Abstract
Imatinib is a tyrosine kinase inhibitor that suppresses the growth of bcr-abl–expressing chronic myeloid leukemia (CML) progenitor cells by blockade of the adenosine triphosphate (ATP)–binding site of the kinase domain of bcr-abl. Imatinib also inhibits the c-abl, platelet-derived growth factor (PDGF) receptor, abl-related gene (ARG) and stem-cell factor (SCF) receptor tyrosine kinases, and has been used clinically to inhibit the growth of malignant cells in patients with CML and gastrointestinal stromal tumors (GISTs). Although initially considered to have minimal effects of normal hematopoiesis, recent studies show that imatinib also inhibits the growth of some nonmalignant hematopoietic cells, including monocyte/macrophages. This inhibition could not be attributed to the known activity profile of imatinib. Here, we demonstrate for the first time that imatinib targets the macrophage colony-stimulating factor (M-CSF) receptor c-fms. Phosphorylation of c-fms was inhibited by therapeutic concentrations of imatinib, and this was not due to down-regulation in c-fms expression. Imatinib was also found to inhibit M-CSF–induced proliferation of a cytokine–dependent cell line, further supporting the hypothesis that imatinib affects the growth and development of monocyte and/or macrophages through inhibition of c-fms signaling. Importantly, these results identify an additional biologic target to those already defined for imatinib. Imatinib should now be assessed for activity in diseases where c-fms activation is implicated, including breast and ovarian cancer and inflammatory conditions.
Introduction
Imatinib (Glivec, formerly STI-571; Novartis, Basel, Switzerland) is a protein tyrosine kinase inhibitor showing high efficacy in the treatment of chronic myeloid leukemia (CML) and gastrointestinal stromal tumors (GISTs). The drug was designed to specifically target the CML-specific tyrosine kinase bcr-abl, but has also shown potency against platelet-derived growth factor receptor (PDGF-R), stem cell factor receptor (c-kit), c-abl, and the abl-related gene ARG.1-4 By targeting bcr-abl in CML, and c-kit in GISTs, imatinib is effective in inhibiting the growth of cancerous cells.1,5
Although clinical trials have demonstrated hematologic and cytogenetic responses in most patients with chronic-phase CML, and clinical trials in patients with GISTs have shown a prevention of disease progression with minimal side effects,5-7 emerging reports show that normal hematopoietic cells are also affected by imatinib.8-10 Growth inhibition was observed in normal CD34+ progenitor cells and cells of the monocyte-macrophage lineage,8-10 and this inhibition was found to be independent of c-kit signaling.8
Inhibition of monocyte and/or macrophage growth and development by imatinib in healthy donors10 cannot be explained by the known activity profile of this drug. In contrast to the receptor tyrosine kinases c-kit and PDGFRβ, phosphorylation of c-fms was reported to be unaffected across the concentration range tested (0-10 μM imatinib).4 c-fms,11 c-kit,12 Fms-like tyrosine kinase 3 (FLT3),13 PDGFRα14 and PDGFRβ15 all belong to the class III receptor tyrosine kinase family, and play a vital role in hematopoiesis. c-fms is crucial for the growth and differentiation of the monocyte-macrophage lineage16 and upon binding of M-CSF to the extracellular domain of c-fms, the receptor dimerizes and transautophosphorylates several cytoplasmic tyrosine residues.17 The relative specificity of the action of imatinib on cells of the monocyte-macrophage lineage in vitro suggests that the signaling pathways of monocyte and/or macrophage development are affected. In this study, we examined the effect of imatinib on signal transduction through c-fms, and demonstrated inhibition of this receptor at therapeutic concentrations of imatinib.
Materials and methods
Isolation of bone marrow mononuclear cells and CD34+ cells
Normal bone marrow (BM) was aspirated from the posterior iliac crest of healthy volunteers following informed consent. The use of BM cells for these studies was approved by the Human Ethics Committee, Royal Adelaide Hospital. Low-density BM mononuclear cells (BMMNCs) were collected by centrifugation over Ficoll-Hypaque (1.077 g/dL; Lymphoprep; Nycomed Pharma, Roskilde, Denmark) at 400g for 30 minutes.
CD34+ progenitor cells (> 90% pure) were isolated from BMMNCs using a magnetic-activated cell-sorting (MACS) CD34+ progenitor cell isolation kit, according to the manufacturer's instructions (Miltenyi Biotech, Bergisch Gladbach, Germany).
Establishment of hemopoietic colony assays
BMMNCs or CD34+ cells were assayed for colony formation in semisolid agar, using a modification by Johnson.18 Briefly, 5.0 × 104 BMMNCs or 7.5 × 103 CD34+ cells were plated per 35-mm cell-culture dish (BD Biosciences, San Jose, CA), in 1.0 mL of Iscove modified Dulbecco medium (IMDM; JRH Biosciences, Lenexa, KS) supplemented with 0.33% agar (Bacto Agar, BD Biosciences, San Jose, CA), 25% fetal calf serum (FCS), and 2 mM l-glutamine. Colony growth was stimulated by the addition of 4 hematopoietic growth factors ([4HGF], interleukin [IL]–3, IL-6, granulocyte colony-stimulating factor [G-CSF], and granulocyte macrophage–CSF [GM-CSF], each at a final concentration of 10 ng/mL) (Peprotech, Rocky Hill, NJ), 5 hematopoietic growth factors ([5HGF], IL-3, IL-6, G-CSF, GM-CSF, and stem-cell factor [SCF], each at a final concentration of 10 ng/mL) (Peprotech), M-CSF (25 ng/mL), or GM-CSF (10 ng/mL) (Peprotech). Imatinib (0.3 μMto30 μM; Novartis), anti–c-fms antibodies (1 μg/mL 2-4A5; Santa Cruz Biotechnology, Santa Cruz, CA), or anti–c-kit antibodies (1 μg/mL; Sigma, St Louis, MO) were also added to colony cultures. Cultures were incubated at 37°C plus 5% CO2 for 2 weeks, then fixed in 3% glutaraldehyde. Fixed cultures were sequentially stained for naphthol acetate esterase19 and chloroacetate esterase,20 then stained with luxol fast blue dye21 (Merck Pty Ltd, Melbourne, Australia), to identify monocyte and/or macrophage, neutrophil, and eosinophil colonies, respectively. Colonies were scored according to standard criteria (> 50 cells).
Detection of M-CSF by ELISA
Monocytes were isolated from buffy coats from healthy donors, as previously described.10 Monocyte cultures (1 × 105/mL) were established in 24-well plates in serum-deprived medium (SDM; IMDM/1% bovine serum albumin [BSA] supplemented with 2 mM l-glutamine, 200 μg/mL transferrin, 10 μg/mL insulin (Actrapid; Novo Nordisk, Sydney, Australia), 10-4 M β-mercaptoethanol, 50 μg/mL low-density lipoproteins (Sigma), and stimulated with 20 ng/mL GM-CSF. Supernatants were harvested at 24-hour intervals for 5 days, and analyzed for M-CSF using a DuoSet enzyme-linked immunosorbent assay (ELISA) development system (R&D Systems, Minneapolis, MN).
Transduction of c-fms into FDC-P1 cells
Stable Ψ2 virus–producing cell lines transfected with MSCV-CFMS-IRES-GFP (kindly provided by Dr M. Roussel, St Jude Children's Research Hospital, Memphis, TN) were produced by Fugene (Roche, Basel, Switzerland) transfection, and sorted on a FACStarPLUS flow cytometer (Becton Dickinson, Franklin Lakes, NJ), collecting cells that expressed green fluorescence protein (GFP). These cells were used to infect FDC-P1 cells by cocultivation, and FDC-P1 cells expressing c-fms protein (FDC-cfms) were selected in Dulbecco modified Eagle medium (DMEM) supplemented with 10% FCS, 200 mM l-glutamine, and 60 ng/mL recombinant human (rh) M-CSF.
Proliferation assays
FDC-cfms cells were resuspended at 5.0 × 104/mL in DMEM containing 10% FCS, and stimulated with murine IL-3 (1:2000; kindly provided by Dr S. Read, Institute of Medical and Veterinary Science [IMVS]) or rhM-CSF (60 ng/mL; Peprotech, Rocky Hill, NJ). Imatinib was added to a final concentration of 0.5 μMto5.0 μM, in triplicate, and cells harvested at 12-, 24-, and 48-hour time points. Cells were fixed in a known volume, and a fixed volume of known density Flow-Check Fluorospheres (Beckman Coulter, Fullerton, CA) were added. Cell number was determined by a Coulter XL-MCS analytical flow cytometer (Beckman Coulter), using analysis based on gates on forward- versus side-scatter plots that corresponded to beads/cells.
Immunoprecipitation of c-fms lysates
FDC-cfms cells were incubated for 1 hour in serum-free medium (DMEM) at 37°C, with and without imatinib. Following starvation, cells were resuspended at 1.5 × 107/mL in DMEM with and without imatinib, stimulated with 60 ng/mL rhM-CSF for 2 minutes at 37°C, and then lysed in 1% Nonidet P40 (NP40) in TSE (50 mM Tris [tris(hydroxymethyl)aminomethane], 100 mM NaCl, 1 mM EDTA [ethylenediaminetetraacetic acid], pH 8.0), supplemented with 0.5 M NaF, 0.1 M NaPPi, 0.5 M NaVO4, 0.1 M PMSF (phenylmethylsulfonyl fluoride), and complete protease inhibitors (Roche, Basel, Switzerland).
c-fms was immunoprecipitated from FDC-cfms cell extracts using 2.5 μg/mL of anti–c-fms antibody (2-4A5; Santa Cruz Biotechnology) and protein G Sepharose (Amersham Biosciences, Uppsala, Sweden). Immunoprecipitations were carried out for 2 hours at 4°C, and samples were washed extensively and resuspended in 30 μL of reducing (antiphosphotyrosine blots) or nonreducing (anti–c-fms blots) loading buffer. Equivalent amounts of protein as determined using a Micro BCA Protein Assay Reagent (Pierce, Rockford, IL) were used in each immunoprecipitation.
Detection of phosphotyrosine and c-fms by Western blot analysis
Immunoprecipitates were run on an 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and electroblotted to polyvinylidene difluoride (PVDF) membrane (Amersham Biosciences). Membranes were probed with antiphosphotyrosine antibodies (mixture of 1/1000 PY20 [Santa Cruz Biotechnology] and 1/2000 4G10 [Cell Signalling Technology, Beverly, MA]) or an anti–c-fms antibody (R&D Systems). Immunoprecipitated proteins were revealed by incubation in an alkaline-phosphatase–conjugated anti–mouse immunoglobulin (Ig) antibody, and developed using enhanced chemifluorescence (ECF) substrate (Amersham Biosciences). The membrane was imaged using a Typhoon 9410 fluorimager (Amersham Biosciences) at 488-nm excitation, and quantitation was performed using ImageQuant software (Amersham Biosciences).
Flow-cytometric analysis of c-fms expression
FDC-cfms cells (5 × 105) were cultured for 1 hour in serum-free IMDM in the presence of imatinib, then stained with 0.5 μg of anti–c-fms antibody (R&D Systems). Bound antibody was detected by staining with an R-phycoerythrin–conjugated anti–mouse antibody (SouthernBiotech, Birmingham, AL), and cells analyzed using a Coulter XL-MCS analytical flow cytometer (Beckman Coulter).
Statistical and pharmacokinetic data analysis
Data were analyzed using analysis of variance (ANOVA), and differences were considered to be statistically significant when the P value was less than .05. The calculation of 50% inhibitory concentration (IC50) values was performed using the Hill equation:
Results
Anti–c-fms, but not anti–c-kit, inhibits monocyte and/or macrophage colony growth
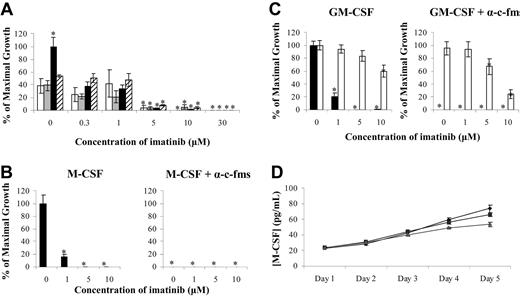
At first, we investigated the potential involvement of the known imatinib target, c-kit, in the growth of monocyte and/or macrophage colonies (Figure 1A). Colony cultures were established using normal BMMNCs stimulated with 4HGF or 5HGF, and the effect of anti–c-kit antibodies in combination with imatinib examined on monocyte and/or macrophage growth (Figure 1A). In the absence of imatinib, addition of anti–c-kit to 4HGF–stimulated cultures had no effect on colony number. The dose of anti–c-kit (1.0 μg/mL) was sufficient to completely block the SCF receptor, as its addition to cultures stimulated with 4HGF plus SCF (5HGF) reduced colony growth to the same level as cultures stimulated with 4HGF alone (Figure 1A).
Imatinib inhibits monocyte and/or macrophage colony formation. (A) Normal BMMNCs were stimulated with 4HGF (IL-3, IL-6, G-CSF, GM-CSF) (□), 4HGF plus α-c-kit antibodies (▦), 5HGF (IL-3, IL-6, G-CSF, GM-CSF, SCF) (▪), or 5HGF plus α–c-kit antibodies (▨), and monocyte and/or macrophage colony growth examined. Growth of monocyte and/or macrophage (▪) and eosinophil (□) colonies from CD34+ progenitors stimulated with M-CSF (B) or GM-CSF (C) was also examined. The 4HGF (A) or 0 μM imatinib controls (B,C) were used to set the value for maximal growth, and all other data points were normalized to this value. ELISA was used to determine whether GM-CSF–stimulated monocyte cultures produced autocrine M-CSF (D) (♦, 0 μM imatinib; ▪, 1.0 μM imatinib, ▴, 5.0 μM imatinib). Results are representative of 3 individual experiments. *P < .05. Error bars represent the standard error of the mean (SEM).
Imatinib inhibits monocyte and/or macrophage colony formation. (A) Normal BMMNCs were stimulated with 4HGF (IL-3, IL-6, G-CSF, GM-CSF) (□), 4HGF plus α-c-kit antibodies (▦), 5HGF (IL-3, IL-6, G-CSF, GM-CSF, SCF) (▪), or 5HGF plus α–c-kit antibodies (▨), and monocyte and/or macrophage colony growth examined. Growth of monocyte and/or macrophage (▪) and eosinophil (□) colonies from CD34+ progenitors stimulated with M-CSF (B) or GM-CSF (C) was also examined. The 4HGF (A) or 0 μM imatinib controls (B,C) were used to set the value for maximal growth, and all other data points were normalized to this value. ELISA was used to determine whether GM-CSF–stimulated monocyte cultures produced autocrine M-CSF (D) (♦, 0 μM imatinib; ▪, 1.0 μM imatinib, ▴, 5.0 μM imatinib). Results are representative of 3 individual experiments. *P < .05. Error bars represent the standard error of the mean (SEM).
To examine the role of c-fms in colony growth, anti–c-fms antibodies were added to cultures stimulated with M-CSF or GM-CSF. In M-CSF cultures, only monocyte and/or macrophage colonies were observed, and growth was inhibited by up to 80% at 1.0 μM imatinib (Figure 1B). Anti–c-fms antibody was sufficient to completely inhibit monocyte and/or macrophage colonies, demonstrating dependence of growth on M-CSF (Figure 1B).
As previously described,10 stimulation of cultures with GM-CSF induced growth of both monocyte and/or macrophage and eosinophil colonies (Figure 1C). The addition of 1.0 μM imatinib to GM-CSF–stimulated cultures reduced monocyte and/or macrophage colony growth by approximately 80%. In contrast, eosinophil growth was unaffected by imatinib at concentrations less than 10.0 μM (Figure 1C). Interestingly, the addition of an anti–c-fms antibody to GM-CSF–stimulated cultures completely abrogated monocyte and/or macrophage colony growth, while eosinophil growth was unaffected (Figure 1C).
Since GM-CSF–stimulated monocyte and/or macrophage colony growth was inhibited by the addition of anti–c-fms antibodies, we examined whether GM-CSF induced autocrine production of M-CSF in our culture system. Low levels (20 pg/mL) of M-CSF were detected 24 hours after cultures were established (Figure 1D), with these levels increasing over the 5 days to approximately 70 pg/mL. The addition of 1.0 μM imatinib had no effect on M-CSF production by the cultured monocytes, while a 30% decrease in M-CSF production at day 5 was seen at 5.0 μM imatinib.
Imatinib inhibits the growth of FDC-cfms cells at therapeutic concentrations
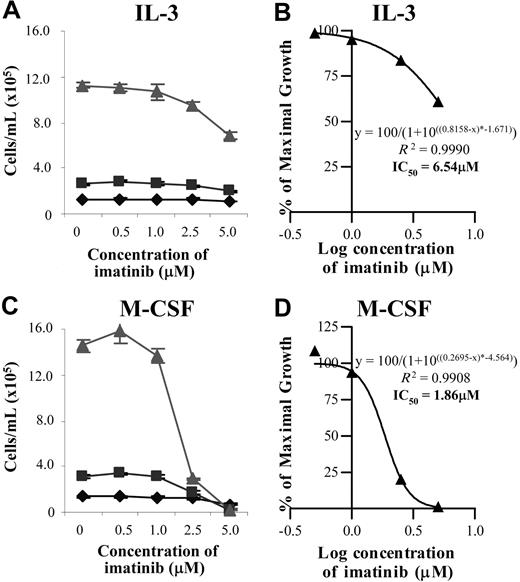
Since imatinib appeared to be mediating its inhibitory effect on monocyte and/or macrophage development through c-fms, the effect of imatinib on a cell line dependent on either murine IL-3 or human M-CSF was investigated. Control cultures stimulated with murine IL-3 showed no imatinib-specific effects on cell growth at 12 or 24 hours across the range of imatinib doses examined (Figure 2A). At 48 hours, FDC-cfms proliferation in the presence of IL-3 was reduced by 15% at 2.5 μM imatinib and 40% at 5.0 μM imatinib, suggesting that imatinib had a mildly toxic effect on these cells (Figure 2A). At the 48-hour time point, inhibition of IL-3–stimulated cell proliferation by imatinib was estimated to occur at an IC50 value of 6.54 μM, reflecting the toxicity of the drug at higher doses (Figure 2B).
Imatinib inhibits M-CSF– but not IL-3–stimulated growth of a c-fms–expressing cell line at therapeutic concentrations. FDC-cfms cells were stimulated with murine IL-3 (A) or rhM-CSF (C) and cell counts performed at 12 (♦), 24 (▪) and 48 ( ) hours. The relationship between imatinib concentration and cell growth was predicted according to a sigmoidal model and used to calculate IC50 values (B,D). Results are representative of 3 individual experiments. Error bars represent SEM.
) hours. The relationship between imatinib concentration and cell growth was predicted according to a sigmoidal model and used to calculate IC50 values (B,D). Results are representative of 3 individual experiments. Error bars represent SEM.
Imatinib inhibits M-CSF– but not IL-3–stimulated growth of a c-fms–expressing cell line at therapeutic concentrations. FDC-cfms cells were stimulated with murine IL-3 (A) or rhM-CSF (C) and cell counts performed at 12 (♦), 24 (▪) and 48 ( ) hours. The relationship between imatinib concentration and cell growth was predicted according to a sigmoidal model and used to calculate IC50 values (B,D). Results are representative of 3 individual experiments. Error bars represent SEM.
) hours. The relationship between imatinib concentration and cell growth was predicted according to a sigmoidal model and used to calculate IC50 values (B,D). Results are representative of 3 individual experiments. Error bars represent SEM.
Where M-CSF was the sole source of stimulation, a 50% inhibition of cell growth was seen at 5.0 μM imatinib 12 hours after the initiation of the culture (Figure 2C). At 24 hours, cell counts were up to 45% lower at 2.5 μM imatinib than cultures not stimulated with imatinib, and at 5.0 μM imatinib, cell counts were lower than seeded values (Figure 2C). The effect of imatinib on M-CSF–stimulated FDC-cfms cultures was most profound at 48 hours, when 2.5 μM imatinib reduced cell counts by 80% compared with controls, and at 5.0 μM imatinib, when the concentration of cells was lower than the seeded level (Figure 2C). The IC50 was calculated to be 1.86 μM imatinib at 48 hours (Figure 2D), which was markedly lower than the IC50 value observed following imatinib treatment of IL-3–stimulated FDC-cfms cells (Figure 2B).
Imatinib inhibits c-fms phosphorylation
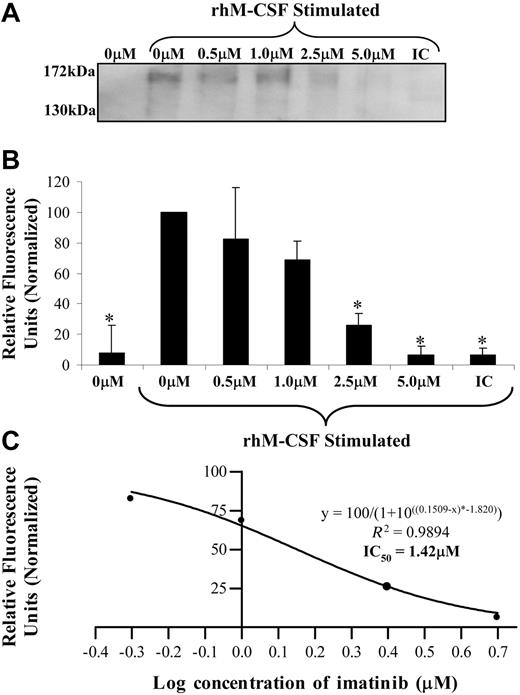
To determine if imatinib mediated an inhibitory effect on the M-CSF receptor directly, the effect of imatinib on the phosphorylation of c-fms was examined on FDC-cfms cells (Figure 3A). Starved FDC-cfms cells that were not stimulated with M-CSF displayed no c-fms phosphorylation (Figure 3A). Starved FDC-cfms cells that were stimulated with M-CSF exhibited receptor phosphorylation, and 1.0 μM imatinib reduced this phosphorylation by approximately 30%. At 2.5 μM imatinib, c-fms phosphorylation was reduced by 75%, and at 5.0 μM imatinib, no significant phosphorylation was observed (Figure 3B). Analysis of the data yielded an IC50 value for imatinib inhibition of c-fms phosphorylation of 1.42 μM (Figure 3C), similar to the value obtained in the proliferation experiments (Figure 2D).
Imatinib inhibits c-fms phosphorylation. (A) FDC-cfms cells were starved for 1 hour in the presence of imatinib, then stimulated with M-CSF for 2 minutes. Immunoprecipitates were examined for α-phosphotyrosine using Western blotting (IC indicates isotype control). (B) Band intensity was normalized from 3 Western blots. (C) The relationship between imatinib concentration and phosphorylation was predicted according to a sigmoidal model and used to calculate an IC50 value. Results are representative of 3 individual experiments. *P < .05). Error bars represent SEM.
Imatinib inhibits c-fms phosphorylation. (A) FDC-cfms cells were starved for 1 hour in the presence of imatinib, then stimulated with M-CSF for 2 minutes. Immunoprecipitates were examined for α-phosphotyrosine using Western blotting (IC indicates isotype control). (B) Band intensity was normalized from 3 Western blots. (C) The relationship between imatinib concentration and phosphorylation was predicted according to a sigmoidal model and used to calculate an IC50 value. Results are representative of 3 individual experiments. *P < .05). Error bars represent SEM.
Imatinib does not affect c-fms protein expression
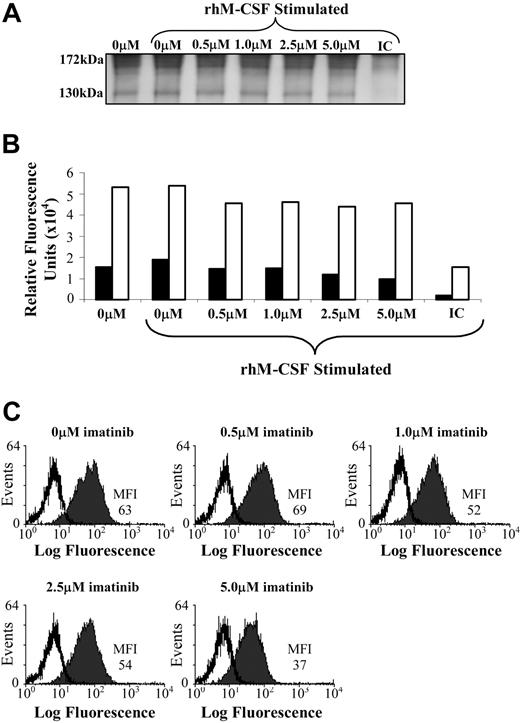
Since phosphorylation of c-fms was inhibited by imatinib treatment, Western blots were also probed for c-fms protein to confirm this effect was not due to decreased c-fms expression (Figure 4A). Two c-fms bands were detected in these blots, with the 170-kD band representing the fully glycosylated c-fms protein, and the 130-kD band representing the immature, nonglycosylated form.22 The intensity of both the 170-kD and 130-kD bands was quantitated, and the expression of both forms of c-fms were unaffected by imatinib treatment (Figure 4A-B). The lack of effect of imatinib on the expression of c-fms was confirmed using flow cytometry, with no difference in surface expression of c-fms in FDC-cfms cells cultured in 0.5 μM to 2.5 μM imatinib, and with marginally lower expression at 5.0 μM imatinib (Figure 4C).
Imatinib does not affect c-fms expression by an M-CSF–dependent cell line. (A,B) FDC-cfms cells were starved for 1 hour in the presence of imatinib, and examined for c-fms expression by Western blotting (IC indicates isotype control) or (C) flow cytometry. Immunoprecipitates were examined for c-fms protein expression, with the 170-kD band (□) representing the mature c-fms protein, and the 130-kD band representing the immature, nonglycosylated form (▪) (A,B). Results are representative of 3 individual experiments. MFI indicates mean fluorescence intensity, and isotype matched controls are represented by unfilled histograms (C).
Imatinib does not affect c-fms expression by an M-CSF–dependent cell line. (A,B) FDC-cfms cells were starved for 1 hour in the presence of imatinib, and examined for c-fms expression by Western blotting (IC indicates isotype control) or (C) flow cytometry. Immunoprecipitates were examined for c-fms protein expression, with the 170-kD band (□) representing the mature c-fms protein, and the 130-kD band representing the immature, nonglycosylated form (▪) (A,B). Results are representative of 3 individual experiments. MFI indicates mean fluorescence intensity, and isotype matched controls are represented by unfilled histograms (C).
Discussion
Here, we show for the first time that the known targets for the protein-tyrosine kinase inhibitor imatinib can be extended to include the M-CSF receptor, c-fms. Although the potency of inhibition is lower than what is observed for Abl (IC50 = 0.25 μM), c-kit (IC50 = 0.1 μM) or PDGF (IC50 = 0.25 μM) receptor tyrosine kinases, as calculated by Western blotting,1,4 inhibition was observed at concentrations of imatinib that are within the therapeutic dose range (IC50 = 1.42 μM). These data contradict published findings reporting that imatinib does not affect tyrosine phosphorylation of c-fms–expressing NIH 3T3 cells at concentrations up to 10.0 μM.4 However, the analysis performed by Buchdunger et al4 involved Western blot analysis on whole-cell lysates, and it was not stated whether specific stimulation of c-fms was performed. In our studies, the effect of imatinib on the phosphorylation of c-fms was examined on c-fms immunoprecipitates from a hematopoietic cell line following specific receptor stimulation with saturating doses of M-CSF. These data were further supported through demonstration that imatinib inhibited the proliferation of an M-CSF–dependent cell line at an IC50 of 1.86 μM.
The receptor c-fms is expressed at low levels on monocytes, and its expression markedly increases during differentiation to macrophages. In the absence of M-CSF, the mature cell-surface form of c-fms is relatively stable and ligand binding down-regulates receptor expression by internalization and degradation within lysosomes.23 Since phosphorylation of c-fms was inhibited by treatment with imatinib, Western blots were also probed for total c-fms protein to confirm this effect was not due to an imatinibmediated decrease in c-fms protein expression. No difference in the expression of c-fms protein in the presence of imatinib was observed using either Western blotting or flow cytometry, indicating that decreased c-fms phosphorylation by imatinib was not attributable to a decrease in c-fms protein expression.
Abrogation of c-fms tyrosine phosphorylation by imatinib provides a direct mechanism for the inhibitory effect of imatinib on monocyte and/or macrophage growth in M-CSF–stimulated colony cultures. This inhibition cannot be attributed to imatinib blockade of c-kit tyrosine kinases in the presence of exogenous SCF. Imatinib inhibition of c-fms signal transduction as a mechanism for the suppression of monocyte and/or macrophage growth is further supported by similarity in the IC50 values for imatinib-mediated suppression of monocyte and/or macrophage growth/function (0.86-1.25 μM imatinib) and c-fms phosphorylation (1.47 μM imatinib). Interestingly, the addition of anti–c-fms antibodies to GM-CSF–stimulated colony cultures also inhibited monocyte and/or macrophage growth, while neither imatinib nor anti–c-fms antibodies affected the growth of eosinophil colonies in GM-CSF–stimulated cultures until high (10.0 μM) concentrations of imatinib were used. These results suggest that whereas GM-CSF directly stimulates eosinophil growth, it indirectly stimulates the growth of monocyte and/or macrophage colonies through autocrine production of M-CSF. This hypothesis is supported by reports that GM-CSF stimulation of monocytes induces M-CSF protein secretion.24,25 To examine the possibility that GM-CSF was acting indirectly in this present study, ELISA for M-CSF was performed on GM-CSF–stimulated monocyte culture supernatants harvested over a 5-day period. The maximum level of M-CSF produced was estimated to be 70 pg/mL, which is 300- to 500-fold lower than the concentration of added M-CSF used in experiments in this study. It is possible that a suboptimal concentration of M-CSF in combination with GM-CSF was sufficient to induce the growth and differentiation of monocytes, with GM-CSF acting synergistically to potentiate the effect of M-CSF.
Although patients with CML treated with imatinib exhibit myelosuppression, particularly at higher doses, there does not appear to be specific suppression of the monocytic lineage.26 Our own assessment of 16 patients treated with either 600 mg or 800 mg imatinib over a period of 1 to 2 years also revealed no differences in peripheral blood monocyte levels when compared with healthy donors (data not shown). However this in vivo assessment of monocyte and/or macrophage numbers is confounded by their relatively short lifespan and their localization as macrophages within tissues.
The absence of an imatinib-specific effect on cells of the monocyte lineage in vivo may be due to redundancy in signaling pathways, such as signaling through the GM-CSF receptor.27 Monocytopenia may become significant where combination therapies that block multiple pathways are used. This could include current treatment strategies where imatinib is administered in combination with interferon-α (IFN-α) or cytarabine.28-30
In conclusion, we demonstrate that the in vitro profile of imatinib can be extended to include c-fms. Our findings have major therapeutic implications and suggest that the clinical application of imatinib may be extended to include the treatment of diseases involving abnormal c-fms activation, including common cancers such as breast and epithelial ovarian cancer, and inflammatory conditions such as rheumatoid arthritis. Abnormal c-fms expression has been demonstrated in a range of cancers including carcinomas of the breast and ovary, and activation of c-fms has been demonstrated to stimulate tumor invasion.31,32 Furthermore, abnormal c-fms expression in breast tumors and advanced epithelial ovarian carcinomas correlates with tumor cell invasiveness and adverse clinical prognosis, and M-CSF produced by breast tumors has been implicated in the promotion of bone metastasis.33,34 Outside the hematopoietic system, c-fms signaling plays an important role in pregnancy, affecting preimplantation embryo development and mammary gland development.35 Through M-CSF stimulation, c-fms also plays an important role in bone metabolism and inflammatory processes.36
Prepublished online as Blood First Edition Paper, January 6, 2005; DOI 10.1182/blood-2004-10-3967.
Supported by a Cancer Council South Australia grant (A.B.L.), an Australian Postgraduate Award (Adelaide University; A.L.D.), and a Lions Medical Research Foundation Scholarship (A.L.D.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank Drs M. Roussel and C. Sherr from the St Jude Children's Research Hospital, Memphis, TN, for providing an MSCV-CSF1Rwt-IRES GFP plasmid, and Novartis for supplying imatinib for research purposes. We would also like to thank the Detmold Family Imaging Centre (Adelaide, Australia) for access to flow cytometers and the Australian Red Cross Blood Service for providing buffy coats.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal