Abstract
Activating fetal liver tyrosine kinase 3 (Flt3) mutations represent the most common genetic aberrations in acute myeloid leukemia (AML). Most commonly, they occur as internal tandem duplications in the juxtamembrane domain (Flt3-ITD) that transform myeloid cells in vitro and in vivo and that induce aberrant signaling and biologic functions. We identified RGS2, a regulator of G-protein signaling, as a gene specifically repressed by Flt3-ITD. Here we demonstrate an important role of RGS2 in Flt3-ITD–mediated transformation. RGS2 was repressed after forced expression of activating Flt3 mutations in 2 myeloid cell lines (32Dcl3 and NB4). Furthermore, RGS2 was repressed in Flt3-mutation–positive AML cases in comparison to Flt3-mutation–negative cases, especially in Flt3-ITD–positive cases with a high ITD-to–wild-type (WT) ratio. Coexpression of RGS2 with Flt3-ITD inhibited Flt3-ITD–induced autonomous proliferation and clonal growth of 32D cells. RGS2 also inhibited Flt3-ITD–induced phosphorylation of Akt and glycogen synthase kinase β (Gsk3-β) without influencing signal transducer and activator of transcription 5 (STAT5) activation. In addition, RGS2 reinduced the expression of Flt3-ITD–repressed CCAAT/enhancer-binding protein α (c/EBPα) and antagonized the Flt3-ITD–induced differentiation block in 32D cells. Expression analyses in myeloid cell lines revealed induction of RGS2 during granulocytic but not during monocytic differentiation. Taken together, RGS2 is a novel mediator of myeloid differentiation, and its repression is an important event in Flt3-ITD–induced transformation.
Introduction
Acute myeloid leukemia (AML) is a malignant disease characterized by uncontrolled proliferation and impaired differentiation of myeloid progenitor cells. Among the genetic alterations involved in the pathogenesis of this disease, activating mutations of the receptor tyrosine kinase fetal liver tyrosine kinase 3 (Flt3) represent the most common group, occurring in 30% of AML patients (reviewed by Gilliland and Griffin1 ). Two major forms of activating Flt3 mutations have been characterized so far. Internal tandem duplications of the Flt3 gene lead to the duplication of a stretch of amino acids in the juxtamembrane region of the receptor (ITD mutations or Flt3-ITD)2 and point mutations in the activation loop in the kinase domain of Flt3, such as the D835 mutations.3,4 Both mutations cause constitutive activation of the receptor.5-7
Flt3-ITDs mediate factor-independent proliferation, clonal growth, and an increase in resistance against radiation-induced apoptosis in the interleukin-3 (IL-3)–dependent murine myeloid progenitor cell line 32Dcl3.6-9 Furthermore, Flt3-ITDs transform myeloid cell lines and primary murine bone marrow in vivo causing a myeloproliferative disease7,10 and cooperate with other AML-associated mutations, such as the inducer of acute promyelocytic leukemia/retinoic acid receptor α (PML/RARα) fusion protein.11 Interestingly, Flt3-ITDs mediate biologic functions that are distinct from those mediated by Flt3 ligand (FL)–stimulated wild-type Flt3 (Flt3-WT), such as a strong activation signal transducer and activator of transcription 5 (STAT5) or clonal growth in the absence of cytokine stimulation in 32D cells.7
In order to identify aberrant Flt3-ITD–induced signaling pathways, we recently performed microarray analyses comparing gene expression profiles of 32D cells transfected with Flt3-WT under FL stimulation with those of Flt3-ITD–transfected cells.12 We found differences between the gene expression profiles induced by Flt3-ITD and ligand-stimulated Flt3-WT. Several proliferation-associated genes such as Pim2 were induced by Flt3-ITD but not by ligand stimulation of Flt3-WT. On the other hand, differentiation-associated myeloid transcription factors such as PU.1 or CCAAT/enhancer-binding protein α (c/EBPα) were repressed by Flt3-ITD but induced by ligand-stimulated Flt3-WT. In general, Flt3-ITD–induced gene expression profiles resembled those observed under IL-3 stimulation but differed from those induced by FL-stimulated Flt3-WT.
RGS2 was one of the genes repressed by Flt3-ITD but induced by the Flt3-WT signal. This molecule belongs to the protein family of the regulators of G-protein signaling (RGS proteins). All members of this protein family share a highly conserved RGS domain with the function to enhance the intrinsic guanosine 5′-triphosphatase (GTPase) activity of Gα subunits of heterotrimeric G-proteins. Thus, RGS proteins function as negative regulators of G-protein–coupled receptors (GPCRs). So far, more than 30 mammalian RGS proteins have been identified. In addition to the core RGS domain, many RGS proteins contain multiple other functional modules that bind various signaling molecules. The accumulated evidence suggests an important role of this protein family in the integration and modulation of GPCR signaling (reviewed by Hollinger and Hepler13 ).
RGS2 has been shown to bind with high affinity to Gαq subunits and to negatively influence their activity.14,15 Furthermore, RGS2 directly binds to and inhibits adenylyl cyclases and thereby decreases cyclic adenosine monophosphate (cAMP) levels independently from its influence on heterotrimeric G-proteins.16 RGS2 is expressed in many tissues and has been shown to be involved in a variety of cellular functions including the regulation of blood pressure17,18 and neurologic functions.19-24 Also, a negative influence of RGS2 on Wnt-induced morphogenesis in Xenopus embryos25 and promotion of adipocyte differentiation by this molecule26,27 have been described. Systematic gene expression profiling revealed that RGS2 is strongly expressed in hematopoietic tissue,28 and more detailed analyses showed strongest RGS2 expression in hematopoietic stem cells without lineage commitment.29-31 Initially, high RGS2 mRNA levels were found in samples from acute leukemias and chronic myeloid leukemia in blast crisis.32 In contrast, more recent investigations indicated repression of RGS2 in bone marrow specimens from patients with different kinds of hematologic diseases.33-35 The physiologic function of RGS2 in hematopoietic tissue is not known.
In this study we show that RGS2 was repressed by AML-typical Flt3 mutations and was also repressed in the majority of AML cases. RGS2 overexpression inhibited Flt3-ITD–induced transformation of myeloid cells and negatively influenced some Flt3-ITD–induced signaling events, such as the phosphorylation of Akt or Gsk3-β. In addition, RGS2 antagonized the Flt3-ITD–mediated block of differentiation in 32D cells. Gene expression analyses in several myeloid cell lines showed induction of RGS2 expression during granulocytic differentiation. These data indicate that RGS2 is involved in myeloid differentiation and that its repression is an important step in the transformation of myeloid cells.
Patients, materials, and methods
Antibodies and reagents
Recombinant human Flt3 ligand (FL) and recombinant murine interleukin-3 (IL-3) were purchased from Pepro Tech (Rocky Hill, NJ). Rabbit polyclonal anti-RGS2 (H-90) and anti-c/EBPα (14AA) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal anti–phospho-Akt (Ser473), anti–total-Akt, anti–phospho–Gsk3-α/β (Ser21/9), and anti–total–Gsk3-β antibodies were purchased from Cell Signaling Technology (Beverly, MA). The mouse monoclonal anti–phospho-STAT5 antibody was obtained from Upstate Technology (Lake Placid, NY). Horseradish peroxidase–coupled goat antirabbit and goat antimouse antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). The mouse monoclonal antiactin antibody, 12-O-tetradecyanoylphorbol 13-acetate (TPA), and all-trans retinoic acid (ATRA) were purchased from Sigma (Taufkirchen, Germany). Dimethyl sulfoxide (DMSO) was purchased from Serva Electrophoresis GmBH (Heidelberg, Germany). The 1,25-dihydroxy vitamin D3 was obtained from Calbiochem (San Diego, CA). Phycoerythrin (PE)–labeled anti-CD11b (Mac1) antibody was purchased from BD Pharmingen (Heidelberg, Germany). SU11248 was kindly provided by Sugen (San Francisco, CA).
Patient samples
All samples were collected from patients enrolled in 2 different treatment optimization trials in Germany.36,37 Control total bone marrow samples were obtained from healthy donors. CD34+ cells were enriched to a purity of greater than 90% from peripheral blood mononuclear cells (after mobilization with granulocyte colony-stimulating factor [G-CSF]) by magnetic bead technology using a directly coupled CD34 antibody. Written informed consent was obtained from all individuals. The use of human material for scientific purposes was approved by the human ethics committee of each participating institution.
Statistical methods
To determine the statistical significance of differential RGS2 expression levels between patient samples, a Mann-Whitney U test was performed.
Construction of plasmids and generation of stable and inducible cell lines
The generation of 32Dcl3 cells stably transfected with wild-type Flt3 or Flt3-ITD was described previously.12 The full-length coding sequence of Flt3 from a patient with ITD mutation as well as the full-length wild-type sequence from the Oci-AML5 cell line were amplified and cloned as described.12 The constructs were cloned into an expression vector (pAL) under the control of the 5′ long-terminal repeat (5′LTR) of the Moloney murine leukemia virus (MoMuSV). The constructs were stably transfected into 32Dcl3 cells. Bulk cultures were used for further experiments. The coding sequence of human RGS2 was amplified from cDNA from peripheral blood of healthy individuals and cloned into pOPRSVI (Stratagene, La Jolla, CA). The construct was sequenced to exclude the introduction of point mutations during the cloning procedure. For stable and permanent coexpression of Flt3-ITD and RGS2 in 32D cells, the stably Flt3-ITD–expressing cell line was cotransfected with pOPRSVI-RGS2. To generate Flt3-ITD–positive 32D cell lines with IPTG-inducible RGS2 expression, pOPRSVI-RGS2 and pLamLac-R, which expresses the Lac repressor,7 were transfected into 32D cells that were already stably transfected with Flt3-ITD. Single clones were generated by limiting dilution and tested for their Lac-inducible expression of human RGS2. Clones that showed tight Lac-inducible RGS2 expression were used for further experiments. NB4 cells were stably transfected with Flt3-ITD in pCDNA3.1(-). Bulk cultures were used for further experiments.
RNA isolation, generation of cDNA, and real-time reverse transcriptase–polymerase chain reaction (RT-PCR)
Total RNA was isolated using the TRIZOL reagent (Invitrogen, Karlsruhe, Germany) according to the manufacturer's recommendations. One microgram total RNA was used for reverse transcription. The cDNA was diluted to 200 μL with ddH20, and 2.5 μL was used for each PCR reaction. The quantification of mRNA levels was carried out using a real-time fluorescence detection method as described before.38 Relative gene expression levels were calculated using standard curves generated by the serial dilutions of cDNA from 32D cells or NB4 cells. All samples were independently analyzed at least twice for each gene. The housekeeping gene GAPDH served as an additional control for the cDNA quality. For patient samples, blasts were enriched from bone marrow samples by density centrifugation of heparinized aspirates at the time of diagnosis and frozen at -80°C until the experiments were performed.
Western blot analysis
Cells were washed once in ice-cold phosphate-buffered saline (PBS) and lysed for 30 minutes on ice in a buffer containing 150 mM NaCl, 1% nonidet P–40 (NP40), 0.5% deoxycholic acid (DOC), 0.1% sodium dodecyl sulfate (SDS), 50 mM Tris (tris(hydroxymethyl)aminomethane; pH 8.0) with proteinase inhibitors (Complete; Boehringer Mannheim, Mannheim, Germany), and 1 mM sodium orthovanadate. Cell lysates were clarified at 20 000g for 15 minutes. After adjustment of protein concentrations, the lysates were boiled in SDS sample buffer for 5 minutes and separated by SDS–polyacrylamide gel electrophoresis (SDS-PAGE). Gels were blotted on a polyvinylidene fluoride (PVDF) membrane (Immobilon P; Millipore, Bedford, MA) and stained with the indicated antibody. Antibody binding was detected with a horseradish peroxidase (HRP)–coupled secondary antibody followed by chemoluminescence detection (ECL Plus; Amersham Pharmacia, Uppsala, Sweden).
3H-thymidine incorporation
A total of 1 × 104 cells per well were starved of IL-3 for 12 hours in a volume of 200 μL. Subsequently, isopropyl-β-D-thiogalactopyranoside (IPTG) was added to a final concentration of 5 mM for RGS2 induction and cells were exposed to either 40 ng/mL FL, 2 ng/mL IL-3, or to control medium without cytokines for 6 hours. Subsequently, 1 μCi (0.037 MBq) of 3H-thymidine was added, and cells were cultured for another 6 hours under the same condition. Cells were harvested on glass fiber filters and β-emission of the bound DNA was detected with a scintillation counter. Experiments were performed in triplicates. Each data point represents mean and standard deviation of 3 independent experiments.
Clonal growth in methylcellulose
Flt3-ITD–expressing 32Dcl3 cells were electroporated with 15 μg of pOPRSVI-RGS2 or the control vector. One day after electroporation, cells were seeded at a concentration of 1 × 105 cells per dish in 1 mL of a culture mix containing Iscoves modified Dulbecco medium (IMDM; Life Technologies, Grand Island, NY), 1% methylcellulose, 20% fetal calf serum (FCS), and 0.6 mg/mL G418. The colonies were counted on day 8. Photographs of the colony assays were taken with an Olympus CKX1 inverted microscope, an Olympus plan 4×/0.1 NA objective lens, and an Olympus C-5050 digital camera (Olympus, Hamburg, Germany).
Flow cytometry
Cells were preincubated at 4°C with mouse immunoglobulin G (IgG) for 15 minutes and subsequently stained for 15 minutes with the indicated antibody. Cells were washed twice with phosphate-buffered saline (PBS) containing 0.1% human serum albumin and analyzed with a FACSCalibur (Becton Dickinson, Heidelberg, Germany).
Differentiation of myeloid cell lines
HL-60 cells were differentiated with DMSO (1.25%) to granulocytes and with TPA (10 ng/mL) and vitamin D3 (10 nM) to macrophages and monocytes, respectively. U937 cells were differentiated to macrophages and monocytes with TPA (10 ng/mL) and vitamin D3 (10 nM), respectively, or to granulocytes with ATRA (10-6 M). Differentiation into granulocytes and macrophages was confirmed by flow cytometry for CD11b and CD14, respectively. Samples for RNA preparation were taken at the indicated time points. RGS2 expression was analyzed by real-time RT-PCR.
Results
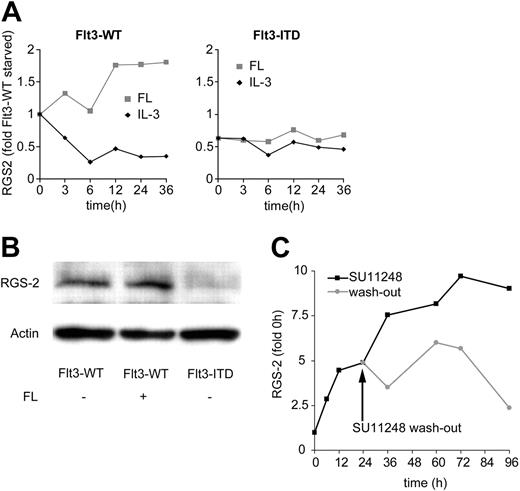
In a microarray study, we identified RGS2 as a repressed gene in response to Flt3-ITD expression.12 In order to characterize the modulation of RGS2 by Flt3-ITD in more detail, we analyzed the influence of Flt3-ITD on RGS2 expression on the mRNA and protein levels. First, we determined RGS2 expression on the mRNA level in 32D cells stably transfected with either Flt3-WT or Flt3-ITD. Figure 1A shows RGS2 mRNA expression levels analyzed by real-time RT-PCR. Following 12-hour growth factor withdrawal, we exposed the cells to FL or IL-3 for up to 36 hours. In 32D/Flt3-WT cells, FL induced RGS2 mRNA about 2-fold, whereas IL-3 repressed RGS2 mRNA levels. The 32D/Flt3-ITD cells expressed significantly less RGS2 mRNA, and no response to FL stimulation was observed. The results were confirmed on the protein level by Western blot analyses, where the presence of Flt3-ITD strongly repressed the RGS2 protein, while the Flt3-WT signal led to a slight induction (Figure 1B). To further confirm the repression of RGS2 by Flt3-ITD, we treated 32D/Flt3-ITD cells with 100 nM SU11248, a tyrosine kinase inhibitor that has been well described to inhibit Flt3-ITD phosphorylation (Figure 1C).39 Upon SU11248 treatment we observed an almost 10-fold induction of RGS2 mRNA. After 24 hours we washed the cells and incubated half of them in the presence and the other half in the absence of SU11248. Cells cultured in the absence of SU11248 showed no further increase of RGS2 levels and after 96 hours RGS2 expression was again as low as under baseline conditions. Point mutations in the activation loop of Flt3 occur in about 7% of AML patients, most commonly at residue D835.3,4 RGS2 mRNA levels in 32D/Flt3-D835Y cells without cytokine stimulation were reduced compared with 32D/Flt3-WT cells. However, in contrast to 32D/Flt3-ITD cells, RGS2 levels were significantly induced upon stimulation with FL to levels found in FL-stimulated 32D/Flt3-WT cells (data not shown). These data indicate that Flt3-ITD mutations, but not Flt3-D835Y, significantly repressed RGS2 expression.
RGS2 is repressed by activating Flt3 mutations. (A) Analysis of RGS2 mRNA levels by real-time RT-PCR. The 32D cells stably transfected with either wild-type Flt3 (Flt3-WT) or Flt3-ITD were growth factor starved for 12 hours. Samples were taken for RNA preparation at the indicated time points after the addition of IL-3 ( ) or FL (▦). (B) RGS2 protein levels. The 32D cells stably transfected with Flt3-WT or Flt3-ITD were cultured for 12 hours in the absence of IL-3 and in the absence or presence of FL as indicated. RGS2 protein levels were determined by Western blot. (C) Flt3-ITD–induced RGS2 repression can be antagonized by inhibition of Flt3 phosphorylation. The 32D/Flt3-ITD cells were cultured in the presence of 100 nM SU11248 (▪), an Flt3-ITD–specific tyrosine kinase inhibitor. After 24 hours, cells were washed, one half was cultured further in the absence (
) or FL (▦). (B) RGS2 protein levels. The 32D cells stably transfected with Flt3-WT or Flt3-ITD were cultured for 12 hours in the absence of IL-3 and in the absence or presence of FL as indicated. RGS2 protein levels were determined by Western blot. (C) Flt3-ITD–induced RGS2 repression can be antagonized by inhibition of Flt3 phosphorylation. The 32D/Flt3-ITD cells were cultured in the presence of 100 nM SU11248 (▪), an Flt3-ITD–specific tyrosine kinase inhibitor. After 24 hours, cells were washed, one half was cultured further in the absence ( ) and the other half in the presence of SU11248. RGS2 mRNA levels were analyzed by real-time RT-PCR.
) and the other half in the presence of SU11248. RGS2 mRNA levels were analyzed by real-time RT-PCR.
RGS2 is repressed by activating Flt3 mutations. (A) Analysis of RGS2 mRNA levels by real-time RT-PCR. The 32D cells stably transfected with either wild-type Flt3 (Flt3-WT) or Flt3-ITD were growth factor starved for 12 hours. Samples were taken for RNA preparation at the indicated time points after the addition of IL-3 ( ) or FL (▦). (B) RGS2 protein levels. The 32D cells stably transfected with Flt3-WT or Flt3-ITD were cultured for 12 hours in the absence of IL-3 and in the absence or presence of FL as indicated. RGS2 protein levels were determined by Western blot. (C) Flt3-ITD–induced RGS2 repression can be antagonized by inhibition of Flt3 phosphorylation. The 32D/Flt3-ITD cells were cultured in the presence of 100 nM SU11248 (▪), an Flt3-ITD–specific tyrosine kinase inhibitor. After 24 hours, cells were washed, one half was cultured further in the absence (
) or FL (▦). (B) RGS2 protein levels. The 32D cells stably transfected with Flt3-WT or Flt3-ITD were cultured for 12 hours in the absence of IL-3 and in the absence or presence of FL as indicated. RGS2 protein levels were determined by Western blot. (C) Flt3-ITD–induced RGS2 repression can be antagonized by inhibition of Flt3 phosphorylation. The 32D/Flt3-ITD cells were cultured in the presence of 100 nM SU11248 (▪), an Flt3-ITD–specific tyrosine kinase inhibitor. After 24 hours, cells were washed, one half was cultured further in the absence ( ) and the other half in the presence of SU11248. RGS2 mRNA levels were analyzed by real-time RT-PCR.
) and the other half in the presence of SU11248. RGS2 mRNA levels were analyzed by real-time RT-PCR.
RGS2 expression in bone marrow of AML patients
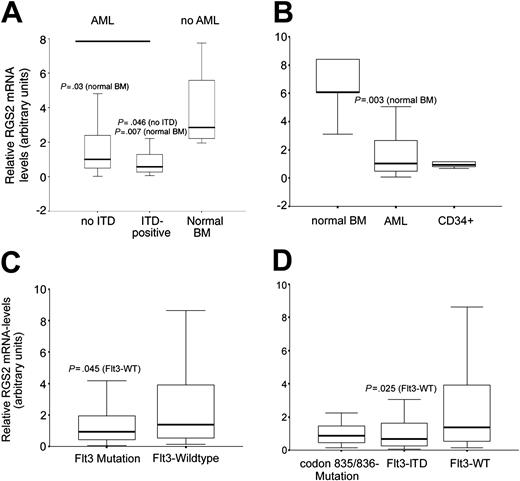
Next, we analyzed the expression of RGS2 mRNA in primary blasts from AML patients. Real-time RT-PCR analyses in bone marrow specimens of 84 newly diagnosed and untreated AML patients revealed significant repression of RGS2 mRNA in comparison to normal bone marrow (Figure 2A). When we compared the relative expression levels of RGS2 in Flt3-ITD–positive versus–negative cases, we found RGS2 expression levels to be slightly but significantly lower in the Flt3-ITD–positive cases (P = .046).
Expression of RGS2 in primary bone marrow of AML patients. (A) Bone marrow samples from 84 AML patients were analyzed for RGS2 expression by real-time RT-PCR and compared with RGS2 levels in bone marrow from healthy donors. Box plots are shown for the relative expression levels of RGS2 normalized to GAPDH. The differences in expression between leukemic samples from patients with and without Flt3-ITD as well as between leukemic samples and normal bone marrow were statistically significant (P < .05, Mann-Whitney U test). (B) Another set of 120 AML bone marrow samples (49 with Flt3-WT, 50 with Flt3-ITD, and 21 with point mutations of codon 835 or 836) was analyzed for RGS2 expression compared with bone marrow of healthy donors and with CD34+ progenitor cells. (C) Comparison of RGS2 mRNA levels between AML samples with and without Flt3 mutation (ITD or point mutation) from the second cohort. (D) RGS2 mRNA expression in AML samples with Flt3-WT compared with Flt3-ITD with high expression of the mutated allele and to cases containing a codon 835/836 point mutation. Statistic analyses were performed with Mann-Whitney U tests. The horizontal lines indicate the median; the boxes, the interquartile range; and the error bars, the extreme values of each cohort.
Expression of RGS2 in primary bone marrow of AML patients. (A) Bone marrow samples from 84 AML patients were analyzed for RGS2 expression by real-time RT-PCR and compared with RGS2 levels in bone marrow from healthy donors. Box plots are shown for the relative expression levels of RGS2 normalized to GAPDH. The differences in expression between leukemic samples from patients with and without Flt3-ITD as well as between leukemic samples and normal bone marrow were statistically significant (P < .05, Mann-Whitney U test). (B) Another set of 120 AML bone marrow samples (49 with Flt3-WT, 50 with Flt3-ITD, and 21 with point mutations of codon 835 or 836) was analyzed for RGS2 expression compared with bone marrow of healthy donors and with CD34+ progenitor cells. (C) Comparison of RGS2 mRNA levels between AML samples with and without Flt3 mutation (ITD or point mutation) from the second cohort. (D) RGS2 mRNA expression in AML samples with Flt3-WT compared with Flt3-ITD with high expression of the mutated allele and to cases containing a codon 835/836 point mutation. Statistic analyses were performed with Mann-Whitney U tests. The horizontal lines indicate the median; the boxes, the interquartile range; and the error bars, the extreme values of each cohort.
In order to confirm these small expression differences, we analyzed 120 samples of another independent set of patients that was diagnosed at a different center. Fifty patients of this series with Flt3-WT status were analyzed, 49 patients with Flt3-ITD expression and 21 patients displaying a mutation in codon 835 or 836 (Flt3-TKD [tyrosine kinase domain]). When we compared the expression of RGS2 in these AML samples with the expression of RGS2 in normal bone marrow, we again saw a significant down-regulation of RGS2 mRNA expression in the AML cases (P = .003). However, expression of RGS2 in normal CD34+ progenitor cells matched the expression in AML samples, indicating that the low RGS2 expression levels in AML samples reflect the lack of differentiation of AML blasts (Figure 2B).
When we compared the expression levels of RGS2 in AML blasts with mutant Flt3 genotype with Flt3-WT samples (Figure 2C), we again found a slight but significant reduction of RGS2 expression in the Flt3-mutant samples (P = .045). For a subgroup of the Flt3-ITD–positive cases,35 the ratio of Flt3-ITD to Flt3-WT on the genomic DNA level was available. In some of the samples, low values of this ratio indicated a relatively low contribution of Flt3-ITD–positive clones to the total analyzed blast population. Interestingly, we found a negative correlation between the ITD/WT ratio and the RGS2 expression level (r = 0.625, P < .001; data not shown). If we excluded samples with a low ITD/WT ratio from the analyses (below a ratio of 0.6), we found a significant repression of RGS2 mRNA in Flt3-ITD–positive versus–negative cases (P = .025; Figure 2D). In contrast, the RGS2 expression in Flt3-TKD mutation–positive cases was not significantly different from Flt3-WT cases. In conclusion, AML blasts as well as CD34+ normal hematopoietic progenitors express markedly less RGS2 than normal bone marrow. Also, in Flt3-ITD–positive AML samples, RGS2 mRNA expression is significantly lower than in Flt3-WT cases, with the most pronounced RGS2 down-regulation in cases with a high ITD/WT ratio.
RGS2 inhibits Flt3-ITD–induced autonomous proliferation
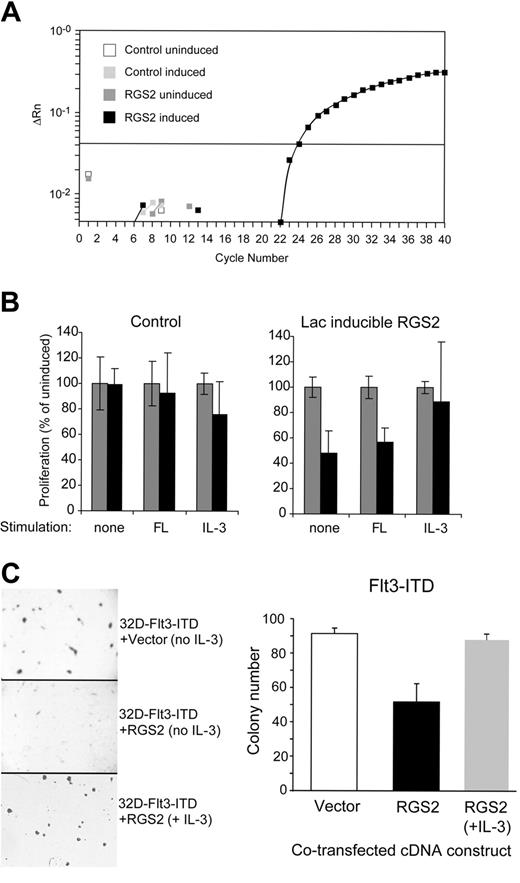
Subsequently, we studied the involvement of RGS2 repression in Flt3-ITD–mediated signal transduction and biologic function. First, we analyzed the effects of RGS2 overexpression on the growth properties of the IL-3–dependent 32D cell line that proliferates factor independently after forced overexpression of Flt3-ITD. We generated a 32D/Flt3-ITD cell line that contained a Lac-inducible RGS2 expression construct. Several clonal cell lines were established and tested for the tight regulation of RGS2 expression. The amplification plot of a real-time RT-PCR for RGS2 for one of these cell lines with and without RGS2 induction is shown in Figure 3A. In these cell lines, proliferation was analyzed by 3H-thymidine incorporation assays in the presence and absence of IPTG for induction of Lac-responsive gene expression. As shown in Figure 3B, coexpression of RGS2 reduced Flt3-ITD–mediated autonomous proliferation by about 50%. Exposure of the cells to FL did not rescue the impaired proliferation after RGS2 induction. However, addition of IL-3 readily restored proliferation in RGS2-expressing 32D/Flt3-ITD cells.
RGS2 inhibits Flt3-ITD– and IL-3–mediated proliferation of 32D cells. (A) Lac-inducible expression of RGS2. The 32D/Flt3-ITD cells were stably transfected with a Lac-inducible RGS2 construct or a control vector. Cells were cultured in the presence or absence of IPTG (for induction of RGS2). RGS2 expression was measured by real-time RT-PCR. The amplification plot shows the amount of RGS2 cDNA (ΔRn) during each cycle of the PCR reaction. (B) RGS2 inhibits Flt3-ITD– and IL-3–mediated autonomous proliferation of 32D cells. The 32D/Flt3-ITD cells were stably transfected with a Lac-inducible RGS2 construct (right) or a control vector (left). Cells were starved of IL-3 for 12 hours. Subsequently, the indicated cytokine with (▪) or without IPTG (▦) was added. DNA synthesis was detected by 3H-thymidine incorporation. (C) RGS2 inhibits Flt3-ITD–induced clonal growth of 32D cells. The 32D cells stably transfected with Flt3-ITD were transiently cotransfected with RGS2 (with or without IL-3 stimulation) or with an empty vector. One day after transfection, 1 × 105 cells were plated per dish in the presence of G418 as selection marker. Colonies were analyzed on day 8.
RGS2 inhibits Flt3-ITD– and IL-3–mediated proliferation of 32D cells. (A) Lac-inducible expression of RGS2. The 32D/Flt3-ITD cells were stably transfected with a Lac-inducible RGS2 construct or a control vector. Cells were cultured in the presence or absence of IPTG (for induction of RGS2). RGS2 expression was measured by real-time RT-PCR. The amplification plot shows the amount of RGS2 cDNA (ΔRn) during each cycle of the PCR reaction. (B) RGS2 inhibits Flt3-ITD– and IL-3–mediated autonomous proliferation of 32D cells. The 32D/Flt3-ITD cells were stably transfected with a Lac-inducible RGS2 construct (right) or a control vector (left). Cells were starved of IL-3 for 12 hours. Subsequently, the indicated cytokine with (▪) or without IPTG (▦) was added. DNA synthesis was detected by 3H-thymidine incorporation. (C) RGS2 inhibits Flt3-ITD–induced clonal growth of 32D cells. The 32D cells stably transfected with Flt3-ITD were transiently cotransfected with RGS2 (with or without IL-3 stimulation) or with an empty vector. One day after transfection, 1 × 105 cells were plated per dish in the presence of G418 as selection marker. Colonies were analyzed on day 8.
RGS2 inhibits Flt3-ITD–mediated clonal growth
In contrast to the ligand-stimulated wild-type receptor, Flt3-ITDs promote clonal growth of 32D cells in methylcellulose in the absence of IL-3.7 Therefore, we transiently overexpressed RGS2 in 32D/Flt3-ITD cells and analyzed its influence on Flt3-ITD–induced colony growth. Figure 3C shows representative areas of dishes with the mock-transfected and RGS2-transfected cells. In addition, RGS2 significantly decreased the number of colonies. Also here, the repression of clonal growth by RGS2 could be rescued by IL-3 stimulation. In summary, enforced expression of RGS2 inhibited Flt3-ITD–mediated transformation of 32D cells.
Influence of RGS2 on Flt3-ITD–induced signaling pathways
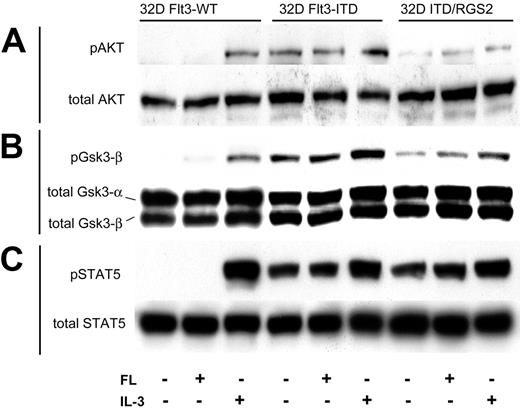
Flt3-ITD employs several signaling pathways in the process of cellular transformation. To investigate which of these pathways is affected by RGS2, we analyzed the phosphorylation of several signaling intermediates previously implicated in Flt3 signal transduction by phospho-specific Western blot analyses (Figure 4).
Influence of RGS2 on Flt3-ITD–induced signaling pathways. The 32D cells stably transfected with either Flt3-WT or Flt3-ITD together with a control vector or with Flt3-ITD together with RGS2 were starved of growth factors for 12 hours and were subsequently exposed to the indicated cytokines for 10 minutes. Western blot analyses were performed with lysates from these cells using phospho-specific antibodies for Akt (A), Gsk3-β (B), or STAT5 (C). The membranes were reprobed with antibodies detecting total proteins.
Influence of RGS2 on Flt3-ITD–induced signaling pathways. The 32D cells stably transfected with either Flt3-WT or Flt3-ITD together with a control vector or with Flt3-ITD together with RGS2 were starved of growth factors for 12 hours and were subsequently exposed to the indicated cytokines for 10 minutes. Western blot analyses were performed with lysates from these cells using phospho-specific antibodies for Akt (A), Gsk3-β (B), or STAT5 (C). The membranes were reprobed with antibodies detecting total proteins.
The protein kinase Akt is an effector of the phosphatidylinositol 3 (PI3)–kinase pathway and an important regulator of cellular proliferation and survival. It is activated by phosphorylation on serine 473. Here, Flt3-WT only very weakly induced Akt phosphorylation (Figure 4A). However, in 32D/Flt3-ITD cells, Akt was phosphorylated, even in the absence of added growth factors. Coexpression of RGS2 significantly diminished Akt phosphorylation. Interestingly, IL-3 could not rescue the RGS2-mediated reduction of Akt activity.
To further confirm the influence of Flt3-ITD and RGS2 on Akt-dependent signaling events, we also determined the phosphorylation of Gsk3-β. This molecule contributes in a complex with APC and axin to the degradation of cytoplasmic β-catenin40,41 and can be directly inactivated by Akt via phosphorylation.42 Here also, Flt3-ITD caused constitutive phosphorylation on a much higher level than observed in cells expressing Flt3-WT. Again, RGS2 coexpression significantly diminished the Flt3-ITD–induced enhancement of Gsk3-β phosphorylation (Figure 4B).
Finally, we analyzed the phosphorylation of STAT5. Again, we observed strong and constitutive phosphorylation of STAT5 in the 32D/Flt3-ITD cells. Ligand-activated Flt3-WT did not cause STAT5 phosphorylation, an observation that we and others have described earlier.7 In contrast to the phosphorylation of Akt and Gsk3-β, RGS2 coexpression did not influence Flt3-ITD–induced STAT5 activation. Taken together, RGS2 coexpression inhibits some Flt3-ITD–induced signaling pathways (Akt, Gsk3-β) but leaves STAT5 activation untouched.
RGS2 reverses the Flt3-ITD–induced block of differentiation in 32D cells
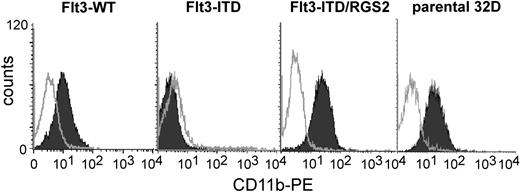
Recently, our group and others reported that Flt3-ITD inhibits G-CSF–induced granulocytic differentiation.12,43 Thus, we analyzed the effects of RGS2 overexpression on the Flt3-ITD–mediated differentiation block. The 32D cells stably transfected with either Flt3-WT or Flt3-ITD or cotransfected with Flt3-ITD and RGS2 and parental 32D cells were cultured in the presence of G-CSF for 12 days. Cells transfected with the wild-type receptor were concomitantly exposed to FL. After 12 days of G-CSF treatment, the differentiation status of the cells was assessed by flow cytometric analysis of CD11b expression. Flt3-ITD, but not FL-activated Flt3-WT, inhibited G-CSF–induced CD11b expression (Figure 5). RGS2 coexpression restored the ability of 32D/Flt3-ITD to express CD11b after G-CSF exposure at levels equal to G-CSF–treated parental 32D cells. Indeed, we observed CD11b expression in RGS2-positive cells even without G-CSF exposure of the cultures (data not shown). This experiment was performed with several independent stable 32D cell lines coexpressing Flt3-ITD and RGS2 with similar results. These analyses showed that RGS2 restored the G-CSF–induced differentiation capacities of 32D/Flt3-ITD cells.
RGS2 reverses the Flt3-ITD–induced block of differentiation. Stable 32D/Flt3-WT, 32D/Flt3-ITD, or 32D/Flt3-ITD cells cotransfected with RGS2 and parental 32D cells were used for these experiments. Cells were grown for 12 days in the presence of G-CSF without IL-3. Granulocytic differentiation was determined by flow cytometric CD11b surface expression.
RGS2 reverses the Flt3-ITD–induced block of differentiation. Stable 32D/Flt3-WT, 32D/Flt3-ITD, or 32D/Flt3-ITD cells cotransfected with RGS2 and parental 32D cells were used for these experiments. Cells were grown for 12 days in the presence of G-CSF without IL-3. Granulocytic differentiation was determined by flow cytometric CD11b surface expression.
RGS2 induces c/EBPα expression in 32D cells
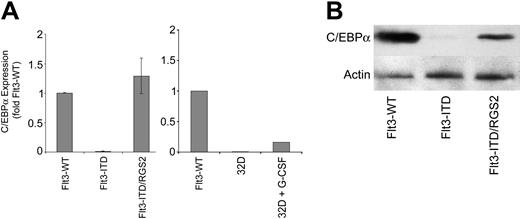
Myeloid differentiation is regulated by a complex interplay of several differentiating myeloid transcription factors. Among these, c/EBP transcription factor family members are highly important. Since c/EBPα is repressed by Flt3-ITD,12 we analyzed the c/EBPα expression in parental 32D cells with and without G-CSF treatment for 12 days and 32D cells transfected with either Flt3-WT (under FL stimulation) or Flt3-ITD or with Flt3-ITD and RGS2 (Figure 6). Flt3-ITD caused strong repression of c/EBPα. However, coexpression of RGS2 with Flt3-ITD completely restored c/EBPα expression on mRNA level in these cells. The c/EBPα mRNA levels in these cells were even higher than in G-CSF–treated parental 32D cells. We further examined c/EBPα expression on the protein levels. Here, we also found strong repression of c/EBPα by Flt3-ITD compared with Flt3-WT and induction of c/EBPα protein expression by coexpression of RGS2 with Flt3-ITD. Therefore, Flt3-ITD–induced RGS2 repression is necessary for the repression of c/EBPα.
RGS2 induces c/EBPα expression in Flt3-ITD–transformed 32D cells. The 32D cells were stably transfected with Flt3-WT or Flt3-ITD or cotransfected with Flt3-ITD and RGS2. The cells were cultured for 12 hours in the absence of IL-3; Flt3-WT transfected cells were exposed to FL. Parental 32D cells were grown either in the presence of IL-3 or stimulated for 12 days with G-CSF as indicated. (A) c/EBPα mRNA expression was determined by real-time RT-PCR. The means and standard deviations of 3 independent experiments are shown. (B) c/EBPα protein expression was determined by Western blot analysis.
RGS2 induces c/EBPα expression in Flt3-ITD–transformed 32D cells. The 32D cells were stably transfected with Flt3-WT or Flt3-ITD or cotransfected with Flt3-ITD and RGS2. The cells were cultured for 12 hours in the absence of IL-3; Flt3-WT transfected cells were exposed to FL. Parental 32D cells were grown either in the presence of IL-3 or stimulated for 12 days with G-CSF as indicated. (A) c/EBPα mRNA expression was determined by real-time RT-PCR. The means and standard deviations of 3 independent experiments are shown. (B) c/EBPα protein expression was determined by Western blot analysis.
RGS2 is induced during granulocytic differentiation of myeloid cell lines
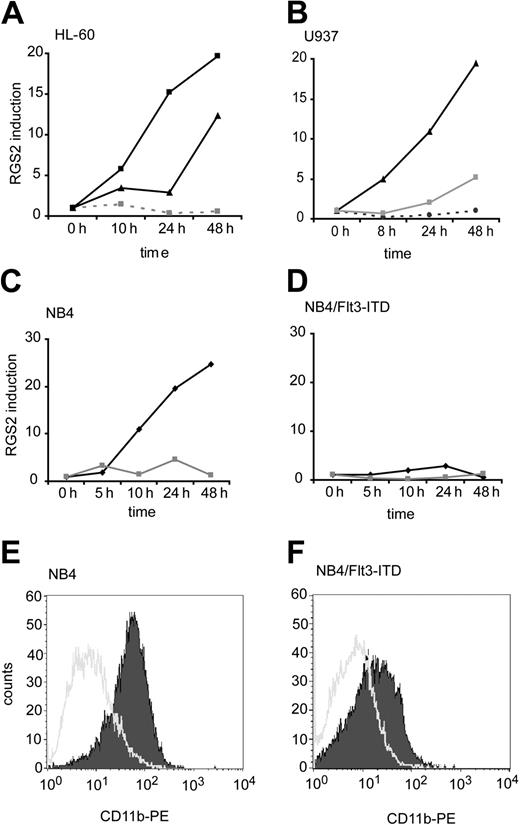
Having established the surprising result that RGS2 mediates the Flt3-ITD–induced differentiation block, we analyzed the RGS2 expression in several model cell lines of myeloid differentiation. Figure 7 depicts a time course of RGS2 mRNA expression as analyzed by real-time RT-PCR during differentiation. HL-60 cells can be differentiated to granulocytes with DMSO, while TPA and vitamin D3 induce monocytic differentiation. RGS2 mRNA was strongly induced by DMSO and TPA but not by vitamin D3. Another myeloid cell line, U937 (Figure 7B), also showed RGS2 induction after exposure to ATRA and TPA but not during vitamin D3–induced monocytic differentiation. As a third model for myeloid differentiation, we used NB4 cells derived from an AML M3 patient with PML/RARα translocation. Exposure of NB4 cells to ATRA induces granulocytic differentiation. Figure 7C shows a more than 20-fold up-regulation of RGS2 under ATRA treatment in NB4 cells.
RGS2 expression during differentiation of myeloid cell lines. (A) HL-60 cells were cultured in the presence of DMSO (▴) for granulocytic differentiation, vitamin D3 (▦ and dotted line) for monocytic differentiation, or TPA (▪) for differentiation into macrophages. At the indicated time points, cells were removed for RNA isolation. RGS2 mRNA levels were determined by real-time RT-PCR. Monocytic differentiation was confirmed by flow cytometry for CD14, granulocytic differentiation by flow cytometry for CD11b. (B) U937 cells were treated with vitamin D3 (⬡ and dotted line) or TPA (▴) for differentiation into monocytes or macrophages, respectively, or with ATRA (▦) for granulocytic differentiation. Samples for the analysis of RGS2 mRNA levels were taken at the indicated time points. Differentiation was confirmed by flow cytometry. (C) NB4 cells were treated with ATRA ( ) to induce granulocytic differentiation. Samples for RGS2 mRNA determination were taken at the indicated time points. ▦ indicates ethyl alcohol. (D) NB4 cells stably transfected with Flt3-ITD were treated with ATRA. Samples for RGS2 mRNA determination were taken at the indicated time points. Symbol meaning is the same as in panel C. (E-F) Flow cytometry for CD11b after 48 hours treatment with NB4 cells (E) or NB4-Flt3-ITD cells (F). Open histograms show ethanol-treated cells and filled histograms ATRA-treated cells.
) to induce granulocytic differentiation. Samples for RGS2 mRNA determination were taken at the indicated time points. ▦ indicates ethyl alcohol. (D) NB4 cells stably transfected with Flt3-ITD were treated with ATRA. Samples for RGS2 mRNA determination were taken at the indicated time points. Symbol meaning is the same as in panel C. (E-F) Flow cytometry for CD11b after 48 hours treatment with NB4 cells (E) or NB4-Flt3-ITD cells (F). Open histograms show ethanol-treated cells and filled histograms ATRA-treated cells.
RGS2 expression during differentiation of myeloid cell lines. (A) HL-60 cells were cultured in the presence of DMSO (▴) for granulocytic differentiation, vitamin D3 (▦ and dotted line) for monocytic differentiation, or TPA (▪) for differentiation into macrophages. At the indicated time points, cells were removed for RNA isolation. RGS2 mRNA levels were determined by real-time RT-PCR. Monocytic differentiation was confirmed by flow cytometry for CD14, granulocytic differentiation by flow cytometry for CD11b. (B) U937 cells were treated with vitamin D3 (⬡ and dotted line) or TPA (▴) for differentiation into monocytes or macrophages, respectively, or with ATRA (▦) for granulocytic differentiation. Samples for the analysis of RGS2 mRNA levels were taken at the indicated time points. Differentiation was confirmed by flow cytometry. (C) NB4 cells were treated with ATRA ( ) to induce granulocytic differentiation. Samples for RGS2 mRNA determination were taken at the indicated time points. ▦ indicates ethyl alcohol. (D) NB4 cells stably transfected with Flt3-ITD were treated with ATRA. Samples for RGS2 mRNA determination were taken at the indicated time points. Symbol meaning is the same as in panel C. (E-F) Flow cytometry for CD11b after 48 hours treatment with NB4 cells (E) or NB4-Flt3-ITD cells (F). Open histograms show ethanol-treated cells and filled histograms ATRA-treated cells.
) to induce granulocytic differentiation. Samples for RGS2 mRNA determination were taken at the indicated time points. ▦ indicates ethyl alcohol. (D) NB4 cells stably transfected with Flt3-ITD were treated with ATRA. Samples for RGS2 mRNA determination were taken at the indicated time points. Symbol meaning is the same as in panel C. (E-F) Flow cytometry for CD11b after 48 hours treatment with NB4 cells (E) or NB4-Flt3-ITD cells (F). Open histograms show ethanol-treated cells and filled histograms ATRA-treated cells.
To further investigate the interplay of Flt3-ITD and RGS2 in myeloid differentiation, we generated an NB4 cell line stably transfected with Flt3-ITD. Compared with the parental cells, these cells have a reduced ability to differentiate in response to ATRA, as shown by CD11b expression (Figure 7E-F). Interestingly Flt3-ITD–transfected NB4 cells also failed to induce RGS2 expression when exposed to ATRA.
Taken together, differentiation of myeloid cell lines induces RGS2 mRNA levels in most cases. RGS2 is up-regulated during granulocytic differentiation (as by DMSO or ATRA) and in response to treatment with TPA but not in response to vitamin D3. Furthermore, Flt3-ITD induces a block of differentiation also in NB4 cells that is accompanied by a lack of RGS2 induction.
Discussion
In this study, we examined the expression and function of the Flt3-ITD target gene RGS2 in the transformation and differentiation of myeloid cells. We demonstrate that RGS2 repression is a constant feature of activating Flt3 mutations in myeloid cell lines and primary AML blasts. In the 32Dcl3 cell line, we found repression of RGS2 by Flt3-ITD, whereas RGS2 was induced by ligand-stimulated wild-type Flt3. In contrast, no consistent effect of Flt3-TKD mutations on the RGS expression was noted. In NB4 cells, Flt3-ITD did not impair basal RGS2 expression but inhibited its induction during ATRA-mediated differentiation. The effects of ATRA on differentiation were also impaired by Flt3-ITD.
RGS2 mRNA expression in primary AML bone marrow samples was repressed in the majority of cases compared with controls from healthy donors, also in the absence of activating Flt3 mutations. The repression of RGS2 in Flt3-ITD–negative AML could be a reflection of the differentiation block in AML blasts. This notion is supported by the equally low expression of RGS2 in normal CD34+ progenitor cells. On the other hand, transforming events other than Flt3-ITD or the activation of cytokine receptors like the IL-3 receptor could cause RGS2 repression in AML blasts. However, when we compared the RGS2 levels in AML samples, Flt3-ITD–positive cases expressed slightly but significantly lower levels of RGS2 mRNA than Flt3-ITD–negative cases. The inconsistent effects of Flt3-D835Y on RGS2 expression in 32D cells were reflected by the lack of statistically significant RGS2 repression in Flt3-TKD mutation–positive AML. This is in line with other experiments from our group that indicate profound differences in the transforming potential and signal transduction of Flt3-TKD versus Flt3-ITD mutations (C.C., J.S., and H.S., unpublished data, 2004). Thus, we conclude that RGS2 repression in AML reflects the low degree of differentiation in AML blasts. Although the observed differences in AML blasts are small, they hint at the possibility that Flt3-ITD mutations not only repress RGS2 in cell line models but also in primary AML blasts.
Forced overexpression of RGS2 inhibited Flt3-ITD–induced growth in suspension as well as in colony assays. These findings establish that RGS2 repression is a necessary prerequisite for Flt3-ITD–induced transformation. Analyses of known Flt3-ITD–activated signaling pathways revealed that RGS2 antagonizes some, but not all, effects of Flt3-ITD. Interestingly, IL-3 stimulation rescued the inhibitory effect of RGS2 on 32D cell proliferation and clonogenic growth. This suggests that IL-3 may use different signaling pathways than Flt3-ITD that are not subject to inhibition by RGS2. The observed inhibition of Akt activation may give some clue as to how RGS2 inhibits Flt3-mediated transformation. RGS2 inhibits the function of several Gα subunits. However, its best-characterized interaction partner is Gαq.14,15 Gαq has been shown to be involved in cellular transformation,44,45 for example by PI3-kinase–mediated Akt activation.46-48 Thus, the modulation of cytoplasmic RGS2 levels could regulate Gαq activity and thus serve as a point of convergence of receptor tyrosine kinase (RTK) and G-protein–coupled signaling in mitogenic pathways. Crosstalk between RTKs and receptors coupled to heterotrimeric G-proteins has frequently been observed, often in models relating to carcinogenesis (reviewed by Wetzker and Bohmer49 ). However, so far little is known about G-protein–coupled receptor function in leukemic transformation.
We have recently shown that the activation of Wnt pathways plays an important role in leukemic transformation.50 Therefore, we were especially intrigued by earlier reports that RGS2 has severe inhibitory effects on the Wnt signaling pathway in Xenopus development. Gsk3-β, a key enzyme in this pathway regulating the free pool of β-catenin, is a direct downstream target of Akt.42 Indeed, in our model, Flt3-ITD induced strong phosphorylation of Gsk3-β that was significantly dependent on RGS2 down-regulation.
Another important finding of the current report is the effect of RGS2 on myeloid differentiation. RGS2 has been shown to be widely expressed in mature28 and immature29-31 hematopoietic cells including very early hematopoietic stem cells. From this expression pattern, a role in myeloid differentiation cannot be easily deducted. However, the data presented here show a strong correlation of RGS2 expression and myeloid differentiation in several leukemia cell line models. Also, the reconstitution of c/EBPα expression and of the 32D cell differentiation capacity by forced overexpression of RGS2 established the functional role of RGS2 in myeloid differentiation.
While our data clearly indicate that RGS2 repression is necessary for Flt3-ITD–mediated repression of differentiation, it seems unlikely that it is sufficient. In RGS2 knockout mice, myelopoiesis is not obviously affected.19 One possibility could be that Flt3 mutations inhibit c/EBPα by switching off several redundant pathways that can induce its expression. Enforced expression of RGS2 could reactivate one of these pathways and reinstitute the differentiation capacities. Alternatively, RGS2 might be part of a positive feedback loop: our results indicate that RGS2 is induced during myeloid differentiation, a process that is enhanced by its own presence. Since RGS2 is a known inhibitor of G-protein signaling, this hypothesis points to a possible role of heterotrimeric G-proteins in blocking c/EBPα expression and thus differentiation. Alternatively, RGS2 might be directly involved in nuclear processes regulating gene transcription. Indeed, immunofluorescence studies revealed its predominant localization in the nucleus.51 Other members of the RGS protein family were described to be directly involved in the regulation of transcription, and a more general role of heterotrimeric G-proteins in the nucleus is emerging.52
We and others have previously reported that Flt3-ITD can block myeloid differentiation in 32D cells by repression of c/EBPα expression.12,43 Others have shown that in human cells, Flt3-ITD induced mitogen-activated protein (MAP) kinase–mediated phosphorylation of c/EBPα and thus inhibition of its function.53 Here, we extend the observation of the effects of Flt3-ITD to NB4 cells, a human acute promyelocytic leukemia (APL) cell line. The analyses of RGS2 expression in these cell lines suggest a possible point of convergence of Flt3-ITD and PML/RARα function. Both proteins repress RGS2 since upon treatment with ATRA, RGS2 is strongly induced but only in the absence of Flt3-ITD. Since Flt3-ITD and PML/RARα have been shown to cooperate in a mouse APL model,11 experiments analyzing the role of RGS2 in this cooperation would be highly interesting.
In conclusion, we describe a new function for a regulator of G-protein signaling, RGS2, as an important mediator of Flt3-dependent leukemic transformation. Our results indicate that signals elicited by mutationally activated receptor tyrosine kinases are closely connected to G-protein signaling in the pathogenesis of AML.
Prepublished online as Blood First Edition Paper, November 9, 2004; DOI 10.1182/blood-2004-03-0940.
Supported by grants from the Deutsche Forschungsgemeinschaft (Se 600/2, Se 600/3, and Mü 1328/2) and the Innovative medizinische Forschung (IMF) Program (Sc119341) at the University of Münster.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Beate Surmann and Marion Baas for their excellent technical assistance and the AML Cooperative Group (AMLCG) and the Süddeutsche Hämoblastose Gruppe (SHG) study groups for providing leukemic samples. SU11248 was kindly provided by Sugen and Pfizer.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal