Abstract
Bone marrow transplantation blocks diabetes pathogenesis and reverses autoimmunity in nonobese diabetic (NOD) mice. However, there is a greater barrier to engraftment in the context of autoimmunity. In the present study, we characterized which recipient cells influence engraftment in prediabetic NOD mice, with the goal to replace myelotoxic conditioning with antigen-specific deletion of reactive host cells. Preconditioning of NOD mice with anti-CD8 and anti-CD154 monoclonal antibodies (mAbs) synergistically enhanced engraftment and significantly reduced the minimum total body irradiation (TBI) dose for engraftment. Strikingly, preconditioning with anti-CD4 mAb significantly impaired engraftment, negating the beneficial effect of anti-CD8, and resulted in a requirement for more TBI-based conditioning compared with controls conditioned with TBI alone. Similarly, more TBI was required when anti–T-cell receptor β (TCRβ) mAb was administered as preconditioning. The addition of anti-CD152 to CD154 preconditioning abrogated the engraftment-enhancing effect of anti-CD154. Taken together, these data indicate a role for CD4+ regulatory T cells in vivo which require signaling via CD152 in the induction of chimerism and tolerance in NOD recipients. Notably, disease prevention and reversal of autoimmunity was absolutely correlated with the establishment of chimerism. These studies have important implications for the design of novel clinical approaches to treat type 1 diabetes.
Introduction
Type 1 diabetes is an immune-mediated disorder associated with defects in the hematopoietic stem cell (HSC) and/or its progeny. A number of autoimmune diseases have been successfully treated via bone marrow transplantation (BMT) in rodents and humans.1-3 Both full2,4 and mixed5 allogeneic chimerism interrupts disease progression and reverses the autoimmune pathogenesis.2,4 Theoretically, BMT can be applied to treat type 1 diabetes via 2 mechanisms: (1) through reversal of the autoimmune processes that lead to islet cell destruction; and (2) through the induction of donor-specific tolerance to islet cell transplants. The morbidity and mortality associated with current conditioning methods has precluded the widespread use of BMT clinically.6
Conditioning for BMT has historically been believed to require cytoreduction to prepare vacant niches and immunosuppression to suppress host-versus-graft alloreactivity. Current cytoreductive approaches use agents with broad and nonspecific myelotoxicity. The relative contribution of myelotoxic agents to preparing vacant niches versus controlling the host-versus-graft response is currently controversial. Recent data from nonmyeloablative conditioning models in nonautoimmune recipients suggest that the main role of myelotoxic agents is to control host-versus-graft alloreactivity rather than prepare vacant niches,7,8 leading to the possibility of developing more benign antigen-driven conditioning strategies. For example, when total body irradiation (TBI) is used as the sole conditioning agent for allogeneic BMT in mice, a minimum of 700 cGy TBI is required.9 The administration of cyclophosphamide 2 days after relative BMT reduces the TBI to 500 cGy.10 The addition of both anti-CD8 and anti–T-cell receptor β (TCRβ) mAb treatment to the conditioning approach decreases the TBI dose to as low as 300 cGy and eliminates the requirement for cyclophosphamide.7 Blocking CD40-CD40L costimulation through anti-CD154 mAb decreases TBI dose to 400 cGy,11 and the addition of cyclosporine A further decreases the TBI dose to 200 cGy.12
Nonobese diabetic (NOD) mice share with humans with type 1 diabetes at least 20 genetic loci associated with diabetes.13,14 The underlying autoimmunity is associated with a number of features that differ from normal mice.15 NOD mice require higher cell doses for engraftment, and the minimal effective TBI dose required to obtain allogeneic engraftment is significantly higher compared to disease-resistant mouse strains.2 In addition, anti-CD154 mAb treatment of NOD mice only decreases the TBI dose to 600 cGy, and only 67% of the treated recipients engraft.11 We therefore hypothesized that specific cell populations in NOD mice are responsible for the increased resistance to allogeneic engraftment.
In the present studies, we examined whether targeting of host natural killer (NK) cells and T cells would reduce the TBI dose required for engraftment in NOD mice. We report that preconditioning of NOD mice with anti-NK cell mAb does not enhance engraftment, in contrast with normal mice; however, the treatment of NOD mice with anti-CD8 mAb and/or costimulatory blockade significantly enhances engraftment and decreases the minimum dose of TBI required. Surprisingly, the elimination of recipient αβ-TCR+ T cells significantly impaired engraftment in NOD mice, in contrast with the enhanced engraftment observed after αβ-TCR+ mAb preconditioning in nonautoimmune mice.7 Similarly, treatment of the NOD recipients with anti-CD4 mAb abrogated the engraftment-enhancing effect of anti-CD8 mAb treatment alone and actually increased the minimum TBI dose to above that for TBI alone. The combination of anti-CD152 and anti-CD154 mAb similarly abrogated the increased engraftment observed when either reagent was used alone. Taken together, these data implicate a role for CD4+/CD25+ regulatory T cells in establishing chimerism and tolerance in vivo. Animals that became chimeric did not progress to diabetes, had reversal of insulitis, and exhibited suppression of autoreactivity in vitro. The development of mechanistically driven nonmyeloablative conditioning approaches will significantly impact the clinical application of chimerism for treatment of type 1 diabetes but must take into account the influence of the underlying autoimmune process.
Materials and methods
Animals
Seven-week-old female NOD (H-2d) mice were purchased from Taconic Farms (Germantown, NY) and B10.BR SgSnJ (B10.BR; H-2k) mice from The Jackson Laboratory (Bar Harbor, ME). Animals were housed in a barrier facility at the Institute for Cellular Therapeutics, University of Louisville.
Reagents and antibodies
Monoclonal antibodies against mouse CD8 (53-6.7), CD4 (GK1.5), TCRβ (H57-597), and NK (NK1.1) cells were purchased from Pharmingen (San Diego, CA). Anti-NK (DX5), Ly49C and Ly49I (5E6), Thy1.2 (30-H12), CD154 (MR1), and CD152 (UC10-4F10-11) antibodies were produced and purified at the Institute for Cellular Therapeutics. These antibodies were used for in vivo treatment with the antibody dose and timing in relation to BMT evaluated in preliminary experiments as described in Current Protocols in Immunology.16 Flow cytometric typing of the peripheral blood lymphocytes used anti–H-2Kd fluorscein isothiocyanate (FITC) (SF1-1.1) and anti–H-2Kk phycoerythrin (PE) (AF3-12.1) mAbs (Pharmingen).
In vivo mAb preconditioning
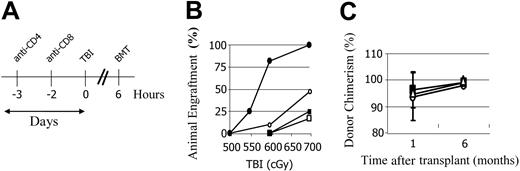
Recipients were given intraperitoneal injections of selected mAb or saline on day -3 or day -2 relative to BMT unless otherwise stated (500-700 μg anti-CD8 mAb, 300 μg anti-CD4 mAb, or a combination of both mAbs). Other recipients were pretreated with 800 to 1000 μg anti-CD154 (depending on the lot of mAb used) on day 0 or 500 μg anti-CD152 mAb on day +2.
Chimera preparation
Chimeras were prepared by a modification of the method previously described.2 Briefly, female NOD (H2g7) recipients were irradiated with a single dose (500-950 cGy) of TBI from a cesium source (Nordion, Ottawa, Ontario, Canada). The femurs and tibiae of donor B10.BR mice were flushed using chimera media (CM; Medium 199 containing 50 μg/mL gentamicin; both from Life Technologies, Grand Island, NY) resuspended via gentle aspiration through an 18-gauge needle and passed through sterile 100 μm nylon mesh (Sefar America, Kansas City, MO). Cells were washed in CM, counted, and resuspended to 30 × 106 cells/mL in CM. Cells (30 × 106) were administered via the lateral tail vein 4-6 hours after irradiation using a 27-gauge needle.
Typing of chimeras
Recipients were analyzed for donor B10.BR (H-2k) and recipient NOD (H-2d) chimerism as previously described.15 Briefly, peripheral blood was collected into heparinized vials, mixed, and aliquoted (100 μL/tube). Cells were stained with directly conjugated mAb for 30 minutes at 4°C in the dark. Red blood cells were lysed using ammonium chloride lysis solution (0.16 M NH4Cl, 10 mM KHCO3, 1 × 10-4 M EDTA [ethylenediaminetetraacetic acid]). Analyses were performed via flow cytometry (FACSCalibur; Becton Dickinson, Mountain View, CA) using CellQuest software (Becton Dickinson).
Lineage-specific mAbs used for flow cytometry included (CD marker, clone): CD4-FITC (H129.19); CD8-allophycocyanin (APC) (53-6.7); CD45R/B220-FITC (RA3-6B2); Ly-6G/GR-1–FITC (RB6-8C5); CD11b-FITC (M1/70); and Pan-NK/DX5-FITC (Pharmingen). Because NOD mice do not express NK1.1, the pan–NK cell marker DX5 was used.
Grading for insulitis
Pancreata were removed from the mice after killing and fixed immediately in 10% buffered formalin. Sections (0.7 μm) were prepared and stained with hematoxylin & eosin (H&E). The stained sections were then assessed for degree of infiltration into the islets in a blinded fashion as per Kagi et al.17 At least 40 randomly chosen islets were assessed per sample and insulitis was scored as follows: 0 indicates no insulitis; 1, mild insulitis (a weak peripheral inflammatory infiltration); 2, peri-insulitis (an infiltration surrounding, but not penetrating the islet architecture); 3, moderate insulitis (a penetrating infiltrate that covers < 50% of the islet area); and 4, severe insulitis (a penetrating infiltrate that covers > 50% of the islet area).
Monitoring for diabetes
Animals were tested weekly for glycosuria using Chemstrip uGK test strips (Roche, Indianapolis, IN). Any animal that tested positive was tested daily. An animal was considered diabetic after 3 consecutive positive urine tests. Animals with sustained (1 week) diabetes were killed and the native pancreas examined histologically and scored for insulitis.
Spleen-cell suppressor assay
The ability of splenocytes to suppress autoimmune or allogeneic MLR responses was assessed as detailed.18 Briefly, 0.1 × 106 naive NOD spleen cells were incubated with 0.1 × 106 irradiated (2500 cGy) stimulator spleen cells from NOD mice (1:1 ratio). To these cultures, either 0.1 × 106 or 0.05 × 106 spleen cells obtained from NOD transplant recipients were added for a final responder cell-to-spleen cell ratio to cells assayed for regulatory function of 1:1:1 or 1:1:0.5, respectively. Triplicate wells were cultured for a total of 90 hours and 0.037 MBq (1 μCi)/well [3H]-thymidine included in the culture for the final 18 hours. Cultures were harvested onto glass filters and radioactive counts enumerated using a 1205 Betaplate scintillation counter (Wallac, Gaithersburg, MD).
Statistics
Statistics applied were from the SASJMP statistical program (SAS Institute, Cary, NC). Nonparametric Wilcoxon/Kruskal-Wallis, Student t test, and analysis of variance (ANOVA) were employed to determine the statistical significance. Nonparametric Kaplan-Meier methods were used to generate survival plots. A P value of less than .05 was considered statistically significant. A logistic regression model was fit to the engraftment data using Stata/SE 8.2 software (StatacorpLP, College Station, TX).
Results
NOD mice express a skewed NK subfamily repertoire
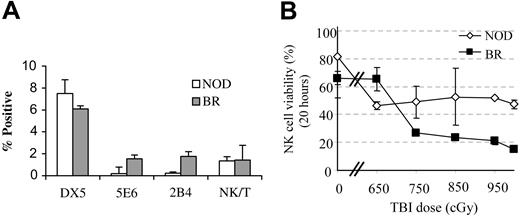
NK cells have been shown to play a significant role in engraftment in mice and humans.19,20 We therefore first evaluated NK cell subfamilies in the bone marrow of NOD mice. As previously reported, NOD mice lack NK1.1+ cells, as well as the 5E6 and 2B4+ NK cell subfamilies (Figure 1A). Only DX5+ (pan-NK) and NK/T cells are present.21,22
NK-cell subfamily analysis and radiation sensitivity. (A) Bone marrow from NOD mice (□) and age-matched B10.BR controls (BR; ▦) was stained for the various subfamilies of NK cells (5E6, 2B4) and pan-NK (DX5, NK1.1) markers as well as NK/T cells (DX5+/αβTCR+) and the relative frequency of the populations was enumerated by flow cytometry. A minimum of 5 animals was analyzed per group. Values represent mean ± SD of positive cells in the lymphoid gate. (B) Radiation sensitivity of NK cells from NOD marrow (⋄) compared with B10.BR controls (▪). Error bars indicate ± SD of averages.
NK-cell subfamily analysis and radiation sensitivity. (A) Bone marrow from NOD mice (□) and age-matched B10.BR controls (BR; ▦) was stained for the various subfamilies of NK cells (5E6, 2B4) and pan-NK (DX5, NK1.1) markers as well as NK/T cells (DX5+/αβTCR+) and the relative frequency of the populations was enumerated by flow cytometry. A minimum of 5 animals was analyzed per group. Values represent mean ± SD of positive cells in the lymphoid gate. (B) Radiation sensitivity of NK cells from NOD marrow (⋄) compared with B10.BR controls (▪). Error bars indicate ± SD of averages.
We then compared the radiation sensitivity of bone marrow NK cells in NOD mice with normal controls. Bone marrow cells were exposed to increasing doses of irradiation (650 cGy-1000 cGy) and cultured for 20 hours. Viability was determined by dye exclusion. At high irradiation doses (≥ 700 cGy), the percentage of viable NK cells left was significantly higher (P ≤ .05) in NOD mice compared with disease-resistant B10.BR controls (Figure 1B).
Targeting of recipient NK-cell subpopulations does not enhance engraftment in NOD mice
Because NOD mice are relatively resistant to engraftment with either syngeneic or allogeneic cells,15 we sought to identify the cell type(s) that mediate this resistance. NOD mice were preconditioned 2 days prior to BMT with anti-DX5, Thy1.2, or CD8 mAbs. They then received 700 cGy TBI 4 to 6 hours prior to BMT with 30 × 106 B10.BR bone marrow cells. Preconditioning with the anti-NK cell mAb did not enhance engraftment of allogeneic B10.BR bone marrow cells in NOD recipients (Table 1), nor did anti-Thy1.2 mAb, a marker expressed on NK cells as well as T cells. In contrast, anti-CD8 mAb preconditioning significantly enhanced engraftment.
Anti-CD8 mAb preconditioning but not anti-NK reduces the effective dose of TBI
mAb . | No. animals . | Engraftment, % . | Donor chimerism in PB of engrafted animals, % ± SD . | P compared with TBI alone . |
|---|---|---|---|---|
| None | 10 | 0.0 | NA | NA |
| anti-DX5 | 4 | 0.0 | NA | NS |
| anti-Thy1.2 | 6 | 0.0 | NA | NS |
| anti-CD8 | 9 | 88.9 | 93.6 ± 2.4 | < .001 |
mAb . | No. animals . | Engraftment, % . | Donor chimerism in PB of engrafted animals, % ± SD . | P compared with TBI alone . |
|---|---|---|---|---|
| None | 10 | 0.0 | NA | NA |
| anti-DX5 | 4 | 0.0 | NA | NS |
| anti-Thy1.2 | 6 | 0.0 | NA | NS |
| anti-CD8 | 9 | 88.9 | 93.6 ± 2.4 | < .001 |
NOD mice were treated with the indicated mAb 2 days prior to conditioning with 700 cGy TBI. Allogeneic B10.BR bone marrow cells (BMCs) were subsequently administered, and the mice were monitored for engraftment and chimerism at 28 days. NA indicates not applicable; NS, nonsignificant.
Anti-CD8 preconditioning enhances allogeneic engraftment
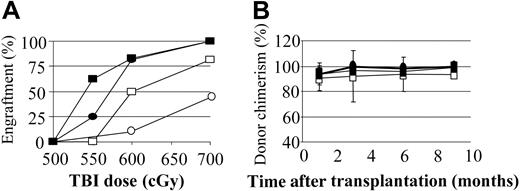
We previously reported that preconditioning of nonautoimmune mice with anti–T-cell mAbs to induce host-versus-graft hyporesponsiveness significantly reduced the need for myelotoxic conditioning. We therefore examined whether a similar result would occur for prediabetic NOD mice. Preconditioning of NOD mice with anti-CD8 mAb on day -2 significantly enhanced engraftment, with 100% (10 of 10) of mice engrafting with 700 cGy of TBI and 77% (11 of 14) with 600 cGy TBI. In contrast, the majority of controls conditioned with 700 cGy or less of TBI did not engraft (Figure 2B).
Anti-CD8 mAb treatment decreases the minimum TBI dose required for allogeneic engraftment in NOD mice, while anti-CD4 impairs conditioning. (A) Mice were preconditioned with anti-CD8 mAb on day -3, and/or anti-CD4 mAb on day -2 relative to the TBI. Recipients received transplants of 30 × 106 B10.BR BMCs 4 to 6 hours after TBI. A minimum of 10 animals per group in at least 3 separate experiments is shown. (B) Percentage of recipients with engraftment at 28 days. ○ indicates no mAb; •, anti-CD8; □, anti-CD4; and ▪, anti-CD8 plus anti-CD4. (C) The percentage of donor cell chimerism in the mice that engrafted with anti-CD8 preconditioning 1 and 6 months after transplantation. ○ indicates recipients of 700 cGy TBI; ▪, 600 cGy; ▵, 550 cGy. Error bars indicate ± SD.
Anti-CD8 mAb treatment decreases the minimum TBI dose required for allogeneic engraftment in NOD mice, while anti-CD4 impairs conditioning. (A) Mice were preconditioned with anti-CD8 mAb on day -3, and/or anti-CD4 mAb on day -2 relative to the TBI. Recipients received transplants of 30 × 106 B10.BR BMCs 4 to 6 hours after TBI. A minimum of 10 animals per group in at least 3 separate experiments is shown. (B) Percentage of recipients with engraftment at 28 days. ○ indicates no mAb; •, anti-CD8; □, anti-CD4; and ▪, anti-CD8 plus anti-CD4. (C) The percentage of donor cell chimerism in the mice that engrafted with anti-CD8 preconditioning 1 and 6 months after transplantation. ○ indicates recipients of 700 cGy TBI; ▪, 600 cGy; ▵, 550 cGy. Error bars indicate ± SD.
Preconditioning of NOD mice with anti-CD4 mAb significantly impairs chimerism
We next examined whether targeting of NOD CD4+ cells would further reduce conditioning. NOD mice received either saline, anti-CD8 mAb, anti-CD4 mAb, or a combination of both anti-CD8 mAb and anti-CD4 mAb 2 to 3 days prior to TBI (Figure 2A). Strikingly, engraftment of mice preconditioned with anti-CD4 mAb was significantly impaired compared with NOD mice conditioned with TBI alone (Figure 2B). Moreover, when anti-CD4 mAb was administered with anti-CD8 mAb, the engraftment-enhancing effect of anti-CD8 mAb was completely lost and engraftment in that group was impaired compared with those without mAb preconditioning. Approximately 50% (10 of 20) of the untreated NOD mice engrafted after conditioning with 700 cGy TBI, whereas only 20% (2 of 10) of the anti-CD4–treated NOD mice engrafted at this TBI dose, regardless of the inclusion of anti-CD8 mAb (Figure 2B).
We previously reported that when mixed chimerism is established ablatively in NOD mice, the percentage of donor chimerism progressively increases until the donor cell engraftment approaches 100% by 4 months.15 Similarly, nonmyeloablatively conditioned NOD chimeras exhibited this same phenotype: the B10.BR → NOD chimeras, which were initially mixed, became 95% or more donor by 6 months (Figure 2C).
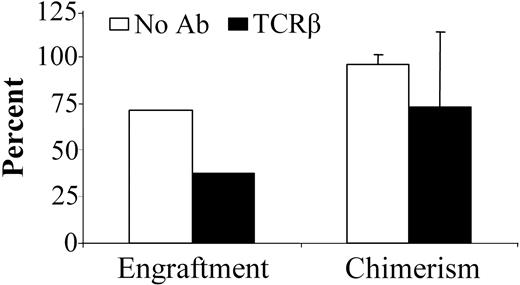
Anti-TCRβ mAb pretreatment of NOD mice impairs engraftment
We previously reported that anti-TCRβ mAb preconditioning significantly decreases the TBI dose for durable engraftment in normal mice.7 Strikingly, when NOD mice were preconditioned with anti-TCRβ mAb, significantly impaired engraftment was observed. As for anti-CD4 mAb preconditioning, a higher TBI dose was required compared with TBI-only treated recipients (Figure 2B). With 700 cGy TBI conditioning, 5 of 7 NOD mice engrafted without mAb treatment. In contrast, only 3 of 8 treated with the anti-TCRβ mAb engrafted at this TBI dose (Figure 3). Anti-TCRβ mAb treatment of recipients did not significantly influence level of allogeneic chimerism in the animals with engraftment. Of the recipients that engrafted, untreated NOD and anti-TCRβ mAb-treated NOD mice exhibited 95.7% ± 5.3% and 72.3% ± 41.9% donor chimerism, respectively, after conditioning with 700 cGy TBI.
Treatment of NOD mice with anti-TCRβ mAb significantly impairs allogeneic engraftment. NOD mice received vehicle (□; n = 7) or anti-TCRβ mAb (▪; n = 8) on day -3 prior to conditioning with 700 cGy TBI. Allogeneic B10.BR BMCs were subsequently administered, and the mice were monitored for engraftment and chimerism. Chimerism data presented is from 28 days after transplantation. Data represents the means ± SD of combined data obtained from 2 separate experiments.
Treatment of NOD mice with anti-TCRβ mAb significantly impairs allogeneic engraftment. NOD mice received vehicle (□; n = 7) or anti-TCRβ mAb (▪; n = 8) on day -3 prior to conditioning with 700 cGy TBI. Allogeneic B10.BR BMCs were subsequently administered, and the mice were monitored for engraftment and chimerism. Chimerism data presented is from 28 days after transplantation. Data represents the means ± SD of combined data obtained from 2 separate experiments.
Costimulatory blockade enhances engraftment in NOD mice
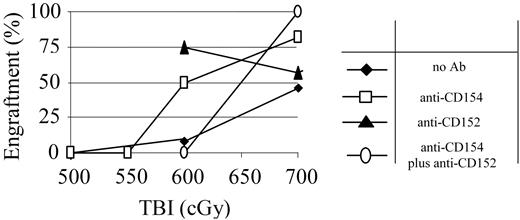
Blockade of costimulatory molecules decreases the TBI conditioning required in normal mice, and anti-CD154 mAb has been shown to decrease the TBI dose required in NOD mice.11 We therefore assessed the ability of anti-CD154 and anti-CD152 mAb to further reduce the TBI dose required in NOD mice via inducing immune deviation. NOD recipients were treated with either anti-CD154 mAb on day 0 or anti-CD152 mAb on day +2 with respect to TBI and BMT. Treatment with either anti-CD154 or anti-CD152 mAb significantly decreased the minimal TBI dose required for engraftment when these mAbs were used singly. Anti-CD154 mAb-conditioned animals exhibited a relatively small increase in engraftment compared with untreated NOD mice (82% vs 48%) when 700 cGy TBI was administered (Figure 4). However, when the TBI was lowered to 600 cGy TBI, 50% of the anti-CD154 conditioned recipients engrafted, while only 1 of 16 TBI-only conditioned recipients engrafted. Anti-CD152 mAb treatment also effectively enhanced engraftment in NOD mice treated with 600 cGy TBI. Notably, when these 2 mAbs were used in combination, no synergism was detected. Although engraftment at the 700 cGy dose was 100%, when the TBI dose was decreased to 600 cGy, the beneficial effect of either antibody alone was lost and, in fact, engraftment was significantly impaired compared with TBI-treated controls (Figure 4).
Costimulatory blockade with anti-CD154 or anti-CD152 mAb potentiates conditioning. NOD mice were pretreated with anti-CD154 (□), anti-CD152 mAb (▴), or the combination of both mAbs (○) and conditioned with decreasing doses of TBI and transplanted with 30 × 106 B10.BR BMC 4-6 hours after TBI.  indicates no mAb. The percentage of recipients that engrafted at each TBI dose is shown for each preconditioning approach. n = at least 8 animals per group.
indicates no mAb. The percentage of recipients that engrafted at each TBI dose is shown for each preconditioning approach. n = at least 8 animals per group.
Costimulatory blockade with anti-CD154 or anti-CD152 mAb potentiates conditioning. NOD mice were pretreated with anti-CD154 (□), anti-CD152 mAb (▴), or the combination of both mAbs (○) and conditioned with decreasing doses of TBI and transplanted with 30 × 106 B10.BR BMC 4-6 hours after TBI.  indicates no mAb. The percentage of recipients that engrafted at each TBI dose is shown for each preconditioning approach. n = at least 8 animals per group.
indicates no mAb. The percentage of recipients that engrafted at each TBI dose is shown for each preconditioning approach. n = at least 8 animals per group.
Preconditioning with anti-CD8 and anti-CD154 mAb synergistically reduces myelotoxic conditioning
In order to determine whether the anti-CD8 or anti-CD154 mAb pretreatment had nonoverlapping specificities or worked via similar mechanisms, NOD mice were treated with both anti-CD8 (day 3) and anti-CD154 (day 0) mAbs and the lowest effective TBI dose determined. The combined treatment increased engraftment in NOD mice at both the 700 cGy and 600 cGy doses over either mAb used singly (P < .001; Figure 5A). By logistic regression analysis, mice pretreated with the combined therapy were found to be 143 times more likely to engraft than those receiving only anti-CD154 or only anti-CD8 and 24.9 times more likely to engraft than with TBI alone. Moreover, this is the first treatment that allowed allogeneic engraftment in NOD mice at a TBI dose less than 600 cGy (550 cGy; 5 of 8). Therefore, the combination of preconditioning with both anti-CD8 and anti-CD154 mAbs synergizes to enhance engraftment and reduce the requirement for myelotoxic agents such as TBI. The percentage chimerism ranged from 90% to 100% donor (Figure 5B).
Combination treatment of NOD mice with anti-CD8 and anti-CD154 mAb further enhances engraftment and reduces the minimum TBI dose to establish chimerism. Percentage of engraftment (A) and chimerism (B) obtained in NOD BMT mice treated with either anti-CD8 mAb on day -3(•), anti-CD154 mAb on day 0 (□), or the combination of both mAbs (▪). ○ indicates no mAb. n = at least 6 and up to 21 animals per group. Error bars indicate ± SD.
Combination treatment of NOD mice with anti-CD8 and anti-CD154 mAb further enhances engraftment and reduces the minimum TBI dose to establish chimerism. Percentage of engraftment (A) and chimerism (B) obtained in NOD BMT mice treated with either anti-CD8 mAb on day -3(•), anti-CD154 mAb on day 0 (□), or the combination of both mAbs (▪). ○ indicates no mAb. n = at least 6 and up to 21 animals per group. Error bars indicate ± SD.
Anti-CD8 preconditioning significantly deletes CD11c+/CD8+ dendritic cells (DCs) and CD11c+/TCR-/CD8+ DCs from host marrow
In order to define which host bone marrow cells (BMCs) were targeted by the anti-CD8 mAb preconditioning, NOD mice were conditioned with 700 cGy TBI alone or after the mAb pretreatment (n = 5 per group). Notably, a significantly higher number of CD11c+ cells (P < .05) were apoptotic in the anti-CD8 treated group compared with TBI alone (CD11c+/TCR-/CD8+ pre-DCs and CD11c+ DC: 25.3% ± 3.1% vs 18.4% ± 3.5% and 27.0% ± 4.9% vs 17.6% ± 5.8%, respectively). The NK/T, CD8+/TCR- facilitating cell (FC), NK, CD8- B cell, and CD11c+/B220+ cell populations were not significantly affected (data not shown).
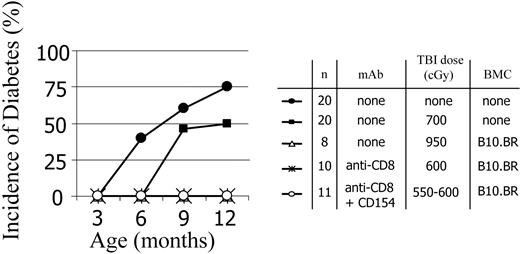
Chimeric NOD mice do not develop diabetes
We next asked whether less aggressive conditioning would still be associated with protection from diabetes. Age-matched control NOD mice as well as conditioned NOD mice that did not receive transplants were monitored for development of diabetes. As expected, naive NOD mice developed diabetes as early as 6 months of age, with an overall 75% progression to diabetes by 12 months (Figure 6). Control ablatively conditioned B10.BR → NOD chimeras did not develop diabetes. Partially conditioned mAb-treated B10.BR → NOD chimeras prepared by 2 approaches did not develop diabetes (Figure 6), indicating that this transplantation strategy is effective at terminating the active diabetes-promoting processes in NOD mice. Although NOD mice that were conditioned with 700 cGy TBI but did not undergo allogeneic BMT were slightly delayed in disease progression, diabetes did occur. The NOD mice conditioned with only 600 cGy TBI, which did not receive anti-CD8 mAb or BMC progressed to diabetes with a kinetic similar to unmanipulated controls (data not shown).
Diabetes progression in age-matched naive and conditioned NOD mice that did/did not receive transplants. Chimeras were prepared using anti-CD8 mAb preconditioning or anti-CD8 mAb plus anti-CD154 plus TBI. The progression to diabetes was monitored in control mice and mice given TBI conditioning doses in the absence and presence of mAb treatments. For all treatment groups, the presence of chimerism was associated with protection from diabetes.
Diabetes progression in age-matched naive and conditioned NOD mice that did/did not receive transplants. Chimeras were prepared using anti-CD8 mAb preconditioning or anti-CD8 mAb plus anti-CD154 plus TBI. The progression to diabetes was monitored in control mice and mice given TBI conditioning doses in the absence and presence of mAb treatments. For all treatment groups, the presence of chimerism was associated with protection from diabetes.
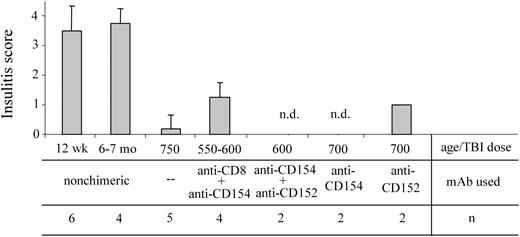
Chimerism established by nonmyeloablative conditioning reverses insulitis in NOD mice with active autoimmunity
We previously reported that when chimerism is established in NOD recipients using ablative conditioning, intra-islet insulitis is resolved.2,15 NOD chimeras may still exhibit peri-insulitis (the lymphocytic infiltrating cells remain outside the islet architecture [grades 1 and 2]), the more aggressive intra-insulitis is reversed.15 In order to determine the effect of nonmyeloablative conditioning on the occurrence of autoimmune insulitis in NOD mice, pancreatic tissue samples from chimeric recipients were analyzed histologically for the extent of insulitis (Figure 7). As expected, the pancreata obtained from 12-week-old NOD mice exhibit moderate to severe insulitis in our colony. However, for as long as 15 months after BMT with 550 cGy TBI and anti-CD8 plus anti-CD154 mAb treatment, no severe insulitis was detected in the chimeras. Only mild to moderate peri-insulitis (grades 1 or 2) was present in any of the treatment groups where chimerism was established.
Nonmyeloablatively conditioned NOD recipients with chimerism exhibit decreased insulitis. The pancreata from 12-week-old and 6- to 7-month-old naive NOD mice as well as from chimeric NOD mice conditioned as indicated, were harvested and fixed in 10% buffered formalin. H&E-stained sections of each pancreas were read by a pathologist in a blinded fashion and each were assigned an insulitis score as noted in “Materials and methods.” Results shown are the mean score ± SD of evaluated sections; n.d. indicates no insulitis detected in the evaluated sections. Error bars indicate ± SD.
Nonmyeloablatively conditioned NOD recipients with chimerism exhibit decreased insulitis. The pancreata from 12-week-old and 6- to 7-month-old naive NOD mice as well as from chimeric NOD mice conditioned as indicated, were harvested and fixed in 10% buffered formalin. H&E-stained sections of each pancreas were read by a pathologist in a blinded fashion and each were assigned an insulitis score as noted in “Materials and methods.” Results shown are the mean score ± SD of evaluated sections; n.d. indicates no insulitis detected in the evaluated sections. Error bars indicate ± SD.
Analysis of regulatory T cells (Treg)
Thus far, we have determined that the minimum effective TBI dose for obtaining allogeneic engraftment in NOD mice is most significantly influenced by preconditioning with anti-CD8 and anti-CD154 mAbs, either alone or in combination, but impaired by anti-CD4 or anti-TCRβ pretreatment. Taken together, these results pointed to a possible role for Treg, the best characterized of which are CD4+/CD25+ T cells, for successful establishment of chimerism. The inhibition of CD4 would theoretically eliminate antigen recognition by CD4+ T cells, including CD4+/CD25+ T cells.23 With this in mind, we evaluated whether Treg were present in the anti-CD8 mAb/600 TBI chimeric mice. The results were compared with anti-CD8 plus anti-CD4 mAb/600 TBI-treated, nonchimeric mice.
In vitro proliferation assays were performed in which responder NOD spleen cells were incubated with irradiated NOD splenocytes as stimulator cells to determine whether the spleen cells from the chimeric mice exhibited suppressor activity. The spleen cells from the chimeric NOD mice specifically inhibited the autoantigen-specific proliferation of NOD splenocytes in the presence of irradiated NOD stimulator cells, while those from nonchimeric mice did not (Figure 8).
Spleen cells from chimeric NOD mice have suppressive activity against autoreactive cells. Naive NOD responder spleen cells (0.1 × 106) were harvested and plated with 0.1 × 106 irradiated NOD stimulator spleen cells. Stimulation index was then normalized to the proliferation detected by [3H]-thymidine incorporated in these cultures. To evaluate the effect of spleen cells from the various conditioned NOD BMT mice, spleen cells were harvested and added to the responder/stimulator cells either 0.1 × 106 spleen cells (1:1 ratio) or 0.05 × 106 spleen cells (1:0.5 ratio). The anti-CD8/600 TBI-treated mice and the no-mAb/750 TBI mice were all chimeric. None of the anti-CD8/αCD4/600 TBI treated mice were chimeric (nonchimeric), and an increase in stimulation is noted when these cells were added to the cultures. Spleen cells obtained from these NOD mice that received transplants were combined with the responder NOD spleen cells at either a 1:1 or a 1:0.5 ratio. Data represent the means obtained from 4 individual spleen cell preparations per BMT group obtained over 2 separate experiments. Horizontal line illustrates the controls on which the stimulation indexes were calculated. Cultures with means above the line proliferated more than the controls, whereas cultures with means below the line proliferated less than the controls.
Spleen cells from chimeric NOD mice have suppressive activity against autoreactive cells. Naive NOD responder spleen cells (0.1 × 106) were harvested and plated with 0.1 × 106 irradiated NOD stimulator spleen cells. Stimulation index was then normalized to the proliferation detected by [3H]-thymidine incorporated in these cultures. To evaluate the effect of spleen cells from the various conditioned NOD BMT mice, spleen cells were harvested and added to the responder/stimulator cells either 0.1 × 106 spleen cells (1:1 ratio) or 0.05 × 106 spleen cells (1:0.5 ratio). The anti-CD8/600 TBI-treated mice and the no-mAb/750 TBI mice were all chimeric. None of the anti-CD8/αCD4/600 TBI treated mice were chimeric (nonchimeric), and an increase in stimulation is noted when these cells were added to the cultures. Spleen cells obtained from these NOD mice that received transplants were combined with the responder NOD spleen cells at either a 1:1 or a 1:0.5 ratio. Data represent the means obtained from 4 individual spleen cell preparations per BMT group obtained over 2 separate experiments. Horizontal line illustrates the controls on which the stimulation indexes were calculated. Cultures with means above the line proliferated more than the controls, whereas cultures with means below the line proliferated less than the controls.
Absolute numbers of CD4+/CD25+ T cells were also enumerated in the pancreatic lymph nodes (LNs) and spleens in the anti-CD8 mAb/600 TBI chimeric mice and compared with the T cells in the anti-CD8 plus anti-CD4 mAb/600 TBI-treated, nonchimeric mice (Table 2). There were more CD4+/CD25+ cells present in the nonchimeric mice. However, because CD25 is also present on activated T cells and there is an abundance of activated T cells in the pancreatic LN during the active phase of autoimmunity against β-cell antigens, this finding could be explained by the fact that one would expect more CD4+/CD25+ T cells in the nonchimeric NOD due to the underlying autoimmune process.
Enumeration of CD4+/CD25+ cells in pancreatic lymph node
. | No. . | No. cells × 103/pan-LN ± SD . | % CD4+/CD25+ ± SD . |
|---|---|---|---|
| NOD | 3 | 28.9 ± 3.7 | 2.4 ± 0.7 |
| B10.BR | 3 | 26.1 ± 1.4 | 1.6 ± 0.3 |
| anti-CD8, chimeric | 3 | 8.2 ± 6.0 | 1.7 ± 0.8 |
| Nonchimeric anti-CD8 + anti-CD4 | 4 | 58.4 ± 4.8 | 3.3 ± 1.6 |
| Full conditioning, chimeric | 2 | 46.4 ± 2.4 | 1.6 ± 0.1 |
. | No. . | No. cells × 103/pan-LN ± SD . | % CD4+/CD25+ ± SD . |
|---|---|---|---|
| NOD | 3 | 28.9 ± 3.7 | 2.4 ± 0.7 |
| B10.BR | 3 | 26.1 ± 1.4 | 1.6 ± 0.3 |
| anti-CD8, chimeric | 3 | 8.2 ± 6.0 | 1.7 ± 0.8 |
| Nonchimeric anti-CD8 + anti-CD4 | 4 | 58.4 ± 4.8 | 3.3 ± 1.6 |
| Full conditioning, chimeric | 2 | 46.4 ± 2.4 | 1.6 ± 0.1 |
Pancreatic lymph nodes were harvested and digested with collagenase. Single-cell suspensions were then analyzed for expression of CD4 and CD25 by flow cytometry. The percentage of CD4+/CD25+ cells per pan-LN ± SD are represented.
Discussion
The NOD mouse has been a valuable model to study the in vivo influence of autoimmunity on therapeutic interventions in type 1 diabetes. Although mixed chimerism reverses insulitis in prediabetic NOD mice and is associated with disease prevention,2 NOD mice require more conditioning and higher marrow cell doses due to the associated autoimmunity.2 In evaluating the underlying mechanism for this alloresistance, we have identified a number of features that distinguish prediabetic NOD mice as BMT recipients. Although host T cells and NK cells play a critical role in allogeneic engraftment in normal recipients19 and targeting of these cells peritransplantation allows a significant reduction in myelotoxic conditioning,7,24 a similar outcome was not observed in the NOD mouse. Targeting of NK cells had no effect on lowering the TBI dose requirement for NOD, and the specific targeting of αβ-TCR+ T cells or CD4+ cells in the NOD had an unexpected adverse effect, resulting in a requirement for more TBI compared with controls conditioned with TBI alone. Strikingly, preconditioning of NOD mice with anti-CD8 mAb significantly reduced the minimum TBI dose required for engraftment, and the addition of anti-CD154 to the anti-CD8 preconditioning regimen synergized to further enhance engraftment. A mechanistic understanding of the role of conditioning for HSC engraftment will allow the replacement of nonspecific myelotoxic approaches with tailored antigen-specific ones to tip the balance in favor of a tolerance-inducing milieu.
T-cell stimulation in the presence of costimulatory blockade induces anergy or antigen-driven apoptosis of alloreactive cells.25-30 CD154 is rapidly upregulated on antigen-activated CD4+ T cells26-30 and blockade of CD154 in the context of antigen inactivates in vitro CD4+ T cell responses.28,31,32 The administration of anti-CD154 mAb with donor-specific transfusion (DST) leads to a depletion of alloreactive host CD8+ T cells and prolonged (but not permanent) survival of skin grafts.33 Wekerle et al previously reported that anti-CD154 conditioning with or without the coadministration of CTLA4-Ig resulted in a requirement for less myelotoxic conditioning for BMT in normal recipients.34 Of specific interest to our current observations regarding anti-CD4 mAb preconditioning in NOD mice, durably engrafted chimeras exhibited long-term deletion of donor-reactive CD4+ cells in peripheral blood lymphocytes (PBLs), spleen, and thymus. Moreover, the addition of anti-CD4 and anti-CD8 depleting T-cell mAb to anti-CD154 enhanced chimerism and tolerance in normal recipients of BMT in the Wekerle model.
The administration of anti-CD154 mAb has been previously shown to decrease conditioning in NOD mice,11 and our current observations confirm this. In our hands, pretreatment of NOD mice with anti-CD154 or anti-CD152 mAb effectively reduced the minimum TBI dose required for engraftment. In striking contrast with normal recipients, the combination of these agents negated the enhanced effect seen when either agent was used singly. These data are similar to the observations made by Taylor et al in normal mice, where host signaling via a CD152-dependent pathway to generate regulatory T cells was required for the engraftment-promoting effect of anti-CD154 mAb for bone marrow.12 Clearly, an understanding of the mechanism by which antigen-specific approaches promote HSC engraftment is critical to the potential clinical application of this approach, especially in autoimmune recipients.
In normal mice, the depletion of alloreactive CD8+ T cells does not enhance the engraftment-enhancing effect of anti-CD154.23 In some models, costimulatory blockade has in fact resulted in depletion of CD8+ alloreactive host cells.25 Interestingly, we found that preconditioning of NOD recipients with anti-CD8 mAb plus anti-CD154 significantly potentiated the engraftment-enhancing effect of either antibody alone, reducing the minimum effective TBI dose to 550 cGy TBI. These data would therefore suggest that the combination of anti-CD8 mAb plus anti-CD154 mAb is targeting nonoverlapping recipient alloreactive cell populations and/or perhaps more efficiently targeting and/or eliminating these cells in the autoimmune mice. Interestingly, the CD8+ population most influenced by anti-CD8 preconditioning was the CD11c+ dendritic cell lineage, in addition to conventional T cells. Our observations regarding a role for CD8+ host cells in tolerance induction are compatible with those of Iwakoshi et al, who demonstrated that CD8+ alloreactive host cells were the primary target of costimulatory blockade when transient tolerance to skin grafts was induced by donor-specific transfusion in the presence of anti-CD154.33 Notably, in their model, CTLA4-Ig abrogated the engraftment-enhancing effect of anti-CD154, as did depletion of CD4+ T cells. They concluded that their model required CD4+ cell signaling via the CD28-B7 pathway for generation of Treg. Our present data suggest a similar mechanism in conditioning of NOD mice for establishing chimerism, but implicate CD152 as the important signaling pathway. Theoretically, the binding of CTLA4-Ig to B7.1 or B7.2 on APC would inhibit the interaction of T cells with APC. Conversely, blocking of anti-CD152 on T cells would block T-cell–APC interactions and therefore negate the beneficial effect of either agent alone.
Preconditioning of NOD mice with anti-CD4 mAb had an unexpected adverse effect on conditioning, in distinct contrast with our previous experience in normal controls,35 as well as that of others.36 To our knowledge, this is the first demonstration that targeting specific host T cells resulted in an increased requirement for conditioning. Inclusion of anti-CD4 mAb in the conditioning regimen was associated with significantly impaired engraftment compared with TBI alone and also negated the engraftment-enhancing effect of anti-CD8 mAb preconditioning. Similarly, and in striking contrast with what we have observed for normal mice,7 preconditioning of NOD recipients with anti-TCR mAb significantly impaired engraftment, again resulting in a requirement for more TBI compared with recipients conditioned with TBI alone. Taken together, these data point to an important, if not critical, role for a subset of CD4+ T cells in the early events that determine success or failure of HSC engraftment in autoimmune recipients.
Taken together, these observations may be informative on the mechanism of tolerance induction in nonmyeloablatively conditioned NOD recipients, since the following specific approaches actually resulted in impaired conditioning compared with TBI alone: (1) deletion of host CD4+ cells; (2) combining anti-CD154 plus anti-CD152; (3) anti-TCRβ mAb treatment of the recipient. A common mechanism underlying these observations is that each of the approaches would disrupt existing or developing CD4+/CD25+ Treg.37-42 Moreover, in some models it has been reported that CD4+ T cells need to signal through CD152 for the development of a regulatory phenotype.12,23 The inhibition of CD152 signaling would therefore also inhibit the generation of Treg in anti-CD154 mAb conditioned BM transplant recipients if signaling via this pathway is the predominant mechanism for chimerism induction in autoimmune recipients.
NOD mice, as well as people with human type 1 diabetes, have been demonstrated to have defects in generating certain types of regulatory cells, including NK/T cells43 and CD4+/CD25+ Treg,44-46 which has been suggested to contribute to their autoimmune process. While in normal mice a number of redundancies are likely to be present in the Treg, subsets that would overcome the impact of transiently inhibiting or depleting one subset of Treg, the more skewed immune repertoire present in the NOD may make the deficits more evident. When in vitro assays for Treg were performed, we found that there was indeed an induction of a suppressor function in splenocytes from the chimeras. This suppressive effect was noted for the autoimmune response, where the naive NOD responder cells were inhibited in a dose-specific manner in response to autoantigen. The fact that there were more CD4+/CD25+ cells in nonchimeric NOD mice with active autoimmunity compared with chimeric controls is not surprising, since activated autoimmune cells would be included as well, and does not negate the strong indirect evidence in vivo for a role for Treg in engraftment.
Notably, anti–NK cell mAb preconditioning of NOD mice had no effect on the amount of TBI conditioning required to establish allogeneic BM cell engraftment. This is in contrast with normal mice in which NK cells, T cells, and NK/T cells have been shown to contribute to bone marrow graft rejection.19,47 Although NOD mice have normal numbers of NK cells, the cytolytic function of these cells is severely impaired.48 Similar impaired NK-cell function has been reported in humans with type 1 diabetes.21,49-51 It is therefore not surprising that NK cells are not the primary effector mediating the increased resistance to allogeneic BMC engraftment observed in the NOD mouse strain.
HSC chimerism has the potential to have tremendous impact on the cure of type 1 diabetes, both to reverse the autoimmune process and to induce tolerance to islet allografts. Although humans with type 1 diabetes in their honeymoon period have been cured of diabetes progression after BMT, the widespread clinical application of this approach is incumbent on development of less-toxic conditioning strategies. A specific definition of the critical host-versus-graft alloreactive populations and their mechanism of action as well as methods to induce transient immune deviation of these populations may provide a novel, nonmyelotoxic form of conditioning to allow translation of this approach to the clinic to benefit the millions living with type 1 diabetes, both for disease prevention and the induction of tolerance to islet allografts.
Prepublished online as Blood First Edition Paper, October 21, 2004 DOI 10.1182/blood-2004-04-1340.
Supported in part by grants from the National Institutes of Health (grant nos. DK52294 and R01 HL 63442) (S.T.I.), The Jewish Hospital Foundation, The Commonwealth of Kentucky Research Challenge Trust Fund, and the University of Louisville Hospital.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Laura Mills, Alan Gutowski, and Jennifer Tanner for technical assistance; Carolyn DeLautre and Kim Nichols for manuscript preparation; the staff of the Research Resources Facility at the University of Louisville for outstanding animal care; and Drs Christina Kaufman and Michele Kosiewicz for helpful suggestions and critical review of the manuscript.

![Figure 8. Spleen cells from chimeric NOD mice have suppressive activity against autoreactive cells. Naive NOD responder spleen cells (0.1 × 106) were harvested and plated with 0.1 × 106 irradiated NOD stimulator spleen cells. Stimulation index was then normalized to the proliferation detected by [3H]-thymidine incorporated in these cultures. To evaluate the effect of spleen cells from the various conditioned NOD BMT mice, spleen cells were harvested and added to the responder/stimulator cells either 0.1 × 106 spleen cells (1:1 ratio) or 0.05 × 106 spleen cells (1:0.5 ratio). The anti-CD8/600 TBI-treated mice and the no-mAb/750 TBI mice were all chimeric. None of the anti-CD8/αCD4/600 TBI treated mice were chimeric (nonchimeric), and an increase in stimulation is noted when these cells were added to the cultures. Spleen cells obtained from these NOD mice that received transplants were combined with the responder NOD spleen cells at either a 1:1 or a 1:0.5 ratio. Data represent the means obtained from 4 individual spleen cell preparations per BMT group obtained over 2 separate experiments. Horizontal line illustrates the controls on which the stimulation indexes were calculated. Cultures with means above the line proliferated more than the controls, whereas cultures with means below the line proliferated less than the controls.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/6/10.1182_blood-2004-04-1340/6/m_zh80060575480008.jpeg?Expires=1769189665&Signature=CMPZvNdPJRac6Crd2j1u845ofCS1~cJx7z4R-gXiBwzLsfWhSyp4MJ2HaoajQBXv0rMlC9SCHddXK-kelDNFSybWtN0S7Q6TdjX~W~akx~WL-iW54UTFQ745IpZ9rcnUOQye8IJIQhvt~RT4tvosCRAmU~0Y-sfHOvnfT74NExE1NojKCpdAOOlkldO4u2DwCR0TwaNxFQDYt0UQNqhSYhJXvTjABJEmbC2u9k79Oq157GnpAcuqu74TjnlqiahtoSDbkASYqvGWmRzX5iDWMK0ind3PtjnI4jW0YunbCAa4pVHy~f-3T71ZBfig5mxbRKWHltlY~1klTIROIVDVUw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal