Abstract
B-cell chronic lymphocytic leukemia (CLL) is characterized by accumulation of clonal lymphocytes resistant to apoptosis. We evaluated the ability of the investigational antileukemic agent adaphostin to induce apoptosis in CLL B cells and synergize with fludarabine in vitro. Analysis by annexin V/propidium iodide (PI) staining revealed that the concentration of adaphostin required to induce 50% cell death (IC50) at 24 hours was 4.2 μM (range, 1.10-11.25 μM; median, 4.25 μM; n = 29) for CLL isolates and more than 10 μM for B and T cells from healthy donors. Immunoblots demonstrated adaphostin induced poly(adenosine diphosphate-ribose) polymerase (PARP) cleavage and cleavage of caspase-3 substrates, suggesting that adaphostin induces apoptosis. Adaphostin increased the level of reactive oxygen species (ROS) within CLL B cells, and the antioxidant N-acetylcysteine blocked both adaphostin-induced ROS generation and apoptosis. Adaphostin also caused a decrease in the level of the antiapoptotic protein Bcl-2. When adaphostin was combined with fludarabine (F-ARA-AMP), a synergistic effect on cell death was observed in all 10 CLL samples. These findings not only indicate that adaphostin induces apoptosis selectively in CLL B cells through a mechanism that involves ROS generation but also demonstrate its ability to augment the effects of fludarabine. Further preclinical development of adaphostin as a novel agent for the treatment of CLL appears warranted.
Introduction
B-cell chronic lymphocytic leukemia (CLL) is the most common leukemia in North America and is incurable with current treatments. Unlike cells from rapidly proliferating tumors, most CLL cells are G0 phase cells that accumulate due to defects in apoptosis rather than enhanced cell division.1-5 Increased levels of specific antiapoptotic proteins, including Bcl-2, Mcl-1, and X-linked inhibitor of apoptosis protein (XIAP), appear to mediate the apoptotic resistance of CLL B cells and contribute to the relative resistance of this disorder to chemotherapeutic agents and monoclonal antibodies.2,3,6-10 This feature of apoptotic resistance distinguishes CLL and other indolent B-cell disorders from other hematologic malignancies and creates the need for tailored therapeutic strategies to treat this disease.1,11
Current CLL therapy often includes purine nucleoside analogs such as fludarabine in combination with monoclonal antibodies. Although fludarabine is superior to monotherapy with chlorambucil, approximately one third of patients experience a complete response (CR) with fludarabine, and all responders eventually relapse.12 Antibody-based therapies such as alemtuzumab and rituximab have expanded therapeutic options for patients with CLL,13-15 and combinations of antibodies with conventional chemotherapy have led to dramatically improved response rates.16-18 Despite these advances, CLL remains an incurable malignancy. Recently identified biologic features of the CLL B cell, including immunoglobulin VH (IgVH) mutational status,19,20 level of CD38 expression,20,21 and cytogenetic abnormalities by fluorescence in situ hybridization (FISH),22-26 identify patients with more aggressive disease who are even less likely to respond to conventional treatments.27 Collectively, these observations highlight the need for new agents for CLL, especially agents that complement existing treatments.
Tyrphostins are a class of small molecules that were designed to act as tyrosine kinase inhibitors.28 One of these compounds, adaphostin (NSC 680410), was originally identified as an inhibitor of p210Bcr/abl kinase and a potent inducer of myeloid cell death in p210Bcr/abl-positive K562 cells in vitro.29 Additional studies have demonstrated, however, that this agent might induce cell death through elevation of reactive oxygen species (ROS)30,31 or down-regulation of vascular endothelial growth factor (VEGF)32,33 rather than inhibition of p210Bcr/abl. Consistent with this possibility, we recently reported that adaphostin can induce cell death in Bcr/abl-negative leukemia cells, including CLL B cells.30
It has been unclear whether the cytotoxic mechanisms identified in tissue culture cell lines29-32,34 accurately describe the action of adaphostin in clinical leukemia specimens. Moreover, the effects of adaphostin in combination with other antileukemic agents, particularly agents used to treat CLL, have not been well explored. In the present study, we examined the effects of adaphostin on CLL B cells of all biologic risk categories, compared its cytotoxicity in CLL B cells versus normal B and T cells, and evaluated the mechanistic basis for adaphostin-induced killing of CLL B cells. We also assessed the effect of combining adaphostin with fludarabine in vitro. The results of these studies have implications for possible future testing of adaphostin in patients with CLL.
Materials and methods
Patient selection and CLL sample processing
Blood was obtained from both healthy donors and CLL patients who had provided written informed consent under a protocol approved by the Mayo Clinic Institutional Review Board according to the regulations of the Declaration of Helsinki. All CLL patients had a confirmed diagnosis using the National Cancer Institute (NCI) Working Group definition.35 Patients in this cohort were from all Rai stages and had not been treated for at least 5 weeks prior to blood processing for this study with the exception of 1 patient on low-dose oral chlorambucil (2 mg orally daily). CLL cells were isolated from heparinized venous blood by density gradient centrifugation. When assessed by flow cytometry (FACScan; Becton Dickinson, Sunnyvale, CA), the isolated cells were predominately CLL B cells (minimum, 80% CD19+; mean, 92.2% CD19+, of which 94.4% were CD19+ or CD5+ positive). Purified lymphocytes from CLL patients were either used immediately (less than 48 hours) for the laboratory studies or suspended in RPMI 1640/20% fetal calf serum/10% dimethyl sulfoxide (DMSO) and stored at -80°C until used. Lymphocytes from healthy donors were separated by density gradient centrifugation followed by plate adherence to separate lymphocytes (B and T cells) from monocytes.
Reagents
Immunological reagents that recognize the following antigens were purchased from the indicated suppliers: Bcl-2 from Dako (Carpinteria, CA), XIAP from R&D Systems (Minneapolis, MN), β-actin and survivin from Novus Biologicals (Littleton, CO), phosphotyrosine (clone 4G10) from Upstate Biotechnology (Lake Placid, NY), poly(adenosine diphosphate-ribose) polymerase (PARP) C-210 from Biomol (Plymouth Meeting, PA), and caspase-3, caspase-8, and caspase-9 from Cell Signaling Technology (Beverly, MA). The following reagents were purchased or obtained as gifts from indicated suppliers: N-acetylcysteine and chlorambucil (Sigma, St Louis, MO), fludarabine (F-ARA-AMP; Berlex, Montville, NJ), AIM V (Gibco, Grand Island, NY), RPMI (Biosource, Camarillo, CA), ZVAD-fmk (Biomol), propidium iodide (Becton Dickinson), and annexin and 5-(and-6)-chloromethyl-2′, 7′-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA) probes (Molecular Probes, Eugene, OR). Adaphostin and anticleaved caspase-3 were prepared as described previously.29,36
Cell culture
Fresh (immediately after purification of whole blood cells) and cryopreserved primary CLL B cells were cultured in serum-free medium. Normal human B and T cells were cultured in RPMI with 10% fetal calf serum and l-glutamine, penicillin, and streptomycin. Cells were maintained at 37°C in an atmosphere containing 95% air and 5% CO2 (vol/vol).
Apoptosis and cell death assay
Primary CLL B cells (about 1 × 106 cells) were treated with either vehicle or drug for 24 to 120 hours as indicated, washed with Dulbecco calcium- and magnesium-free phosphate-buffered saline (PBS), and evaluated for dual annexin V and propidium iodide (PI) staining with fluorescence-activated cell sorter (FACS) analysis.37 From 5000 to 10 000 events were collected. Cells staining with annexin V fluorescein isothiocyanate (FITC) or PI were considered positive. Storing and processing of data were done with FACScan software.
Detection of ROS
Primary CLL lymphocytes (about 1 × 106 cells) were treated with either vehicle or drug for 30 minutes. After treatment, cells were washed with PBS. CM-H2DCFDA (10 μM) was used to measure intracellular reactive oxygen species as previously described.30,38 This agent diffuses into cells and is sequestered intracellularly by deesterification. Subsequent reaction with peroxides generates fluorescent 5-chloromethyl-2′,7′-dichlorofluorescein (DCF). Cells were read on the FL1 channel of a Becton Dickinson FACScan. Mean fluorescence intensities were compared between treated and untreated CLL B cells or normal blood cells.
Assessment of Bcl-2 polypeptide levels
Primary CLL B cells (about 1 × 106 cells) were treated with either vehicle or drug for 24 hours. After treatment, cells were washed with PBS, fixed and permeablized using the Pharmingen method (Pharmingen, San Diego, CA), and stained with FITC-conjugated anti–Bcl-2 antibody with FACS analysis (sample size, 5000 to 10 000 events). Mean fluorescence for control cells (ie, vehicle only) and at each dose level was compared. Storing and processing of data were done with FACScan software.
Immunoblotting
Primary CLL B cells (3.3 × 107 to 7.3 × 107 cells) were cultured in 70- to 100-mL flasks and treated with either vehicle or drug for 24 to 96 hours as indicated. Treated cells were then washed with PBS and solubilized in alkylation buffer (6 M guanidine hydrochloride, 250 mM Tris-HCl, pH 7.4 at 21°C, and 10 mM EDTA [ethylenediaminetetraacetic acid] supplemented immediately before use with 150 mM 2-mecaptoethanol and 1 mM phenylmethylsulfonyl fluoride). After reaction with iodoacetamide to block free sulfhydryl groups, samples were dialyzed into 4 M urea and then 0.1% sodium dodecyl sulfate (SDS). Aliquots containing 50 μg protein were separated on 5% to 15% SDS polyacrylamide gels, transferred to nitrocellulose, and probed with antibodies as previously described.39 Bound antibodies were visualized using enhanced chemiluminescence reagents (Amersham Pharmacia, Piscataway, NJ).
Assessment of the effect of combination treatment
CLL B cells were treated with serial dilutions of adaphostin (0.5 μM to 8 μM) or the active moiety of fludarabine (F-ARA-AMP; 0.25 μM to 8 μM) individually or in combination using a constant ratio (adaphostin/F-ARA-AMP, 2:1) at doses that typically correspond to ¼, ½, ¾, 1, 1¼, 1½, 2, 4, and 8 times the dose required to induce 50% cell death (IC50) of the individual agents. After concentration-effect curves were generated for each agent, data were analyzed using the CalcuSyn software program (Biosoft, Cambridge, United Kingdom), which uses the method of Chou and Talalay,40 to determine whether combined treatment yields greater effects than expected from summation alone. A combination index (CI) of 1 indicates an additive effect, a CI above 1 indicates an antagonistic effect, and a CI below 1 indicates a synergistic effect.41-43
Prognostic markers
Rai stage, absolute lymphocyte count, and treatment status were abstracted from clinical records. CLL B-cell CD38 status, IgVH mutation status, and cytogenetic abnormalities by FISH were determined as previously described.21,23 CD38 status and FISH testing are part of routine clinical practice for CLL patients at the Mayo Clinic, while testing for IgVH mutation status is performed as part of investigational protocols.
VEGF ELISA
A commercially available enzyme-linked immunosorbent assay (ELISA) was used according to instructions of the supplier (R&D Systems) to assay for VEGF secreted into the culture medium of CLL B cells (1 × 106/mL) during a 24-hour exposure to diluent or various adaphostin concentrations in vitro.
Statistics
The percent kill and percentage viable cells were evaluated across all CLL patients (n = 29) and were summarized both graphically and quantitatively. These percentages were analyzed for each dose level independently as well as across dose levels. Percentage viable cells across all dose levels and patients was assessed graphically, where the dose-effect curves were evaluated for each patient across the dose levels. In addition, repeated measure models were used to identify the presence of variability in these dose-effect relationships across patients. Prognostic factors were summarized across these patients, and differences in percentage viable cells with the different adaphostin dose levels were assessed graphically. Given the relatively limited sample size, differences in the median percentage viable cells were also evaluated between groups using the nonparametric Wilcoxon rank sum test (for factors with 2 levels) and the Kruskal-Wallis test (for factors with more than 2 levels). Spearman rank correlation coefficients were used to evaluate relationships between quantitative variables such as percent cell kill induced by various agents.
Overall, these analyses were largely hypothesis generating for future studies; therefore, no corrections were made for multiple comparisons. All P values represent 2-sided tests, where significance was determined at P < .05. All quantitative and graphical analyses were done using statistical software Splus 6.1 for Windows (Insightful, Seattle, WA).
Results
Adaphostin induces apoptosis in primary CLL B cells
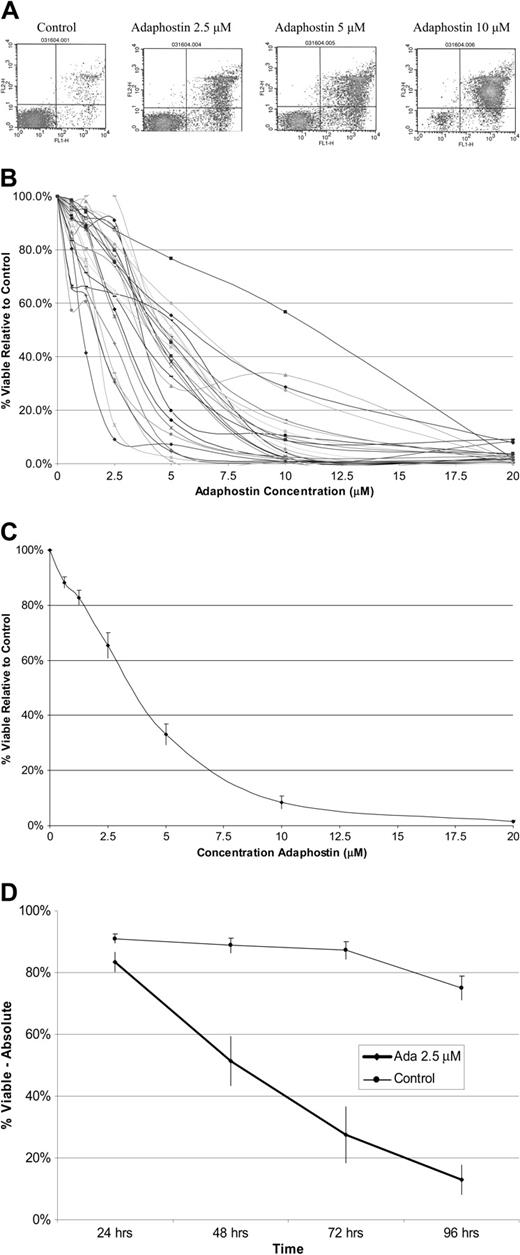
Experiments with human leukemia cell lines suggest adaphostin induces cell death through an apoptotic mechanism.29,30,34,44 To assess the effect of adaphostin on primary CLL B cells, freshly isolated and frozen lymphocytes from patients with CLL (n = 29) were cultured in the absence or presence of adaphostin (0.625 μM to 20 μM) for 24 hours. The percentage of cells staining with annexin V and PI was assessed by flow cytometry. These experiments indicated that the concentration required to induce death in 50% of CLL B cells (IC50) relative to control averaged 4.2 μM (range, 1.10-11.25 μM) (Table 1 and Figure 1A-C). Sensitivity was not significantly different in fresh (median IC50, 4.55; n = 14) and frozen (median IC50, 4.25; n = 15) samples (P = .32). All CLL B cells tested responded at the 10 μM dose in vitro, with an average of more than 90% cell death relative to control at this dose (Table 1). Even though the dose effect differed between patients, the overall trend that higher adaphostin doses induced higher percent cell kill remained consistent—that is, the relationship between percent cell kill and adaphostin dose level was significantly correlated (P < .0001).
Summary statistics for percentage viable cells by adaphostin dose level (n = 29)
Dose level . | Mean, % . | Range, % . | Median, % . |
|---|---|---|---|
| Control | 100 | 100-100 | 100 |
| 0.625 μM | 88 | 57-99 | 91 |
| 1.25 μM | 83 | 41-104 | 88 |
| 2.5 μM | 66 | 9-106 | 75 |
| 5 μM | 33 | 0.9-77 | 39 |
| 10 μM | 8 | 0.1-57 | 4 |
| 20 μM | 1 | 0.06-9 | 2 |
Dose level . | Mean, % . | Range, % . | Median, % . |
|---|---|---|---|
| Control | 100 | 100-100 | 100 |
| 0.625 μM | 88 | 57-99 | 91 |
| 1.25 μM | 83 | 41-104 | 88 |
| 2.5 μM | 66 | 9-106 | 75 |
| 5 μM | 33 | 0.9-77 | 39 |
| 10 μM | 8 | 0.1-57 | 4 |
| 20 μM | 1 | 0.06-9 | 2 |
Effect of adaphostin on CLL B cells. (A) Examples of dot plots for CLL cells stained with annexin (x-axis) and propidium iodine (y-axis) assessed by flow cytometry demonstrating increased cell death as doses of adaphostin are escalated. Dots in the lower right quadrants represent early apoptotic cells, and dots in the upper right quadrants represent late apoptotic cells. (B) Dose response curves for CLL B cells isolated from patients (n = 29) cultured with increasing doses of adaphostin for 24 hours. Percent viable cells relative to control (vehicle alone) is expressed on the y-axis. (C) Mean dose response curve for samples from all 29 patients in panel A. Y bars indicate the standard error of the mean at each dose level. (D) Mean percent viable CLL B cells for samples (n = 12) cultured with 2.5 μM adaphostin or vehicle at 24, 48, 72, and 96 hours. Y bars indicate the standard error of the mean at each dose level.
Effect of adaphostin on CLL B cells. (A) Examples of dot plots for CLL cells stained with annexin (x-axis) and propidium iodine (y-axis) assessed by flow cytometry demonstrating increased cell death as doses of adaphostin are escalated. Dots in the lower right quadrants represent early apoptotic cells, and dots in the upper right quadrants represent late apoptotic cells. (B) Dose response curves for CLL B cells isolated from patients (n = 29) cultured with increasing doses of adaphostin for 24 hours. Percent viable cells relative to control (vehicle alone) is expressed on the y-axis. (C) Mean dose response curve for samples from all 29 patients in panel A. Y bars indicate the standard error of the mean at each dose level. (D) Mean percent viable CLL B cells for samples (n = 12) cultured with 2.5 μM adaphostin or vehicle at 24, 48, 72, and 96 hours. Y bars indicate the standard error of the mean at each dose level.
To evaluate the effect of longer adaphostin exposure, freshly isolated CLL B cells (n = 4) were cultured in the presence or absence of adaphostin (0.625 μM to 20 μM) for 5 days and again examined for annexin V/PI staining by flow cytometry. With a 5-day exposure, the average IC50 decreased to 2 μM. To evaluate the timing of CLL B-cell death during prolonged exposure, we evaluated cell viability at 24-hour intervals using a 2.5 μM adaphostin dose (n = 12). As shown in Figure 1D, cell death at this dose level occurred primarily between 24 and 72 hours.
Because a number of biologic characteristics of CLL cells affect prognosis, we assessed the impact of Rai stage, IgVH mutation status, CD38 expression, and cytogenetic abnormalities on sensitivity to adaphostin-induced cell death in vitro. Median IC50 levels for adaphostin were not significantly different based on IgVH mutation status, level of CD38 expression, or cytogenetic abnormalities by FISH testing (Table 2). There did appear to be statistically significant differences in the median IC50 dose levels based on Rai stage (III-IV, 3.05; 0-II, 4.7; P = .018) with patients with higher Rai stage more sensitive (ie, lower IC50 dose level and higher percent cell kill) to adaphostin than individuals with lower Rai stage.
IC50 adaphostin dose levels by prognostic groups
Factor . | No. . | Median IC50 adaphostin . | Range . | P . |
|---|---|---|---|---|
| Rai stage group | .018 | |||
| Low/intermediate: 0-II | 19 | 4.70 | 1.8-11.25 | |
| High: III-IV | 10 | 3.05 | 1.1-5.4 | |
| IgVH mutation status | .72 | |||
| Mutated* | 11 | 4.45 | 1.1-11.25 | |
| Nonmutated | 10 | 3.93 | 1.8-5.8 | |
| CD38 status† | .74 | |||
| Negative | 20 | 4.55 | 1.1-11.25 | |
| Positive | 9 | 4.25 | 1.8-6.6 | |
| FISH defects‡ | .83 | |||
| 13q- | 8 | 4.70 | 1.75-11.25 | |
| Normal | 6 | 3.53 | 1.1-5.8 | |
| 12+ | 4 | 3.93 | 2.25-6.6 | |
| 17p/11q | 5 | 4.20 | 2.0-5.4 |
Factor . | No. . | Median IC50 adaphostin . | Range . | P . |
|---|---|---|---|---|
| Rai stage group | .018 | |||
| Low/intermediate: 0-II | 19 | 4.70 | 1.8-11.25 | |
| High: III-IV | 10 | 3.05 | 1.1-5.4 | |
| IgVH mutation status | .72 | |||
| Mutated* | 11 | 4.45 | 1.1-11.25 | |
| Nonmutated | 10 | 3.93 | 1.8-5.8 | |
| CD38 status† | .74 | |||
| Negative | 20 | 4.55 | 1.1-11.25 | |
| Positive | 9 | 4.25 | 1.8-6.6 | |
| FISH defects‡ | .83 | |||
| 13q- | 8 | 4.70 | 1.75-11.25 | |
| Normal | 6 | 3.53 | 1.1-5.8 | |
| 12+ | 4 | 3.93 | 2.25-6.6 | |
| 17p/11q | 5 | 4.20 | 2.0-5.4 |
Two percent or more deviation from germline IgVH sequence
Negative indicates less than 30% of CLL B cells expressing CD38; positive, 30% or more CLL B cells expressing CD38
FISH defects were found using a CLL FISH panel using cutoffs from normal controls as previously described23
CLL B-cell sensitivity to adaphostin relative to sensitivity to fludarabine and chlorambucil
We next evaluated whether the CLL clones most sensitive to adaphostin were also those most sensitive to fludarabine and chlorambucil. CLL B cells (n = 19) were cultured with or without the active moiety of fludarabine (F-ARA-AMP; 1 μM) or chlorambucil (1 μM) for 24 hours. These latter doses were chosen based on their ability to provide reliable levels of killing for most CLL B-cell clones in vitro (data not shown). When the percent of viable cells (as determined by annexin V/PI) from these experiments was compared with the percent of viable cells from the same patient at the adaphostin 5 μM dose (about IC50), no relationship between resistance or sensitivity to conventional chemotherapeutic agents and resistance or sensitivity to adaphostin was observed. In other words, adaphostin induced killing in CLL B cells whether they were sensitive to purine nucleoside or alkylating agents or not. The correlation between percent cell kill by fludarabine and percent cell kill by adaphostin was low (r = 0.3) and was not significantly different from 0 (ie, no correlation; P = .19). Similarly, no significant correlation was found between percent cell kill induced by chlorambucil and by adaphostin (r = 0.25; P = .29). In contrast, there was a strong and statistically significant positive correlation between cell death induced by fludarabine versus chlorambucil for these CLL B-cell clones (r = 0.61; P = .01), suggesting the CLL B-cell clones most sensitive to fludarabine are also those most sensitive to chlorambucil.
Adaphostin induces B-CLL cell death through induction of apoptosis
In view of the cytotoxicity of adaphostin in a wide range of CLL B cells, including samples displaying poor prognostic features or fludarabine/chlorambucil resistance, the cytotoxic action of this agent was examined in greater detail. The presence of annexin V–positive/PI-negative cells (Figure 1A), which reflects phosphatidylserine externalization early in the apoptotic process,37 suggested that adaphostin was inducing death in CLL B cells through an apoptotic mechanism. To confirm this hypothesis, Western blotting for PARP cleavage was performed on cell lysates from CLL B cells after culture with or without adaphostin for 24 hours. Adaphostin induced PARP cleavage to its signature 89-kDa caspase-generated fragment45 in 7 of 7 samples assayed, confirming that adaphostin-mediated killing is accompanied by caspase activation (Figure 2A-B). PARP cleavage was accompanied by the appearance of readily detectable cleaved caspase-3 fragments (17 kDa) in most cases (6 of 7), suggesting that apoptosis was mediated through the conventional, caspase-dependent pathway.
Adaphostin induces apoptosis in CLL B cells. (A) Western blot analysis for PARP cleavage, cleaved caspase-3, and levels of antiapoptotic proteins (Bcl-2, Mcl-1, XIAP, survivin) at increasing doses of adaphostin. (B) Western blot analysis demonstrating the timing of PARP cleavage in CLL B cells exposed to 2.5 μM adaphostin. C indicates control treated cells; A, adaphostin-treated cells (2.5 μM). PARP cleavage became evident in this experiment at 48 hours, coinciding with the onset of cell death as seen in Figure 1D.
Adaphostin induces apoptosis in CLL B cells. (A) Western blot analysis for PARP cleavage, cleaved caspase-3, and levels of antiapoptotic proteins (Bcl-2, Mcl-1, XIAP, survivin) at increasing doses of adaphostin. (B) Western blot analysis demonstrating the timing of PARP cleavage in CLL B cells exposed to 2.5 μM adaphostin. C indicates control treated cells; A, adaphostin-treated cells (2.5 μM). PARP cleavage became evident in this experiment at 48 hours, coinciding with the onset of cell death as seen in Figure 1D.
Adaphostin-induced cell death was also associated in most samples with decreases in Bcl-2 protein expression, an antiapoptotic protein known to be up-regulated in CLL (Figure 2A). This finding was confirmed by flow cytometry, which demonstrated a dose-dependent decrease of Bcl-2 (Table 3). Variable effects on levels of Mcl-1, XIAP, and survivin were observed by Western blotting (Figure 2A and data not shown).
Effect of adaphostin on level of Bcl-2 in CLL B-cells
Adaphostin concentration (μM) . | Patient A . | Patient B . |
|---|---|---|
| 0 | 526 | 275 |
| 1.25 | 400 | 225 |
| 2.5 | 369 | 176 |
| 5 | 261 | 150 |
| 10 | 214 | 163 |
| 20 | 223 | 134 |
Adaphostin concentration (μM) . | Patient A . | Patient B . |
|---|---|---|
| 0 | 526 | 275 |
| 1.25 | 400 | 225 |
| 2.5 | 369 | 176 |
| 5 | 261 | 150 |
| 10 | 214 | 163 |
| 20 | 223 | 134 |
Mean fluorescent values of Bcl-2 in CLL B cells treated with increasing doses of adaphostin.
Effect of adaphostin on VEGF secretion by CLL B cells
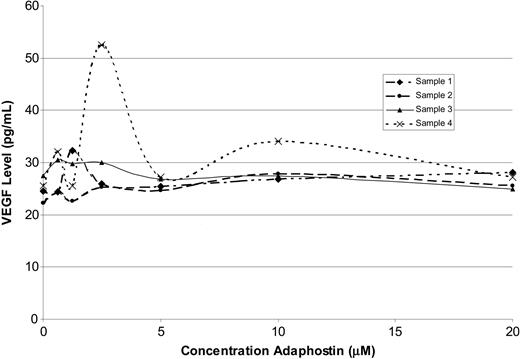
It has been reported that adaphostin inhibits VEGF secretion in human leukemia cell lines.32,33 In view of the importance of VEGF as a survival factor for B-CLL cells,46-48 we investigated the effect of adaphostin on VEGF secretion by primary CLL B cells. This analysis failed to demonstrate any effect of adaphostin on VEGF secretion by ELISA after 24 hours of exposure at doses as high as 20 μM (n = 4; Figure 3). These observations not only appeared to rule out down-regulation of VEGF secretion as a mechanism of adaphostin cytotoxicity in B-CLL cells but also indicated that results obtained in cell lines might not be an accurate reflection of the in vitro cytotoxic mechanism of adaphostin in primary leukemia cells.
Adaphostin does not affect VEGF secretion by CLL B cells. Effect of adaphostin on VEGF secretion by lymphocytes isolated from patients (n = 4) with CLL with increasing doses of adaphostin for 24 hours.
Adaphostin does not affect VEGF secretion by CLL B cells. Effect of adaphostin on VEGF secretion by lymphocytes isolated from patients (n = 4) with CLL with increasing doses of adaphostin for 24 hours.
Adaphostin induces production of ROS in CLL B cells
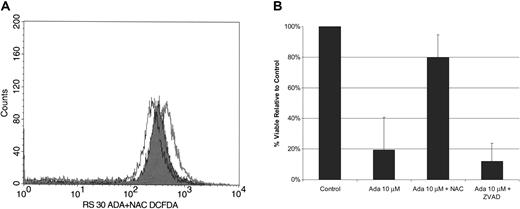
Recent studies in human leukemia cell lines have also suggested that adaphostin might exert its effects through ROS generation.30,31 To assess the potential importance of this effect in primary B-CLL cells, we measured ROS generation by flow cytometry on freshly collected B cells from CLL patients after a 30-minute exposure to 30 μM adaphostin. An increase in ROS by mean fluorescence was detected in 5 of 7 specimens (mean values for all 7 patients: control, 244; 30 μM adaphostin, 318). As expected, preincubation of CLL B cells with N-acetylcysteine (NAC) (20 mM) for 30 to 120 minutes prior to adaphostin treatment attenuated this increase in ROS (mean: 30 μM adaphostin plus NAC, 228; Figure 4A).
Adaphostin induces cell death through generation of ROS. (A) ROS generation. Example of a mean fluorescence histogram for CLL cells. CLL lymphocytes were incubated for 30 minutes in diluent (open black line), 30 μM adaphostin (open gray line), or 30 μM adaphostin added about 2 hours after 20 mM NAC (black line with gray shading). (B) NAC attenuates adaphostin-induced cell death. CLL B cells were cultured in the presence or absence of NAC (20 mM) or the broad-spectrum caspase inhibitor N-benzyloxycarbonyl-Val-Ala-Asp fluoromethylketone (z-VAD-fmk) (50 μM). After 2 hours, adaphostin (10 M) was added to some cultures. CLL B cells were recovered from cultures after 24 hours, and the percentage of viable cells was determined by annexin/PI staining using flow cytometry analysis (mean ± SD; n=6). Y error bars indicate standard deviation.
Adaphostin induces cell death through generation of ROS. (A) ROS generation. Example of a mean fluorescence histogram for CLL cells. CLL lymphocytes were incubated for 30 minutes in diluent (open black line), 30 μM adaphostin (open gray line), or 30 μM adaphostin added about 2 hours after 20 mM NAC (black line with gray shading). (B) NAC attenuates adaphostin-induced cell death. CLL B cells were cultured in the presence or absence of NAC (20 mM) or the broad-spectrum caspase inhibitor N-benzyloxycarbonyl-Val-Ala-Asp fluoromethylketone (z-VAD-fmk) (50 μM). After 2 hours, adaphostin (10 M) was added to some cultures. CLL B cells were recovered from cultures after 24 hours, and the percentage of viable cells was determined by annexin/PI staining using flow cytometry analysis (mean ± SD; n=6). Y error bars indicate standard deviation.
To further investigate the role of ROS in the mechanism of adaphostin-induced cell death, we cultured CLL B cells (n = 6) in the presence or absence of NAC (20 mM) or the broad-spectrum caspase inhibitor z-VAD(OMe)-fmk 49 for 2 hours prior to 24-hour culture with adaphostin (10 μM). As is the case with other cytotoxic in vitro exposures,50 the caspase inhibitor did not protect against adaphostin-induced cell death (Figure 4B). In contrast, preincubation of CLL B cells with NAC provided dramatic protection (mean reduction of cell death, 60%; range, 23-99% reduction) against adaphostin-mediated killing (Figure 4B).
Effect of adaphostin on normal lymphocytes
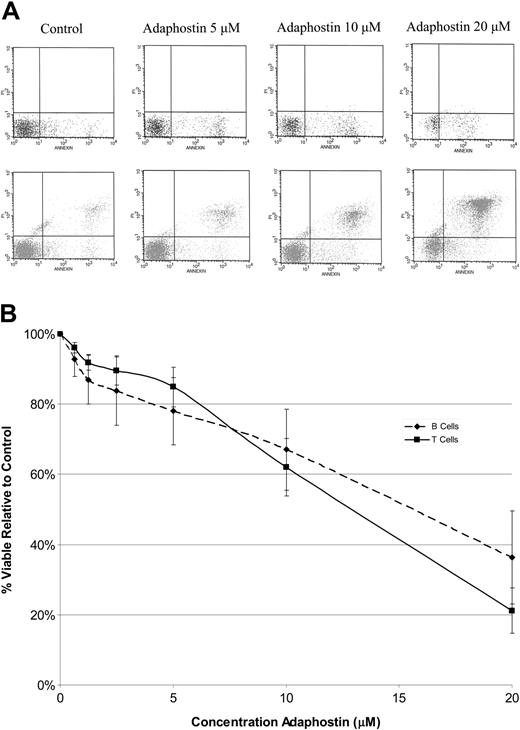
To evaluate the effect of adaphostin on normal lymphocytes, freshly collected peripheral blood mononuclear cells were isolated from healthy controls (n = 3). B cells and T cells were separated from monocytes by plate adherence. Normal B cells and T cells were then cultured with or without adaphostin (0.625 μM to 20 μM) for 24 hours. After drug exposure, viabilities of normal B and T cells were assessed using 2 separate 3-color flow strategies (for B cells: CD19, annexin, and PI; for T cells: CD3, annexin, and PI). Both normal B and T cells were markedly less sensitive to adaphostin than CLL B cells (Figure 5A-B). The IC50s for normal B and T cells were both more than 10 μM, a concentration that killed about 90% of CLL B cells. These observations suggest that adaphostin exhibits some degree of specificity for CLL B cells relative to normal lymphocytes.
Effect of adaphostin on normal B cells and T cells. (A) Representative dot plots for normal B (top row) and T cells (bottom row) stained with annexin (x-axis) and propidium iodine (y-axis) assessed by flow cytometry for increasing doses of adaphostin. Dots in the lower right quadrants represent early apoptotic cells, and dots in the upper right quadrants represent late apoptotic cells. (B) Mean dose response curve for B ( ) and T cells (▪) from normal controls (n = 3) cultured with increasing doses of adaphostin. Y bars indicate the standard deviation at each dose level.
) and T cells (▪) from normal controls (n = 3) cultured with increasing doses of adaphostin. Y bars indicate the standard deviation at each dose level.
Effect of adaphostin on normal B cells and T cells. (A) Representative dot plots for normal B (top row) and T cells (bottom row) stained with annexin (x-axis) and propidium iodine (y-axis) assessed by flow cytometry for increasing doses of adaphostin. Dots in the lower right quadrants represent early apoptotic cells, and dots in the upper right quadrants represent late apoptotic cells. (B) Mean dose response curve for B ( ) and T cells (▪) from normal controls (n = 3) cultured with increasing doses of adaphostin. Y bars indicate the standard deviation at each dose level.
) and T cells (▪) from normal controls (n = 3) cultured with increasing doses of adaphostin. Y bars indicate the standard deviation at each dose level.
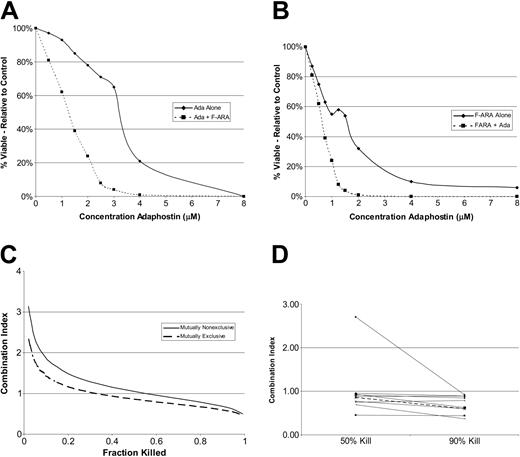
Adaphostin synergizes with fludarabine to induce death in CLL B cells
In further experiments, we investigated the effect of combining adaphostin with the active moiety of fludarabine (F-ARA-AMP) in CLL B cells in vitro. CLL B cells were cultured with increasing concentrations of adaphostin alone (0.5 μM to 8 μM), F-ARA-AMP alone (0.25 μM to 8 μM), or increasing concentrations of a constant ratio of adaphostin to F-ARA-AMP (2:1) for 48 hours. Annexin V/PI staining was again assessed by flow cytometry (Figure 6A-B). When data were analyzed by the method of Chou and Talalay,40 synergy of the 2 agents was observed in 9 of 10 CLL B-cell samples at the IC50 of the combination and in 10 of 10 samples at the IC90 (Figure 6C-D).
Adaphostin synergizes with fludarabine to induce cell death in CLL B cells. (A-B) Sample dose response curves for lymphocytes isolated from a patient with CLL cultured with increasing doses of adaphostin alone (A;  and solid line), the active component of fludarabine alone (B;
and solid line), the active component of fludarabine alone (B;  and solid line), or fixed 2:1 ratio of adaphostin plus the active component of fludarabine (A-B; ▪ and dashed line) for 24 hours. Percent viable cells relative to control (vehicle alone) is expressed on the y-axis. (C) Combination index (CI) calculated from data for the patient in panels A and B under assumption agents are mutually nonexclusive (solid line) or mutually exclusive (dashed line). (D) Combination index 50 (50% cell kill) and combination index 90 (90% cell kill) values for adaphostin and fludarabine (constant ratio, 2:1) for CLL B cells from 10 patients (calculated using Calcusyn software). Value less than 1 implies synergy. The dark dashed line indicates CLL sample highlighted in Figure 5A-B.
and solid line), or fixed 2:1 ratio of adaphostin plus the active component of fludarabine (A-B; ▪ and dashed line) for 24 hours. Percent viable cells relative to control (vehicle alone) is expressed on the y-axis. (C) Combination index (CI) calculated from data for the patient in panels A and B under assumption agents are mutually nonexclusive (solid line) or mutually exclusive (dashed line). (D) Combination index 50 (50% cell kill) and combination index 90 (90% cell kill) values for adaphostin and fludarabine (constant ratio, 2:1) for CLL B cells from 10 patients (calculated using Calcusyn software). Value less than 1 implies synergy. The dark dashed line indicates CLL sample highlighted in Figure 5A-B.
Adaphostin synergizes with fludarabine to induce cell death in CLL B cells. (A-B) Sample dose response curves for lymphocytes isolated from a patient with CLL cultured with increasing doses of adaphostin alone (A;  and solid line), the active component of fludarabine alone (B;
and solid line), the active component of fludarabine alone (B;  and solid line), or fixed 2:1 ratio of adaphostin plus the active component of fludarabine (A-B; ▪ and dashed line) for 24 hours. Percent viable cells relative to control (vehicle alone) is expressed on the y-axis. (C) Combination index (CI) calculated from data for the patient in panels A and B under assumption agents are mutually nonexclusive (solid line) or mutually exclusive (dashed line). (D) Combination index 50 (50% cell kill) and combination index 90 (90% cell kill) values for adaphostin and fludarabine (constant ratio, 2:1) for CLL B cells from 10 patients (calculated using Calcusyn software). Value less than 1 implies synergy. The dark dashed line indicates CLL sample highlighted in Figure 5A-B.
and solid line), or fixed 2:1 ratio of adaphostin plus the active component of fludarabine (A-B; ▪ and dashed line) for 24 hours. Percent viable cells relative to control (vehicle alone) is expressed on the y-axis. (C) Combination index (CI) calculated from data for the patient in panels A and B under assumption agents are mutually nonexclusive (solid line) or mutually exclusive (dashed line). (D) Combination index 50 (50% cell kill) and combination index 90 (90% cell kill) values for adaphostin and fludarabine (constant ratio, 2:1) for CLL B cells from 10 patients (calculated using Calcusyn software). Value less than 1 implies synergy. The dark dashed line indicates CLL sample highlighted in Figure 5A-B.
Discussion
CLL B cells have well-characterized defects in apoptotic pathways, including overexpression of the antiapoptotic proteins Bcl-2, Mcl-1, and XIAP, which confer resistance to cell death, and decreased sensitivity to many conventional chemotherapeutic agents.2,3,6-10 A number of recently identified biologic characteristics, including IgVH mutation status, level of CD38 expression, and presence of certain cytogenetic abnormalities (17p-, 11q-), have also been found to identify CLL clones that are even less likely to respond to conventional chemotherapeutic agents.19-26 These features highlight the need for new agents with activity against resistant CLL clones and that are able to modulate the antiapoptotic protein repertoire of CLL B cells.
A number of recent studies suggest that various neoplastic cells generate a greater quantity of free radicals than their normal counterparts and are under increased oxidative stress.51-54 Previous studies have also demonstrated that B lymphocytes are more sensitive to oxidative stress than T lymphocytes and that CLL B cells are more sensitive to oxidative stress than normal lymphocytes.55-58 These observations provide a rationale for developing agents that induce oxidative stress as a potentially selective therapeutic strategy for CLL.
We recently reported adaphostin appeared to induce cell death in the K562 human leukemia cell line through generation of reactive oxygen species.30 In the present study we extend these earlier results by studying the effect, mechanism of action, predictors of response, and potential for synergistic activity of adaphostin on CLL B cells in vitro. Our results suggest that adaphostin induces apoptotic cell death in primary CLL B cells in a time- and dose-dependent manner, with an average IC50 of about 4 μM at 24 hours (Figure 1C-D). This compares favorably with the IC50 of primary chronic myelogenous leukemia (CML) cells (7.3 μM)29 and acute myelogenous leukemia (AML) cells (6 μM).30 Importantly, CLL B cells from a variety of high-risk categories were equally sensitive to adaphostin, including cells from patients with high-risk chromosome abnormalities (11q-, 17p-) and nonmutated IgVH genes (Table 2). We have demonstrated that intracellular ROS are generated by CLL B cells within 30 minutes of exposure to adaphostin (Figure 4A) and that adaphostin-induced ROS appear to be critical for killing CLL cells (Figure 4B) in vitro. Although based on a small number of patients, our results also suggest that individuals with higher Rai stage may be more sensitive to adaphostin than those with low Rai stage. It is possible that CLL B cells from patients with higher Rai stage have higher basal levels of ROS,57 rendering them more sensitive to oxidative stress. Importantly, normal B and T cells were less sensitive to adaphostin (IC50 more than 10 μM), suggesting a potentially favorable therapeutic ratio. Finally, we found that adaphostin demonstrated consistent ability to synergize with fludarabine in vitro.
Although adaphostin appears to induce CLL cell death through an apoptotic mechanism involving caspase-3 activation,29-31 a broad-spectrum caspase inhibitor failed to prevent adaphostin-induced cell death. Similar findings have been observed for other agents used in the treatment of CLL59 and indicate that caspase inhibition downstream of fatal mitochondrial injury affects the mode but not the extent of cell death.60 In leukemic cell lines, Yu and colleagues131 recently demonstrated that adaphostin induces multiple perturbations in cell signaling, including dose-dependent release of cytochrome c and apoptosis inhibiting factor (AIF), inactivation of cytoprotective pathways (Raf1, mitogen-activated protein kinase [MEK], extracellular signal-regulated kinase 1/2 [ERK1/2], and Akt), activation of stress-induced pathways (Jun N-terminal kinase [JNK] and p38 mitogen-activated protein kinase [MAPK]), and dephosphorylation of retinoblastoma protein (pRb). In these studies, caspase inhibition failed to block adaphostin-induced mitochondrial injury or most of the alterations in cell signaling described. This is consistent with our previous observation that caspase inhibition failed to alter generation of ROS30 and the present finding that caspase inhibitors failed to rescue adaphostin-treated CLL cells. In contrast, in our study of CLL B cells and previous studies of primary CML cells, NAC prevented both generation of ROS and adaphostin-induced cell death. Together, these results suggest that adaphostin-induced alterations in redox state induce mitochondrial injury and impact a broad range of cell-signaling pathways that result in cell death in CLL B cells whether caspases can be activated or not.
Two other compounds currently under investigation as possible agents to treat CLL are also thought to act by elevating cellular ROS. Byrd and colleagues13 recently reported treatment of CLL B cells with Hu1D10, an antibody that targets the major histocompatibility complex (MHC) class II antigen61 (a molecule expressed on normal B cells,62 monocytes,62 dendritic cells,62 and CLL B cells61 ), was associated with generation of ROS (after secondary cross-linking), mitochondrial injury, and cell death that could be attenuated by preincubation with NAC.61 In contrast to the present findings with adaphostin, however, Hu1D10-induced cell death was not associated with caspase activation or PARP cleavage. Moreover, at the same time Hu1D10 elevated ROS, it simultaneously activated a survival pathway involving the antiapoptotic kinase Akt,61 a finding that contrasts with the reported ability of adaphostin to down-regulate Akt.31 Thus, despite the fact both agents induce oxidative stress in CLL B cells, adaphostin and Hu1D10 appear to act through distinct mechanisms.
Increased levels of ROS are also induced by 2-methoxyestradiol (2-ME), possibly by inhibiting superoxide dismutase63 (for an alternative view, see Kachadourian et al64 ). Huang and colleagues63 recently reported that 2-ME has some ability to induce cell death in CLL B cells, but relatively high drug concentrations (IC50 about 30 μM) were required to achieve this effect.57 Combining 2-ME with ROS-generating agents enhanced 2-ME–induced cell death.57,58 The observation that 2-ME does not augment fludarabine-induced cell death in vitro57 suggests adaphostin might be a better candidate for combination with this chemotherapeutic agent.
Finally, it is notable that in in vitro testing imatinib mesylate (Gleevec; Novartis, Basel, Switzerland) was recently reported to act synergistically with chlorambucil in primary CLL B cells despite the fact it had minimal activity as a single agent at physiologically relevant doses.65 Despite the fact imatinib and adaphostin were both originally developed as rationally designed therapy for CML targeting the constitutively activated Bcr/abl tyrosine kinase, both agents have a number of additional effects and act through distinct and potentially even synergistic mechanisms in primary CML cells.44 Imatinib's ability to enhance conventional chemotherapeutic agents in CLL B cells is likely related to inhibition of c-abl kinase,65 a mechanism in no way related to the oxidative stress induced by adaphostin.30,44
In conclusion, the present results suggest that adaphostin is cytotoxic to primary CLL B cells, has a novel mechanism of action (generation of ROS), and exhibits some selectivity for leukemic B cells. Importantly, the agent shows activity against both low- and high-risk subtypes of CLL B cells and synergizes with fludarabine in vitro. Additional preclinical evaluation of adaphostin as a potential agent for the treatment of CLL appears warranted.
Prepublished online as Blood First Edition Paper, September 23, 2004; DOI 10.1182/blood-2004-06-2205.
Supported in part by National Institutes of Health grants R01 CA91542 and R01 CA85972.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal