pg/mL). Each graph represents 1 of 3 similar experiments. Data represent mean ± SD. wt versus cdd, *P < .05 by Mann-Whitney U test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/5/10.1182_blood-2004-08-3036/6/m_zh80050574970002.jpeg?Expires=1764965743&Signature=bQ5wjlh1CIdJD5~cbtgIZLf7L3uHIaTJ5PTYqGh1eSOJUt0fEZ2uQu908w0eU4CR4IYWieGcbE8rl9Nxz0r3SIEy8AMGJ4nwmLHYIDJEHE7otrTut9qv6sbYPX1LP-bIFjJOGBoyn8jUNyHQwP1oxNPOyT5Er49xNhkVmeNBBNRVXUU31JIpAyPh0DcU341BENJHpAwiYOh5DWyJl5Mp1KxbRNEWhA2ObbQzejKWmpLPdd0gT6SFQk~13fGg~SVBpvVeqAFpB0-2CBKTbcPXpCY2xsNOJ4T2ku-qNWXbViF~TQaDdOHh50Hf7U8w7hBma~L9XC3WpG~8cCdS4LOG0w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Deficiency of both perforin and FasL causes decreased apoptosis of donor CD8+ T cells after BMT. (A) Wild-type (•) and cdd (▴) CD8+ T cells at 2 × 105/well were cultured with irradiated bm1 DCs (1 × 104/well) for 2 to 4 days. During the final 12 hours of culture, cells were pulsed with [3H] thymidine and assayed for proliferation. *P < .05 by Mann-Whitney U test. (B) Mice underwent transplantation as in Figure 1 and splenocytes were analyzed on day 10 after BMT by 2-color flow cytometry for expression of annexin and CD8+ cells. Gates were set for CD8+ cells, and the percentage of cells expressing annexin was determined. Data represent mean ± SD. wt versus cdd, *P < .05 by Mann-Whitney U test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/5/10.1182_blood-2004-08-3036/6/m_zh80050574970004.jpeg?Expires=1764965743&Signature=QY6XnKop3RSZIheKDjHwvQLyEulsP7vlGTjVl730KYy6VbHe8HSHB4PdOm8rcQR3-xtorjl3Un31E72dFx~DZPFnI3okOhI5hlLf6x3qz8JWGkrPjxaP5Zaz8STR5k5f9Bs9NfWEVwwdWBvJtdk1XjLO7s6FH5fvJVg74z0ca75-5~vBuMSC3N5Irw6H6OIlovX~HuSYGMttxB5L4o9WwDQBW2zOHW-c9LDwFgR2ng7amH16~brt~ulNPbtE5uMTmpTcC60odvaExxCWk0gRB5pB9przIfCjawOwf9QNfeoAwzBq9EueW0Unpwc6VCzq9fGHXYV9uKY9fcAwpHx0lw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal