Abstract
Myeloid-related protein 8 (MRP8) and MRP14, S100 proteins secreted by activated phagocytes, bind specifically to endothelial cells. The endothelial response to MRP8/MRP14, however, is unknown. Using oligonucleotide microarray analysis, we show for the first time that MRP8/MRP14 induce a thrombogenic, inflammatory response in human microvascular endothelial cells by increasing the transcription of proinflammatory chemokines and adhesion molecules and by decreasing the expression of cell junction proteins and molecules involved in monolayer integrity. All changes on the gene expression level could be confirmed using biochemical and functional assays. We demonstrated that the expression of MRP8/MRP14 closely correlated with the inflammatory activity in systemic vasculitis, confirming the important role of these proteins for distinct inflammatory reactions in endothelia. MRP8/MRP14 may represent novel targets for anti-inflammatory strategies.
Introduction
Myeloid-related protein 8 (MRP8) and MRP14, both S100 proteins, are the major calcium-binding proteins expressed in phagocytes during specific stages of differentiation.1,2 They form stable complexes and are present in circulating neutrophils and monocytes, representing the first cells invading inflammatory lesions.3 The protein complex is found in inflammatory fluids in distinct inflammatory conditions, including rheumatoid arthritis, allograft rejection, inflammatory bowel disease, and lung disease.4-9 Prerequisite for its secretion is the contact of phagocytes with extracellular matrix proteins or inflamed endothelium, resulting in elevated intracellular calcium levels and activated protein kinase C.10,11 MRP8/MRP14 is thereby released specifically at inflammatory sites and leads to increased serum levels in correlation with the degree of inflammation, indicating an extracellular role of these molecules in inflammatory processes. However, little is known about the extracellular functions of MRP8/MRP14. The protein complex is deposited on endothelia for which different mechanisms are proposed. MRP14 has been shown to bind specifically to human microvascular endothelial cells (HMECs) by way of heparan sulphate proteoglycans.12 Another group13 reported that MRP8/MRP14 binds to novel carboxylated N-glycans expressed on inflammatory activated endothelial cells (ECs). Blocking these N-glycans with specific antibodies inhibited leukocyte extravasation in a murine model.13 The hypothesis of a prominent role of MRP8/MRP14 for leukocyte recruitment is further supported by the finding that MRP8/MRP14 increases the binding capacity of CD11b-CD18 on leukocytes to intracellular adhesion molecule-1 (ICAM-1) on endothelium.14 A recently identified inflammatory disorder, with the hallmark of an extraordinarily high abundance of MRP8 and MRP14, finally underscores a direct pathogenetic role for these 2 molecules in inflammation in vivo.15 Thus, multiple findings indicate important interactions between MRP8/MRP14 and ECs, whereas the functional consequences and the underlying molecular mechanisms are completely unknown.
In our study, oligonucleotide microarray analysis of HMECs demonstrated that MRP8/MRP14 directly induces a distinct inflammatory, thrombogenic response in microvascular ECs. The inflammatory response is characterized by the induction of proinflammatory chemokines and adhesion molecules and by increased vascular permeability.
Patients, materials, and methods
Purification of MRP8 and MRP14
MRP8 and MRP14 were purified from human granulocytes as described previously.16 The purity of the protein was greater than 98%, as verified by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and mass spectrometry (MALDI-MS) (Figure 1A), as described elsewhere.17 MRP8/MRP14-containing stock solutions (1.5 mg/mL) were essentially free of endotoxin, as tested by a limulus lysate assay (E-Toxate Reagent Kit, sensitive to 0.05-0.1 endotoxin U/mL; Sigma, Deisenhofen, Germany). Before use in cell culture, the purified proteins were dialyzed against culture medium (dialysis medium) using a semipermeable dialysis membrane with a molecular weight cutoff of 10 kDa. Subsequently, in each assay, the dialysed protein complex was added to the culture medium of HMECs in indicated concentrations. The same volume of dialysis medium without MRP8/MRP14 was added to the medium of control HMECs, which we defined as the control medium. This procedure excluded that effects ascribed to MRP8/MRP14 might be caused by low-molecular contaminants.
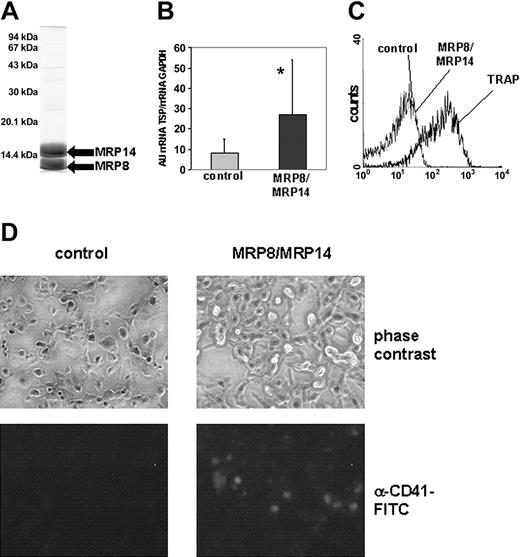
Thrombogenic effect of MRP8/MRP14. (A) After purification of MRP8 and MRP14 from human granulocytes, a sample containing 25 μg purified protein was separated on a 15% SDS gel. (B) In 4 independent experiments, the amount of TSP-1 transcription in control (▦) and MRP8/MRP14-treated HMECs (▪) was determined by quantitative RT-PCR and set in relation to GAPDH expression. Columns represent mean ± SD (*P < .005). (C) Isolated platelets were stimulated with TRAP (25 μM) and MRP8/MRP14 (200 μg/mL) or were kept in control medium. Binding of FITC-conjugated fibrinogen to platelets was analyzed by flow cytometry. (D) HMEC monolayers were incubated with whole blood under defined shear stress and were assessed for platelet binding. Platelets were identified by specific staining with an FITC-coupled anti-CD41 mAb but could also be visualized on monolayers by phase-contrast microscopy (magnification/numerical aperture of the objective lenses were 20/0.5).
Thrombogenic effect of MRP8/MRP14. (A) After purification of MRP8 and MRP14 from human granulocytes, a sample containing 25 μg purified protein was separated on a 15% SDS gel. (B) In 4 independent experiments, the amount of TSP-1 transcription in control (▦) and MRP8/MRP14-treated HMECs (▪) was determined by quantitative RT-PCR and set in relation to GAPDH expression. Columns represent mean ± SD (*P < .005). (C) Isolated platelets were stimulated with TRAP (25 μM) and MRP8/MRP14 (200 μg/mL) or were kept in control medium. Binding of FITC-conjugated fibrinogen to platelets was analyzed by flow cytometry. (D) HMEC monolayers were incubated with whole blood under defined shear stress and were assessed for platelet binding. Platelets were identified by specific staining with an FITC-coupled anti-CD41 mAb but could also be visualized on monolayers by phase-contrast microscopy (magnification/numerical aperture of the objective lenses were 20/0.5).
Cell culture, RNA isolation, and microarray analysis
HMEC-1 was cultured as described earlier.18 Five micrograms total RNA of HMECs from 4 independent experiments were processed for microarray hybridization (Human Genome U95A Array; Affymetrix, Santa Clara, CA), as described elsewhere.19
Using MAS 5.0 software, we created background-adjusted raw intensities accounting for unspecific binding by removing probe sets with insignificant differences between single perfect-matching (PM) and mismatching (MM) probes. Single raw values were calculated for each probe set from the median of PM/MM discrimination values. Fold-changes (log ratio changes) and “change P values” based on a signed rank test were determined for each experiment. The complete data set of these analyses is provided as supplemental data (please see the Blood website for the supplemental data set link at the top of the online article). We selected only genes with a change P less than .05 for up-regulated or a change P less than .95 for down-regulated genes in at least 3 of 4 experiments. The log ratio change for single genes was calculated as mean of log ratio changes of all experiments with significant change P values. We selected only genes with mean log ratio changes of at least 0.7 or -0.7.
For more sophisticated statistical analysis, we used the Expressionist Suite software package (GeneData, Martinsried, Germany). For this analysis, data from all experiments were normalized to a logarithmic mean of 270. We retained only genes presenting a significant fold-change with a t test–based P < .05. For details, see Viemann et al.19
Real-time RT-PCR
Real-time reverse transcription–polymerase chain reaction (RT-PCR) was performed in duplicate, as described.19 Primers (MWG Biotech, Ebersberg, Germany) were thrombospondin-1 (TSP-1) forward, 5′-GCCAGAATTAGGGAATCAGAATCAA-3′, TSP-1 reverse, 5′-CTGGTGCTCACTGAGATGGTAGGT-3′, interleukin-8 (IL-8) forward, 5′-ACCACCGGAAGGAACCATTC-3′, IL-8 reverse, 5′-TTCACACAGAGCTGCAGAAATCA-3′, ICAM-1 forward, 5′-ACCTCCCCACCCACATACATTT-3′, and ICAM-1 reverse, 5′-GGCATAGCTTGGGCATATTCC-3′. Relative gene expression was calculated by using the comparative threshold cycle (CT) method, as described.20 Gene expression was normalized with respect to the endogenous housekeeping control gene GAPDH and was expressed in arbitrary units (AU) of mRNA levels of the respective gene related to the mRNA level of GAPDH. Significant differences were determined using the Mann-Whitney U test.
Platelet adhesion to HMEC monolayer under shear stress
HMECs were grown to confluence in 4-well culture plates (Nunc, Wiesbaden, Germany). Monolayers were treated overnight with 200 μg/mL MRP8/MRP14 or with 1 U/mL α-thrombin (Sigma) for 20 minutes and were washed with phosphate-buffered saline (PBS) with Ca2+ and Mg2+. Citrate-anticoagulated whole blood obtained from healthy volunteers was layered on HMEC monolayers under shear stress (shear rate, 200 seconds-1) for 5 minutes, as described previously.21 Monolayers were then washed carefully 3 times with PBS to remove unbound cells, fixed with 4% paraformaldehyde in PBS, and stained with a platelet-specific, fluorescein isothiocyanate (FITC)–conjugated anti-CD41 monoclonal antibody (mAb), as described (Beckmann Coulter, Marseilles, France). Coverslips were mounted in Moviol containing 1% n-propyl gallate. Images were recorded by fluorescence microscopy and phase-contrast using the Axioskop microscope (Zeiss, Goettingen, Germany). Objectives (10/0.5) were obtained from Zeiss. Images were recorded by the Axiocam Coulour camera (Zeiss) and processed by the Axiovision software (Zeiss).
Flow cytometry
Platelets (5 × 107/mL) were preincubated with 150 μg/mL fibrinogen-FITC for 3 minutes at room temperature (RT), followed by treatment with increasing concentrations of MRP8/MRP14 (25, 50, 100m and 200 μg/mL) or thrombin receptor–activated peptide (TRAP SFLLRN; Bachem, Weil am Rhein, Germany) (25, 50, and 100 μM) for 10 minutes at RT or were left untreated. Platelets were analyzed by a FACScan flow cytometer (Becton Dickinson [BD], Heidelberg, Germany) for binding of soluble fibrinogen. We used a mouse mAb against ICAM-1 (Immunotech, Marseilles, France) for the detection of ICAM-1 on ECs stimulated overnight in serum-free medium with 200 μg/mL MRP8/MRP14 or 300 pg/mL lipopolysaccharide (LPS).
Quantitation of IL-8 serum concentration and LDH activity
IL-8 serum concentrations were determined using a commercial chemoluminescence immunoassay (Immulite; DPC Biermann, Bad Nauheim, Germany). Lactate dehydrogenase (LDH) activity was measured using a chemistry analyzer (Cobas Mira S; Axon Lab, Reichenbach, Germany) using a commercial assay (Axon Lab LDH IFCC).
Transendothelial resistance and permeability assay
Transendothelial resistance (TER) and permeability assays were analyzed as described.22 HMECs were seeded on fibronectin-coated, 6.5-mm Transwell filters (5-μm pore size). After a 48-hour incubation, HMEC monolayers were treated overnight with MRP8/MRP14 in indicated concentrations, with 200 μg/mL MRP8/MRP14 and 10 μg/mL Polymyxin B (Sigma), with 300 pg/mL LPS (Escherichia coli 055:B5; Sigma), or with 200 μg/mL MRP8/MRP14 and polyclonal anti-MRP8 and anti-MRP14 antibodies23 or a polyclonal rabbit immunoglobulin G (IgG) isotype control antibody (Jackson ImmunoResearch, Soham, Cambridgeshire, United Kingdom) in a molar ratio of 1:5. Furthermore, we treated HMEC monolayers with protein preparations from which MRP8/MRP14 had been preabsorbed by immunoprecipitation. Polyclonal anti-MRP8 and anti-MRP14 antibodies23 and a rabbit IgG isotype control antibody (Jackson ImmunoResearch) were coupled to N-hydroxysuccinimide (NHS)–activated Sepharose 4 Fast Flow media according to the manufacturer's instructions (Pharmacia Biotech, Uppsala, Sweden). Purified MRP8/MRP14 preparations (200 μg/mL) and antibody-coupled beads were incubated overnight at 4°C on a rotor to allow absorption of MRP8/MRP14. Afterward the protein solutions were concentrated to the original volume of the MRP8/MRP14 solution using Centriplus filter devices with a molecular cutoff of 3000 kDa (Millipore, Billerica, MA). For the permeability assay, 600 μL medium was added to the lower chamber. Horseradish peroxidase (HRP) (6 mg/mL) in 100 μL medium was placed in the upper compartment, and cells were incubated for 1 hour at 37°C in a humidified atmosphere (3% CO2). Endothelial permeability was measured by quantifying HRP activity in the lower chamber.22
Immunofluorescence, immunoblotting, and immunohistochemistry
Immunofluorescence staining of HMECs was performed as described18 using mAbs against VE-cadherin, β-catenin, α-catenin, ZO-1 (all Transduction Laboratories, Lexington, KY), occludin, and ZO-2 (both Zymed, San Francisco, CA), followed by a Cychrome 2–labeled secondary antibody. Filipin was obtained from Sigma. After staining and washing procedures, coverslips were mounted in Moviol containing 1% n-propyl gallate and analyzed by epifluorescence (Axioskop microscope and objective lenses 40/0.75 from Zeiss). Images were recorded by AxioCam Colour camera (Zeiss) and processed by AxioVision software (Zeiss).
Cellular lysates of HMECs were prepared on ice for 30 minutes with lysis buffer (100 mM NaCl, 5 mM EDTA [ethylenediaminetetraacetic acid], 10 mM EGTA [ethyleneglycotetraacetic acid], 20 mM HEPES (N-2-hydroxyethylenepiperazine-N′-2-ethanesulfonic acid], and Triton X-100 1% supplemented with 1 μM pepstatin, 1 μM leupeptin, 1 mM benzamidin, 1 mM phenylmethylsulfonyl fluoride (PMSF), 50 mM NaF, and 10 mM Na3VO4). Postnuclear supernatants (PNSs) were homogenized and fractionated into cytosol and membranes by ultracentrifugation (30 minutes, 4°C, 100 000g). Samples were adjusted to equal protein concentrations and were immunoblotted. Blots were stained with anti–VE-cadherin, anti–ZO-1, anti–β-catenin, anti–α-catenin, anti-occludin, and anti–ZO-2, followed by secondary antibodies conjugated to HRP (Dianova) and were developed with enhanced chemiluminescence (AppliChem, Darmstadt, Germany). Band intensity was quantified using reflectance scanning densitometry (LumiAnalyst; Boehringer Mannheim, Germany).
Sections of formalin-fixed, paraffin-embedded myocard biopsy specimens obtained from 3 patients who died of Kawasaki disease (KD) were kindly provided by Anne Rowly (Northwestern University, Chicago, IL). Sections of formalin-fixed, paraffin-embedded myocard biopsy specimens of control subjects obtained after explantation for cardiac allograft transplantation were kindly provided by Christian August (University Hospital of Muenster, Germany). Sections were stained with monospecific affinity-purified rabbit antisera to MRP8 and MRP1423 or with polyclonal IgG isotype control antibody (Jackson ImmunoResearch) using a standard immunoperoxidase method, as previously described.10 Images were recorded using the Axioskop microscope with objective lens of × 63 oil magnification and numerical aperture of 1.25 (both Zeiss), and the AxioCam Colour camera and AxioVision software from Zeiss.
Determination of MRP8/MRP14 concentrations in sera of patients with Kawasaki disease and supernatants of isolated granulocytes
We measured MRP8/MRP14 in 21 patients fulfilling the criteria for KD who had been treated with intravenous immunoglobulin (IVIG) (2 g/kg body weight over 1-5 days). Sera were taken before treatment, within 24 hours of treatment, after 2 weeks, and after 1 month. Approval was obtained from the research ethics committee of Toyama University. Informed consent was provided according to the Declaration of Helsinki. Thirty-three healthy, age-matched children served as controls.
Granulocytes were isolated from the peripheral blood of a healthy volunteer, as described earlier.16 Granulocytes were fixed in 3.5% formalin in PBS or were resuspended in PBS for 30 minutes and centrifuged, and supernatants were processed for enzyme-linked immunosorbent assay (ELISA).
MRP8/MRP14 concentrations were determined in serum samples or granulocyte supernatants using sandwich ELISA, as described previously.23 Data were expressed as nanogram per milliliter MRP8/MRP14 and represented the mean of duplicates of each of 3 dilutions within the linear range.
Results
Differential gene expression in microvascular ECs after treatment with MRP8/MRP14
In 4 independent experiments, HMECs were grown to 90% confluence and were incubated for 6 hours with 200 μg/mL MRP8/MRP14 or control medium. Total RNA was isolated and processed for oligonucleotide microarray analysis using microarrays covering approximately 12 000 full-length human transcripts. Hence, 102 genes could be identified whose expression is regulated by MRP8/MRP14 (Table S1). Most of the transcripts consisted of still uncharacterized genes, transcription and translation factors, single factors of signal transduction pathways, or seemingly unconnected genes. We identified a common functional denominator for 22 genes and assigned the genes to 3 functional categories, platelet aggregation, inflammation, and endothelial permeability (Table 1). For functional characterization of MRP8/MRP14 effects on HMECs, we focused our analyses on these 3 functional categories uncovered by the identification of 22 characterized and apparently functionally connected genes.
Genes differentially expressed in HMECs after treatment with MRP8/MRP14
Functional groups . | Up-regulated . | FC . | Down-regulated . | FC . |
|---|---|---|---|---|
| Platelet aggregation | TSP-1 | 2 | ||
| Inflammation | IL-8 | 2 | Heatshock factor 2 | 2 |
| Gro-α (CXCL1) | 2 | Complement component C3 | 2 | |
| Gro-β (CXCL2) | 2 | |||
| Monocyte secretory protein | 2 | |||
| VCAM-1 | 2 | |||
| ICAM-1 | 2 | |||
| Manganese superoxide dismutase | 3 | |||
| Cytochrome b light-chain p22 | 2 | |||
| Endothelial integrity | Trad/Duet | 4 | Catenin α1 | 2 |
| Ras-related rho | 2 | Coxsackievirus and adenovirus receptor | 2 | |
| Cadherin (protocadherin β17) | 2 | |||
| Bullous pemphigoid antigen 1 | 2 | |||
| Matrilin-2 | 2 | |||
| αV-β3 integrin | 2 | |||
| Formyl peptide receptor–like 2 | 3 | |||
| TGF-β2 receptor α | 2 | |||
| Krev-1 (Rap1) | 2 |
Functional groups . | Up-regulated . | FC . | Down-regulated . | FC . |
|---|---|---|---|---|
| Platelet aggregation | TSP-1 | 2 | ||
| Inflammation | IL-8 | 2 | Heatshock factor 2 | 2 |
| Gro-α (CXCL1) | 2 | Complement component C3 | 2 | |
| Gro-β (CXCL2) | 2 | |||
| Monocyte secretory protein | 2 | |||
| VCAM-1 | 2 | |||
| ICAM-1 | 2 | |||
| Manganese superoxide dismutase | 3 | |||
| Cytochrome b light-chain p22 | 2 | |||
| Endothelial integrity | Trad/Duet | 4 | Catenin α1 | 2 |
| Ras-related rho | 2 | Coxsackievirus and adenovirus receptor | 2 | |
| Cadherin (protocadherin β17) | 2 | |||
| Bullous pemphigoid antigen 1 | 2 | |||
| Matrilin-2 | 2 | |||
| αV-β3 integrin | 2 | |||
| Formyl peptide receptor–like 2 | 3 | |||
| TGF-β2 receptor α | 2 | |||
| Krev-1 (Rap1) | 2 |
FC indicates fold change of gene expression after treatment with MRP8/MRP14.
Thrombospondin-1 (TSP-1) is an MRP8/MRP14-induced gene of the category platelet aggregation. It is a matricellular protein required for the secondary phase of firm platelet aggregation under shear stress.21,24,25 The inflammation group comprised multiple inflammatory response genes whose transcription in ECs was primarily increased by MRP8/MRP14 treatment. These were proinflammatory chemokines, such as IL-8, Gro-α, Gro-β, and monocyte chemoattractant protein 1 (MCP-1),26-28 adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1) and ICAM-1, and 2 enzymes involved in superoxide metabolism, manganese superoxide dismutase (MnSOD)29 and mitochondrial cytochrome b light chain p22.30
In contrast, MRP8/MRP14-mediated down-regulation of gene expression was predominantly seen in genes that contribute to growth, differentiation, and endothelial monolayer integrity. Down-regulated genes belonging to this group were cell junction proteins (α-catenin, coxsackievirus receptor, adenovirus receptor [CAR], and cadherin),31,32 matrix proteins involved in monolayer integrity (bullous pemphigoid antigen 1 and matrilin-2),33,34 cell surface receptors mediating EC spreading and chemotactic trafficking (vitronectin α receptor, formyl peptide receptor–like 2),35,36 and components associated with EC growth and differentiation (transforming growth factor–β2 [TGF-β2] receptor α, Krev-1).37,38 Two genes in this group whose products control cytoskeleton-dependent cell functions, Trad/Duet and ras-related rho, were up-regulated.39,40
Thrombogenic properties of MRP8/MRP14
Because microarray results suggested that MRP8/MRP14 exhibited thrombogenic effects in ECs, we first tested for the induction of TSP-1 gene expression by a second independent method (quantitative RT-PCR) in all 4 independent experiments. The amount of TSP-1 mRNA averaged 3.1-fold higher in MRP8/MRP14-stimulated HMECs compared with HMECs incubated with control medium (P < .005), confirming the microarray data (Figure 1B).
To exclude direct activating effects of MRP8/MRP14 on platelets, we analyzed the binding of soluble FITC-conjugated fibrinogen to platelets, which represents a sensitive marker for the activation of the fibrinogen receptor GPIIb/IIIa on platelets.41 Binding of fibrinogen to MRP8/MRP14-treated platelets was comparable to that for control platelets, whereas TRAP-stimulated platelets showed a significant increase in fibrinogen binding (Figure 1C). Therefore, major activating effects of MRP8/MRP14 on platelets could be ruled out.
To evaluate thrombogenic effects of MRP8/MRP14, we layered citrate-anticoagulated whole blood onto confluent HMEC monolayers under defined shear stress to simulate in vivo conditions of blood flow. Platelets bound to the endothelial monolayer were stained with an FITC-coupled, anti-CD41 mAb. Almost no platelets bound to HMEC monolayers cultured with control medium. In contrast, pretreatment with MRP8/MRP14 resulted in the adhesion of numerous single platelets and small aggregates (Figure 1D), comparable to thrombin pretreatment used as positive control regarding platelet adhesion (data not shown).
MRP8/MRP14 induce IL-8 and ICAM-1 in endothelium
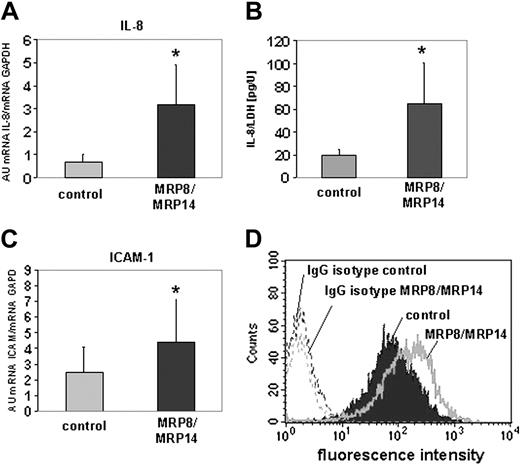
From the group of inflammatory response genes identified by microarray analysis to be induced by MRP8/MRP14, we exemplarily selected IL-8 and ICAM-1 to validate the results by quantitative RT-PCR. The amounts of IL-8 and ICAM-1 gene transcripts were 3.3-fold and 2.5-fold higher in HMEC monolayers after MRP8/MRP14 treatment compared with control HMECs (P < .001) (Figure 2A, C). To study expression changes at the protein level, we determined the concentration of IL-8 in supernatants of control medium– and MRP8/MRP14-treated HMEC monolayers in 3 independent experiments. To exclude changes resulting from cell death and cell lysis, we measured LDH activity in the supernatants and set it in relation to the IL-8 concentration. This analysis revealed that HMEC monolayers secreted significantly higher amounts of IL-8 on stimulation with MRP8/MRP14 than control cells (Figure 2B). ICAM-1 expression was analyzed by flow cytometry. MRP8/MRP14 treatment increased ICAM-1 expression on HMECs (Figure 2D), whereas stimulation with 300 pg/mL LPS had no effect on the ICAM-1 expression of HMECs excluding an MRP8/MRP14 effect caused by LPS contamination (data not shown).
Induction of IL-8 gene expression and secretion in HMECs. Quantitative RT-PCR of IL-8 (A) and ICAM-1 (C) determined their amount of transcription in MRP8/MRP14-treated (▪) and control HMECs (▦). Columns represent mean ± SD of 4 independent experiments (*P < .001). (B) The concentration of IL-8 was determined in supernatants of MRP8/MRP14-treated and control HMEC monolayers and set in relation to LDH activity to exclude differences caused by cell death. Columns represent mean ± SD of 3 independent experiments (*P < .05). (D) MRP8/MRP14-treated and control HMECs were immunostained for ICAM-1 or incubated with IgG isotype control mAb. ICAM-1 expression was analyzed by flow cytometry.
Induction of IL-8 gene expression and secretion in HMECs. Quantitative RT-PCR of IL-8 (A) and ICAM-1 (C) determined their amount of transcription in MRP8/MRP14-treated (▪) and control HMECs (▦). Columns represent mean ± SD of 4 independent experiments (*P < .001). (B) The concentration of IL-8 was determined in supernatants of MRP8/MRP14-treated and control HMEC monolayers and set in relation to LDH activity to exclude differences caused by cell death. Columns represent mean ± SD of 3 independent experiments (*P < .05). (D) MRP8/MRP14-treated and control HMECs were immunostained for ICAM-1 or incubated with IgG isotype control mAb. ICAM-1 expression was analyzed by flow cytometry.
MRP8/MRP14 affect the integrity of the endothelial monolayer
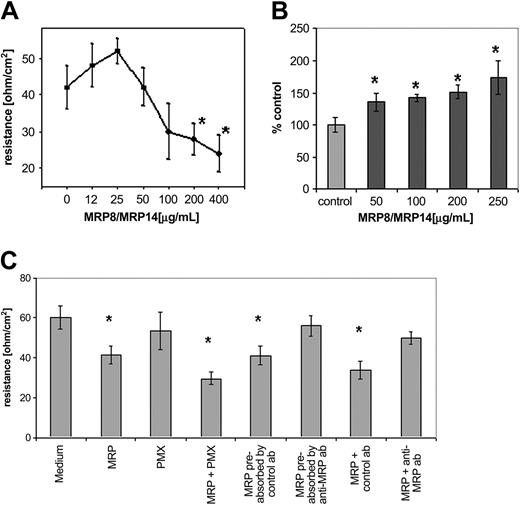
Microarray analysis revealed that several genes encoding endothelial cell junction proteins belonged to the group of MRP8/MRP14 down-regulated genes. Therefore, we predicted a negative effect of MRP8/MRP14 on endothelial monolayer integrity and first examined the influence of these proteins on TER. Treating HMEC monolayers with low concentrations of MRP8/MRP14 (0-25 μg/mL) led to a minimal and insignificant increase in TER, whereas concentrations greater than 25 μg/mL induced a dose-dependent, impressive decrease of TER, supporting a negative influence of MRP8/MRP14 on monolayer integrity (Figure 3A). Analysis of the permeability of the HMEC monolayer treated with MRP8/MRP14 verified a concentration-dependent increase in endothelial permeability (Figure 3B). MRP8/MRP14 also decreased TER in the presence of polymyxin B, whereas stimulation with 300 pg/mL LPS caused no TER decrease (data not shown), excluding contaminating LPS in the purified protein (Figure 3C). The TER-decreasing effect of MRP8/MRP14 could be abrogated by preabsorption of MRP8/MRP14 with polyclonal anti-MRP8 and anti-MRP14 antibodies, whereas isotype-matched control antibodies had no effect (Figure 3C). Furthermore, we treated HMEC monolayers directly with MRP8/MRP14, together with anti-MRP8 and anti-MRP14, antibodies and revealed in the TER assay a blocking effect on MRP8/MRP14 compared with an isotype control antibody (Figure 3C).
Influence of MRP8/MRP14 on transendothelial resistance and permeability. Filter-grown HMEC monolayers were treated for 16 hours with different concentrations of MRP8/MRP14 (A-B) or with 200 μg/mL MRP8/MRP14 (MRP), together with 10 μg/mL polymyxin B (PMX), isotype control antibody (control ab), anti-MRP8 and anti-MRP14 antibodies (anti-MRP ab), or MRP8/MRP14 preabsorbed by isotype control antibody or anti-MRP8 and anti-MRP14 antibodies (C). Subsequently, the TER of the monolayer, expressed as ohm/cm2 (A,C), and the permeability, expressed as percentage of control (B), were determined. All data are presented as mean ± SD of 3 experiments (P < .05). Asterisks represent significant differences to control samples with P < .05.
Influence of MRP8/MRP14 on transendothelial resistance and permeability. Filter-grown HMEC monolayers were treated for 16 hours with different concentrations of MRP8/MRP14 (A-B) or with 200 μg/mL MRP8/MRP14 (MRP), together with 10 μg/mL polymyxin B (PMX), isotype control antibody (control ab), anti-MRP8 and anti-MRP14 antibodies (anti-MRP ab), or MRP8/MRP14 preabsorbed by isotype control antibody or anti-MRP8 and anti-MRP14 antibodies (C). Subsequently, the TER of the monolayer, expressed as ohm/cm2 (A,C), and the permeability, expressed as percentage of control (B), were determined. All data are presented as mean ± SD of 3 experiments (P < .05). Asterisks represent significant differences to control samples with P < .05.
To confirm the disruption of the endothelial monolayer morphologically, MRP8/MRP14- and control medium–treated HMEC monolayers were stained with Filipin, a fluorescent antibiotic that detects unesterified cholesterol in membranous structures. By outlining membranous structures, we found that MRP8/MRP14 treatment caused impressing holes at intercellular boundaries, suggesting the interruption of intercellular contacts. In contrast, HMEC monolayers incubated with control medium (Figure 4A) showed intact membranous structures with no evidence of disrupted intercellular junctions.
Loss of monolayer integrity and cell junction proteins after MRP8/MRP14 treatment. HMEC monolayers were cultured on coverslips and were treated for 16 hours with MRP8/MRP14 or control medium. Immunofluorescence staining was performed for membranous structures with filipin (A) and for VE-cadherin (B), β-catenin (C), and ZO-1 (D). Loss of cell junction proteins and substantial holes are indicated by arrows (A-D). Scale bar, 10 μm. Occludin (E), ZO-2 (F), β-catenin (G), and ZO-1 (H) were analyzed in postnuclear supernatants (P), cytosol (C), and membrane (M) fractions of whole cell lysates of MRP8/MRP14-treated and control HMECs by immunoblotting of equal amounts of protein.
Loss of monolayer integrity and cell junction proteins after MRP8/MRP14 treatment. HMEC monolayers were cultured on coverslips and were treated for 16 hours with MRP8/MRP14 or control medium. Immunofluorescence staining was performed for membranous structures with filipin (A) and for VE-cadherin (B), β-catenin (C), and ZO-1 (D). Loss of cell junction proteins and substantial holes are indicated by arrows (A-D). Scale bar, 10 μm. Occludin (E), ZO-2 (F), β-catenin (G), and ZO-1 (H) were analyzed in postnuclear supernatants (P), cytosol (C), and membrane (M) fractions of whole cell lysates of MRP8/MRP14-treated and control HMECs by immunoblotting of equal amounts of protein.
Loss of endothelial cell junction proteins after MRP8/MRP14 treatment
Loss of the endothelial monolayer integrity as a result of MRP8/MRP14 treatment prompted us to perform more detailed analysis of the impact of these S100 molecules on abundance and subcellular distribution of cell junction proteins. Microarray analysis identified genes coding for 2 classic cell junction proteins, α-catenin and VE-cadherin, the transcription of which was inhibited by MRP8/MRP14 (Table 1). We examined the expression of these proteins and that of β-catenin, occludin, ZO-1, and ZO-2 by immunofluorescence staining of control medium– and MRP8/ MRP14-treated HMEC monolayers. For VE-cadherin, the down-regulation of transcription correlated well at the protein level with a loss of this molecule (Figure 4B). Equally we found a clear loss of β-catenin and ZO-1 out of adjacent EC membranes (Figure 4C-D). The fluorescence staining of ZO-2 showed no changes after MRP8/MRP14 treatment (data not shown). Monoclonal antibodies against α-catenin and occludin did not yield reliable immunofluorescence staining in MRP8/MRP14- or control medium–treated HMEC monolayers.
Furthermore, in 3 independent experiments, we separated lysates of HMEC monolayers into cytosol and membrane fractions and immunoblotted them for the different cell junction proteins. Densitometric scanning of the blots revealed an apparent loss of occludin and ZO-2 and a minor loss of β-catenin in the membrane fractions (reductions to 12%, 43%, and 64% of control, respectively) (Figure 4E-G). For occludin, we also saw a reduction in the cytosol fraction (to 62% of control) (Figure 4E), whereas no changes were detectable for ZO-2 and β-catenin in the cytosol fractions after MRP8/MRP14 treatment (Figure 4F-G). Immunoblotting of ZO-1 indicated an MRP8/MRP14-induced loss from the cytosol fraction. In membrane fractions, ZO-1 was virtually undetectable (Figure 4H). Detection of VE-cadherin was not achieved in either cell fraction. Immunoblotting against α-catenin did not reveal a protein loss in membrane or in cytosol fractions of HMECs after MRP8/MRP14 treatment (data not shown). Thus, the transcriptional down-regulation of α-catenin detected by microarray analysis did not correlate with its expression at the protein level.
Prominent expression of MRP8/MRP14 in KD
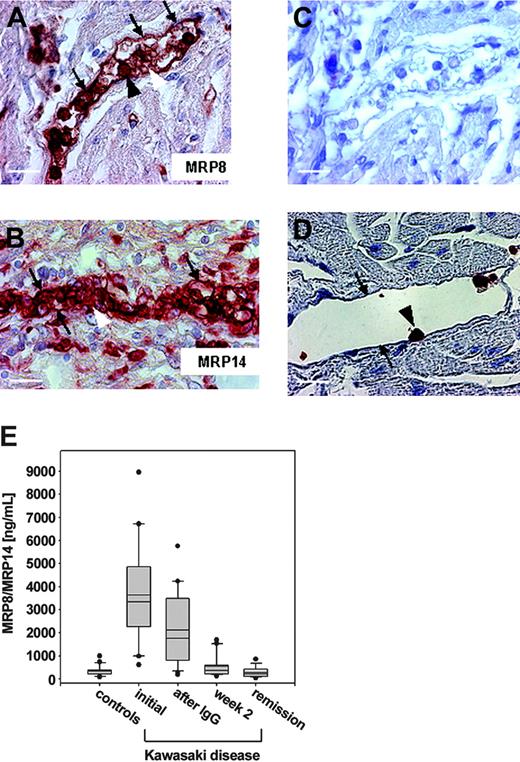
To determine whether MRP8/MRP14 is associated with a human disease characterized by microthromboembolic complications and endothelial disintegration, we analyzed the expression of these proteins in KD, an acute form of systemic vasculitis occurring in young children that particularly affects coronary arteries. Myocard sections from 3 deceased KD patients were immunohistochemically stained with antibodies against MRP8 and MRP14. Endothelia of the vessels were almost completely coated with each protein, whereas other parts of the vascular walls remained free of MRP8/MRP14. Furthermore, staining revealed a massive adhesion of MRP8/MRP14-positive granulocytes to the endothelia (Figure 5A-B). Staining with the isotype control antibody remained completely negative (Figure 5C). In myocard biopsy specimens of controls without signs of inflammation, only granulocytes attaching to the endothelium, which are rarely detectable, showed positive staining for MRP8 (data not shown) and MRP14, but the endothelium itself did not (Figure 5D). To exclude that MRP8/MRP14 was artificially released from granulocytes by the fixation process, we isolated granulocytes from peripheral blood and fixed them with 3.5% formalin or left them untreated. Analysis of the concentration of MRP8/MRP14 in the supernatants of the cells did not show a substantial difference between fixed granulocytes and unfixed control cells (data not shown). Next, we determined serum concentrations of MRP8/MRP14 in 21 patients with KD, all of whom underwent treatment with high-dose IVIG. Results are summarized in Figure 5C. Before treatment, patients with KD had significantly increased concentrations of MRP8/MRP14 (3630 ± 480 ng/mL; mean ± SEM) compared with healthy controls (220 ± 40 ng/mL; P < .001). Initial concentrations of MRP8/MRP14 dropped significantly within 24 hours of IVIG (2110 ± 360 ng/mL; P < .01) and reached a normal range in all patients during the later course. The reduction of MRP8/MRP14 serum concentrations was closely associated with subsiding signs of acute vasculitis.
MRP8/MRP14 in Kawasaki disease. Paraffin sections of myocard biopsy specimens from patients with KD (A-C) and a control decedent (D) were stained against MRP8 (A), MRP14 (B,D), and control antibody (C). Labeled are the endothelial layer of a small vessel (small arrows), granulocytes (large black arrows) adherent to endothelial cells, and microthrombi (large white arrows). Scale bars, 20 μm (A,C-D) and 30 μm (B). (E) Serum concentrations of MRP8/MRP14 were determined in 33 healthy children and in 21 patients with KD at initial presentation, 1 day after therapy with IVIG, in the second week after treatment, and in complete clinical remission. Box plots represent median and mean values, quartiles, and the 10th and 90th percentiles (*P < .05).
MRP8/MRP14 in Kawasaki disease. Paraffin sections of myocard biopsy specimens from patients with KD (A-C) and a control decedent (D) were stained against MRP8 (A), MRP14 (B,D), and control antibody (C). Labeled are the endothelial layer of a small vessel (small arrows), granulocytes (large black arrows) adherent to endothelial cells, and microthrombi (large white arrows). Scale bars, 20 μm (A,C-D) and 30 μm (B). (E) Serum concentrations of MRP8/MRP14 were determined in 33 healthy children and in 21 patients with KD at initial presentation, 1 day after therapy with IVIG, in the second week after treatment, and in complete clinical remission. Box plots represent median and mean values, quartiles, and the 10th and 90th percentiles (*P < .05).
Discussion
MRP8 and MRP14 are the major calcium-binding proteins in phagocytes and comprise up to 40% of detergent-soluble proteins in neutrophils. Noncovalently associated MRP8/MRP14 complexes are secreted by activated phagocytes under inflammatory conditions after contact with inflamed endothelium.10,11 Extracellular concentrations of these proteins are increased to several hundred micrograms per milliliter at local sites of inflammation and correlate well with disease activity in various inflammatory diseases.5-9 An important functional role in inflammatory processes is assumed for MRP8/MRP14 because an extraordinarily high expression of each protein defines a novel, recently identified inflammatory disorder.15 The MRP8/MRP14 protein complex can bind to endothelium through the interaction of MRP14 with heparan sulphate proteoglycans or of the MRP8/MRP14 complex with carboxylated N-glycans exclusively expressed by ECs after inflammatory activation.12,13 It remains unclear whether these N-glycans are part of a specific receptor coupled to a distinct signal transduction pathway. Blocking the interaction of MRP8/MRP14 with carboxylated N-glycans on endothelium results in the inhibition of leukocyte extravasation.13 However, functional effects of MRP8/MRP14 on ECs and their underlying molecular mechanisms have not yet been identified.
Therefore, we analyzed the effects of MRP8/MRP14 on ECs using oligonucleotide microarray technology to approach systematically changes in the gene expression program of ECs elicited by MRP8/MRP14. Twenty-two endothelial genes significantly altered in their transcription could easily be assigned to 3 functional categories: platelet aggregation, inflammation, and endothelial permeability. TSP-1 assigned to the first category is one of the major proteins stored in human platelet α-granules and is secreted by many different cell types, including ECs.42,43 It is considered a matricellular protein, which, among other functions, mediates platelet aggregation at high shear force.21,24 Our microarray data showed an up-regulation of TSP-1 transcription in MRP8/MRP14-treated endothelium that was confirmed by quantitative RT-PCR. Functionally, MRP8/MRP14 showed no direct activating influence on platelets but exhibited a specific thrombogenic reaction in ECs, leading to platelet adhesion and aggregation under shear stress. Our model demonstrates for the first time that MRP8/MRP14 provides a distinct inflammatory milieu in which platelets tend to aggregate despite high flow rates.
In the second category of inflammatory response genes, MRP8/MRP14-induced up-regulation concerned mainly proinflammatory chemokines such as IL-8, Gro-α, and MCP-1 and adhesion molecules such as VCAM-1 and ICAM-1, all of which have the ability to promote further leukocyte recruitment. Furthermore, enzymes of the mitochondrial superoxide metabolism (MnSOD) were induced.44-46 Of these genes, we selected IL-8 and ICAM-1 as prototypical proinflammatory molecules and confirmed their induction at the transcriptional level and at the protein level. Keeping in mind the conditions leading to the secretion of MRP8/MRP14, these findings point to a novel positive feedback mechanism by which phagocytes promote further recruitment of leukocytes to sites of inflammatory reaction.
Microarray analysis revealed a third new aspect of MRP8/MRP14 actions on endothelium. MRP8/MRP14 primarily suppressed the expression of genes involved in monolayer integrity of ECs. We identified cell surface receptors mediating monolayer formation, spreading, probably migration, and trafficking (vitronectin α receptor and formyl peptide receptor–like 2),35,36 growth, and differentiation (TGF-β 2 receptor α)37 and cell junction–associated proteins (α-catenin, CAR, and cadherin).31,32 The resultant hypothesis that MRP8/MRP14 could negatively affect endothelial monolayer integrity was confirmed by demonstrating a dose-dependent loss of the TER and a dose-dependent increase of the endothelial permeability by MRP8/MRP14 in a concentration range typically found at local sites of inflammatory reactions.5 Immunoprecipitation of MRP8/MRP14 and blocking experiments using inhibiting antibodies confirmed the specificity of MRP8/MRP14-induced effects on endothelial monolayers. Given that MRP8 and MRP14 belong to the family of calcium-binding S100 proteins, calcium depletion had to be considered a possible mechanism. However, the calcium content of the HMEC medium (1.6 mM) far exceeds the maximal calcium-binding capacity of MRP8/MRP14 used in a concentration of 200 μg/mL (33 nM).17
A more detailed morphologic and biochemical analysis of different cell junction proteins revealed that MRP8/MRP14 treatment down-regulated the expression or membrane association of VE-cadherin, β-catenin, occludin, ZO-1, and ZO-2. For α-catenin alone, down-regulation at the transcriptional level could not be correlated with its expression at the protein level, possibly because of delayed catabolic metabolism of this protein. Interestingly, a recent study47 showed that components of endothelial adherens junctions (β-catenin, VE-cadherin, α-catenin, and γ-catenin) are sequentially lost at sites where granulocytes adhere to inflammatory stimulated ECs but not further away from EC granulocyte adhesive interactions. A granulocyte-derived elastase is at most partially responsible for this effect.47 Our results imply that MRP8/MRP14, known to be specifically released from primed phagocytes on interaction with ECs at inflammatory sites,10,11 may play an important role in this process.
To get evidence for the pathophysiological relevance of our in vitro experiments, we analyzed the expression of MRP8 and MRP14 in systemic vasculitis. Clinical hallmarks of this inflammatory condition are neutrophil adherence to activated ECs, increased microvascular permeability, and formation of microthrombi, all of which correspond to the identified effects of MRP8/MRP14 on ECs. KD is an especially interesting model of vasculitis syndromes because it is usually responsive to treatment with high doses of γ-globulin within a few days, allowing easy follow-up of patients.48,49 Indeed we found a high expression of MRP8 and MRP14 in neutrophils attaching to the affected vessels and a strong deposition of both molecules at the endothelial surface of the vessels within the inflamed tissue. Furthermore, the rapid decrease of initially high serum concentrations of MRP8/MRP14, in association with the clinical response to treatment, indicates a functional role for these S100 proteins in systemic vasculitis.
In conclusion, this study demonstrates for the first time a direct influence of MRP8/MRP14 on endothelial cell functions. MRP8/MRP14 confers a strong thrombogenic property to ECs. It stimulates an inflammatory response with increased production of distinct proinflammatory chemokines and increased expression of adhesion molecules. Furthermore, MRP8/MRP14 impairs the monolayer integrity of ECs and induces a loosening of endothelial cell junctions. The expression pattern of MRP8/MRP14 in systemic vasculitis supports a relevant role for MRP8/MRP14 as a key molecule of inflammation and indicates that these proteins could present potential targets for novel anti-inflammatory interventions.
Prepublished online as Blood First Edition Paper, December 14, 2004; DOI 10.1182/blood-2004-07-2520.
Supported by grants from the German Research Association and the Interdisciplinary Clinical Research Center of the University of Muenster (FO2/26/04).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Anne Rowly for providing sections of coronary artery aneurysms from patients with Kawasaki disease and Christian August for providing sections of healthy coronary arteries.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal