Abstract
HIV-1 Nef protein is a major determinant of the pathogenicity of the virus. It has been shown that Nef activates Hck, a member of Src family kinase, in monocytes/macrophages and that the interaction is critical for AIDS-like disease progression in a mouse model. However, it was unclear how the molecular interaction in monocytes/macrophages leads to disease progression. Here, we show for the first time that Nef interferes with the macrophage colony-stimulating factor (M-CSF)/M-CSF receptor signal pathway. In this study, we introduced a conditionally active Nef into myeloid leukemia TF-1-fms cells and analyzed their responsiveness to M-CSF. We found that Nef-activated Hck constitutively associated with the M-CSF receptor complex. The formation of the molecular complex should occur under physiologic conditions, that is, on M-CSF stimulation. Because of aberrant molecular association, the tyrosine-phosphorylation/activation of the receptor in response to M-CSF was markedly diminished in Nef-active cells. Consequently, Nef activation caused the inhibition of M-CSF-mediated proliferation of TF-1-fms cells and macrophage differentiation of the cells induced by M-CSF and 12-O-tetradecanoylphorbol 13-acetate. These results indicate that HIV-1 Nef interferes with M-CSF receptor signaling through Hck activation and thereby inhibits M-CSF functions in monocytes/macrophages. (Blood. 2005;105:3230-3237)
Introduction
Nef is an accessory protein of HIV-1, a causative virus for AIDS. A number of reports, including studies of HIV-1-infected patients and of animal models, have demonstrated that the Nef protein is a major determinant of the pathogenicity of HIV-1.1-4 Transgenic mice expressing the complete coding sequences of HIV-1 under the regulatory sequences of human CD4 gene developed severe AIDS-like abnormalities: loss of CD4+ T cells, thymus atrophy, failure to thrive, diarrhea, wasting, premature death, interstitial pneumonitis, and tubulo-interstitial nephritis.4 Using this mouse model and the introduction of mutation into selected HIV-1 gene(s), Hanna et al4 clearly demonstrated that Nef harbored a major disease determinant. Therefore, much attention has been given to Nef to explain its contribution to HIV-1 pathogenesis and to investigate it as a target for antiviral drug development.
CD4+ T cells and monocytes/macrophages are the principal target cells for HIV-1, and the functions of Nef in CD4+ T cells are generally accepted as accounting for many aspects of viral pathogenesis (reviewed in Fackler and Baur5 and Peterlin and Trono6 ). For example, Nef has been shown to cause the downregulation of cell surface molecules such as cell surface receptor CD47 and major histocompatibility complex (MHC) class I in CD4+ T cells.8 The down-regulation of MHC class I is considered to diminish the recognition of HIV-1-infected cells by cytotoxic T cells. In contrast, the contribution of monocytes/macrophages to viral pathogenesis is less well understood. Several lines of evidence support the idea that monocytes/macrophages and CD4+ T cells are important for the development and progression of AIDS. Recent studies have demonstrated that Nef induces the production of CC chemokines (macrophage inflammatory proteins-1α and -1β) and soluble forms of CD23 and intracellular adhesion molecule-1 by macrophages.9,10 These Nef-induced factors from macrophages might stimulate the chemotaxis and activation of resting CD4+ T cells, thereby promoting the permissiveness of CD4+ T cells to HIV-1 infection.9,10 This Nef function is likely to be mediated by the activation of NF-κB transcription factor.10
Another important feature of Nef is the binding at high affinity to myeloid lineage-specific Src family kinase Hck to activate its kinase activity.11,12 The proline-rich (PxxP) motif in Nef binds to the Src homology 3 (SH3) domain of Hck.11 Interestingly, Hck was found to bind preferentially and with higher affinity to Nef than other Src kinases.11 The pathologic relevance of the molecular interaction in vivo was revealed by studies of HIV-1 transgenic mice. The mutation in the SH3-binding motif of Nef abolished the development of AIDS-like disease in the HIV-1 transgenic mice.13 Moreover, the breeding of transgenic mice expressing the complete coding sequences of HIV-1 on a hck-/- background resulted in the delay of disease development.13 These studies suggest that the modulation of macrophage functions by Nef is important for disease development because the molecular interaction between Nef and Hck occurs in cells of macrophage lineage but not in CD4+ T cells. However, it is unclear how the molecular interaction in monocytes/macrophages contributes to HIV-1 pathogenesis. To answer the question, it is necessary to identify intracellular signaling pathways that would be perturbed by the Nef-Hck interaction.
Macrophage colony-stimulating factor (M-CSF) is a primary cytokine for monocytes/macrophages and regulates their development and various effector functions (reviewed in Roth and Stanley14 ). The importance of M-CSF in macrophage development in vivo has been clearly proven using a naturally occurring osteopetrotic (op/op) mouse.15 The M-CSF biologic effects are mediated by a unique receptor encoded by the proto-oncogene c-fms, a receptor tyrosine kinase (reviewed in Bourette and Rohrschneider16 ). The binding of M-CSF induces the dimerization of the receptor and the activation of its kinase activity.16 This leads to the autophosphorylation of specific tyrosine residues in the cytoplasmic domain and the subsequent interactions of the phosphorylated residues with other proteins, each initiating signaling along specific pathways.16 The Src family is one of the well-documented partners for the activated M-CSF receptor. M-CSF stimulation results in the activation of Src kinases, including Hck; simultaneously, these kinases associate with the ligand-activated M-CSF receptor.17-21 We and other investigators17-21 have demonstrated that the activation of Src kinases is required for biologic effects of M-CSF such as mitogenic signal and differentiation-inducing signal. This finding prompted us to examine whether the molecular interaction between HIV-1 Nef and Hck would affect M-CSF receptor signaling and thereby modulate M-CSF bioactivities. In this paper, we demonstrate for the first time that Nef interferes with M-CSF-induced activation of its receptor, the earliest signaling event induced by the ligand, through Hck activation and thereby inhibits M-CSF functions on monocytes/macrophages.
Materials and methods
Cells and culture conditions
TF-1-fms cells were established by introducing the human M-CSF receptor (c-fms) gene into a human myeloid leukemia cell line, TF-1, as described previously.22 Original TF-1 cells were growth factor-dependent cells,23 and the proliferation of TF-1-fms cells was dependent on M-CSF.22 In this study, we established a TF-1-fms clone expressing a fusion protein composed of Nef and murine estrogen receptor hormone-binding domain (Nef-ER) by introducing pEBB-Nef-ER-IRES-puro plasmid24 using Effectene reagent (Qiagen, Valencia, CA). The Nef gene was derived from NL4-3 strain of HIV-1,24 and its product has been shown to activate the kinase activity of Hck.11,12 The transfection was performed according to the manufacturer's recommendations. Transfected cells were selected in medium containing 1.5 μg/mL puromycin (Sigma, St Louis, MO), followed by limiting dilution to establish stable cell lines. The stable TF-1-fms clones were analyzed for Nef-ER expression by Western blotting using anti-Nef rabbit antiserum.25 We also established a TF-1-fms clone expressing enhanced green fluorescent protein (EGFP). This was achieved with a retrovirus vector (MSCV2.2/IRES-EGFP),26 as described previously.27 In brief, the amphotropic packaging PT67 cells (Clontech, Palo Alto, CA) were transfected with MSCV2.2/IRES-EGFP vector using LipofectAMINE2000 reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's recommendations. Then, TF-1-fms cells were cocultured with the transfected PT67 cells in the presence of 8 μg/mL Polybrene (Sigma). The stable TF-1-fms clones were obtained by limiting dilution and were analyzed for EGFP expression by flow cytometry. Parental TF-1-fms cells and EGFP-expressing cells were maintained with RPMI 1640 (Sigma)-10% fetal calf serum (FCS) in the presence of recombinant human M-CSF (100 ng/mL)22 and 200 μg/mL G418 (Life Technologies, Grand Island, NY). Nef-ER-expressing cells were maintained with RPMI 1640-10% FCS in the presence of M-CSF, G418, and puromycin. To activate the Nef-ER protein, an estrogen analog, 4-hydroxytamoxifen (4-HT; Sigma), was added to the cultures.24 Unless otherwise stated, 4-HT was used at a final concentration of 1 μM. To induce the differentiation of TF-1-fms-Nef-ER cells into macrophages, cells were cultured with M-CSF and 100 ng/mL 12-O-tetradecanoylphorbol 13-acetate (TPA; Sigma).
Cell count and flow cytometry
Viable cell counts were obtained by enumerating cells that excluded trypan blue dye. Adherent cells were harvested by trypsinization.
To detect apoptotic cells, the flow cytometric analysis of propidium iodide (PI)-stained nuclei was performed as described previously.28 In brief, cells were washed with phosphate-buffered saline (PBS) and then incubated in hypotonic lysing buffer (0.1% sodium citrate, 0.3% Nonidet P-40, and 50 μg/mL PI) at 4°C for 30 minutes. Alternatively, cells were stained with annexin V conjugated to phycoerythrin (PE) (PharMingen, San Jose, CA) and vital dye 7-aminoactinomycin D (7-AAD; PharMingen). Cultured cells were washed with PBS, resuspended in binding buffer (10 mM HEPES [N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid], 140 mM NaCl, and 2.5 mM CaCl2), and incubated with annexin V-PE and 7-AAD (1.25 μg/mL) at room temperature for 15 minutes. Flow cytometry analyses were performed with FACSCalibur using Cell Quest Software (Becton Dickinson, Mountain View, CA).
In this study, we analyzed the expression level of M-CSF receptor on TF-1-fms-Nef-ER cells by flow cytometry using Flag-tagged M-CSF protein. The Flag (DYKDDDDK) sequence was introduced into the C-terminus of mature M-CSF polypeptides22 by polymerase chain reaction (PCR) amplification with an upstream primer (5′-TGCCGGGACCCAGCTGCCCGTATGA-3′) and a downstream primer (5′-CTCACTTGTCGTCATCGTCTTTGTAGTCTCGGCTAGAGCA-3′). Codons specifying the Flag sequence are underscored. The PCR product was subcloned into an expression vector, pEF-BOS.29 The resultant plasmid was transfected into COS7 cells using LipofectAMINE2000 reagent (Invitrogen),30 and the culture supernatant was used as a source of Flag-tagged M-CSF. For analysis, cells were incubated with the Flag-tagged M-CSF, followed by biotin-labeled anti-Flag M2 antibody (10 μg/mL; Sigma) and PE-labeled streptavidin (5 μg/mL; PharMingen).31
Immunoprecipitation and Western blotting
Immunoprecipitation and Western blotting were performed essentially as described previously.17 Cells were growth-factor depleted for 14 hours in RPMI 1640-10% FCS and then stimulated with M-CSF (100 ng/mL) for the indicated periods. For the activation of Nef, 4-HT (1 μM) was added to the culture at the initiation of M-CSF deprivation. Then cells were solubilized with Nonidet P-40 lysis buffer (1% Nonidet P-40, 50 mM Tris HCl, 150 mM NaCl) containing protease inhibitors (1 mM EDTA [ethylenediaminetetraacetic acid], 1 mM phenylmethylsulfonyl fluoride, 1 μg/mL aprotinin, 1 μg/mL leupeptin, and 1 μg/mL pepstatin) and phosphatase inhibitors (1 mM Na3VO4 and 1 mM NaF). Cell lysates were centrifuged, and the resultant supernatants were subjected to Western blotting or immunoprecipitation. Antibodies (purchased from Santa Cruz Biotechnology, Santa Cruz, CA) used for Western blotting were as follows: anti-M-CSF receptor rabbit immunoglobulin G (IgG) (C-20), antiphosphotyrosine mouse IgG (PY99), anti-ERK rabbit IgG (K-23), antiphosphorylated ERK mouse IgG (E-4), and anti-Hck rabbit IgG (N-30). Anti-Hck mouse IgG (clone 18; Transduction Laboratories, Lexington, KY) was also used. Antibodies used for immunoprecipitation were as follows: anti-M-CSF receptor rat IgG (12-2D6; Zymed, South San Francisco, CA), anti-Hck rabbit IgG (Santa Cruz Biotechnology), and antiphosphotyrosine mouse IgG (PY99; Santa Cruz Biotechnology). Immune complexes were precipitated with Protein A/G PLUS-Agarose (Santa Cruz Biotechnology). Cleared cell lysates and immunoprecipitates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions, and proteins were transferred to a nylon membrane (Hybond-P; Amersham, Buckinghamshire, United Kingdom). Detection was performed using the Enhanced Chemiluminescence Western Blotting Detection System (Amersham) with horseradish peroxidase-coupled antiimmunoglobulin (Amersham).
Expression of M-CSF receptor, Nef, and Hck in 293T cells
Expression plasmid for human M-CSF receptor was prepared as described previously.22 The plasmids for Nef and human Hck were provided by E. T. Sawai32 and Y. Murakami,33 respectively. The Nef gene was derived from the SF2 strain of HIV-1,32 and its product has been shown to activate the kinase activity of Hck.34 The human embryonic kidney cell line 293T was obtained from the American Type Culture Collection (Manassas, VA) and was maintained with Dulbecco modified Eagle medium (DMEM; Sigma)-10% FCS. Cells were grown on a 6-well tissue culture plate and were transfected with plasmids for M-CSF receptor (0.4 μg), Nef (0.8 μg), and Hck (0.4 μg), using LipofectAMINE2000 reagent (Invitrogen). After transfection, the cells were cultured in DMEM-10% FCS for 36 hours and then cultured in DMEM-0.1% FCS for an additional 12 hours. In a selected experiment, cells were stimulated with M-CSF for 2 minutes. Cells were solubilized with Nonidet P-40 lysis buffer, and the cleared cell lysates were subjected to immunoprecipitation with anti-M-CSF receptor rat IgG (Zymed) or anti-Hck rabbit IgG (Santa Cruz Biotechnology). Immunoprecipitates were analyzed by Western blotting with antiphosphotyrosine mouse IgG (Santa Cruz Biotechnology), anti-M-CSF receptor rabbit IgG (Santa Cruz Biotechnology), or anti-Hck mouse IgG (Transduction Laboratories).
Results
Inhibition of M-CSF-mediated cell proliferation and differentiation by Nef
We initially attempted to determine whether Nef would affect the biologic effects of M-CSF. To achieve this, we used the human myeloid leukemia cell line TF-1-fms, the proliferation of which was dependent on M-CSF.22 We established the TF-1-fms clone expressing Nef-murine estrogen receptor hormone-binding domain fusion protein (TF-1-fms-Nef-ER) (Figure 1A). The Nef-ER fusion protein could be seen as a 60-kDa band on Western blot (Figure 1B). In this system, Nef was basally inactive, but its function could be inducibly activated by the estrogen analog 4-HT.24 As shown in Figure 1C, we found that the proliferation of TF-1-fms-Nef-ER cells mediated by M-CSF was markedly inhibited on the addition of 4-HT (ie, the activation of Nef). The inhibitory effect was dependent on the concentrations of 4-HT added to the cultures and was specific because 4-HT never affected the proliferation rate of the parental TF-1-fms cells (Figure 1C). Furthermore, we found that Nef activation was associated with the appearance of apoptotic subdiploid cells in the culture of TF-1-fms-Nef-ER cells (Figure 1D). In parallel, Nef activation increased the percentage of annexin V-positive but 7-AAD-negative cells in the culture of TF-1-fms-Nef-ER cells (Figure 1E). These findings indicated that Nef induced apoptotic cell death in TF-1-fms-Nef-ER cells.
TF-1- fms cells expressing Nef-ER show impaired proliferation and apoptotic cell death on Nef activation. (A) Schematic diagram of the Nef-ER-IRES-puro construct. ER indicates estrogen receptor hormone-binding domain; IRES, internal ribosomal entry sequence; puro, puromycin resistance gene. (B) Total cell lysates from the parental TF-1-fms cells (parent) or the TF-1-fms clone stably expressing Nef-ER (Nef-ER) were analyzed for the expression of Nef-ER by Western blotting with -Nef rabbit antiserum. (C) Parental TF-1-fms (□) or TF-1-fms-Nef-ER cells (▦) were seeded at a density of 1 × 104 cells/mL in the presence of M-CSF (100 ng/mL) and increasing concentrations of 4-HT. Cells were cultured for 2 days (top row) or 4 days (bottom row), and viable cells were enumerated. Error bars from triplicate assays are shown. Results are representative of 3 independent experiments. Error bars indicate standard deviation (SD). (D) TF-1-fms-Nef-ER cells were cultured with M-CSF in the absence (top) or the presence of 1 μM 4-HT (bottom) for 48 hours. Apoptotic subdiploid cells was detected by flow cytometry. The percentages of subdiploid cells are shown. (E) Parental TF-1-fms (top row) or TF-1-fms-Nef-ER cells (bottom row) were cultured with M-CSF in the absence (left column) or the presence of 1 μM 4-HT (right column) for 24 hours. Cells were analyzed for the presence of apoptotic cells by staining with annexin V-PE, and 7-AAD (lower right quadrant). The percentages in cells of the upper right corners and lower right corners are shown.
TF-1- fms cells expressing Nef-ER show impaired proliferation and apoptotic cell death on Nef activation. (A) Schematic diagram of the Nef-ER-IRES-puro construct. ER indicates estrogen receptor hormone-binding domain; IRES, internal ribosomal entry sequence; puro, puromycin resistance gene. (B) Total cell lysates from the parental TF-1-fms cells (parent) or the TF-1-fms clone stably expressing Nef-ER (Nef-ER) were analyzed for the expression of Nef-ER by Western blotting with -Nef rabbit antiserum. (C) Parental TF-1-fms (□) or TF-1-fms-Nef-ER cells (▦) were seeded at a density of 1 × 104 cells/mL in the presence of M-CSF (100 ng/mL) and increasing concentrations of 4-HT. Cells were cultured for 2 days (top row) or 4 days (bottom row), and viable cells were enumerated. Error bars from triplicate assays are shown. Results are representative of 3 independent experiments. Error bars indicate standard deviation (SD). (D) TF-1-fms-Nef-ER cells were cultured with M-CSF in the absence (top) or the presence of 1 μM 4-HT (bottom) for 48 hours. Apoptotic subdiploid cells was detected by flow cytometry. The percentages of subdiploid cells are shown. (E) Parental TF-1-fms (top row) or TF-1-fms-Nef-ER cells (bottom row) were cultured with M-CSF in the absence (left column) or the presence of 1 μM 4-HT (right column) for 24 hours. Cells were analyzed for the presence of apoptotic cells by staining with annexin V-PE, and 7-AAD (lower right quadrant). The percentages in cells of the upper right corners and lower right corners are shown.
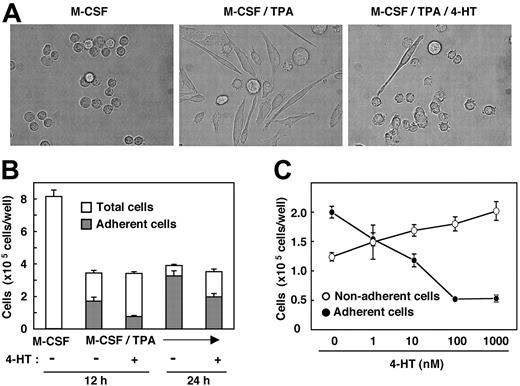
We next attempted to determine whether Nef also could inhibit another biologic activity of M-CSF. The phorbol ester TPA is well known to induce macrophage differentiation of various myeloid leukemia cell lines.23 Recently, we found that, in the presence of TPA, M-CSF markedly stimulates the differentiation of TF-1-fms cells into mature macrophages but that it does not stimulate the proliferation of the cells (S. Suzu et al, unpublished results, February 2004). This was based on several observations. First, the combination of TPA and M-CSF caused more drastic morphologic changes in TF-1-fms cells than did treatment with TPA alone. Second, culture in the presence of TPA and M-CSF contained more adherent cells than in the presence of TPA alone. Third, the phagocytic activity of cells treated with TPA and M-CSF was significantly higher than that of cells treated with TPA alone. Using this culture system, we examined the effect of Nef on the macrophage differentiation-inducing activity of M-CSF. As shown, the morphologic changes in TF-1-fms-Nef-ER cells induced by TPA and M-CSF were markedly inhibited on the activation of Nef (Figure 2A). Furthermore, the number of cells adhering to the dishes in the 4-HT-containing culture was lower than that in the 4-HT-free culture (Figure 2B). The inhibitory effect was dependent on the concentration of 4-HT added to the cultures (Figure 2C).
Nef activation causes inhibition in macrophage differentiation. (A) Morphologies of TF-1-fms-Nef-ER cells cultured for 24 hours with M-CSF (100 ng/mL), M-CSF/TPA (100 ng/mL), or M-CSF/TPA/4-HT (1 μM), as indicated in each case. (B) TF-1-fms-Nef-ER cells were seeded into 6-well culture plates at a density of 2 × 105 cells/well. Cells were cultured in the presence of M-CSF, M-CSF/TPA, or M-CSF/TPA/4-HT for 12 hours or 24 hours. After culture, total cells in the wells (□) and cells that adhered to the dishes (▦) were enumerated. (C) TF-1-fms-Nef-ER cells were seeded as in panel B. Cells were cultured for 12 hours in the presence of M-CSF, TPA, and increasing concentrations of 4-HT. Cells adhering to the dishes (•) or remaining in suspension (○) were enumerated. (B-C) Error bars from triplicate assays are shown and represent SD. Results are representative of 3 independent experiments.
Nef activation causes inhibition in macrophage differentiation. (A) Morphologies of TF-1-fms-Nef-ER cells cultured for 24 hours with M-CSF (100 ng/mL), M-CSF/TPA (100 ng/mL), or M-CSF/TPA/4-HT (1 μM), as indicated in each case. (B) TF-1-fms-Nef-ER cells were seeded into 6-well culture plates at a density of 2 × 105 cells/well. Cells were cultured in the presence of M-CSF, M-CSF/TPA, or M-CSF/TPA/4-HT for 12 hours or 24 hours. After culture, total cells in the wells (□) and cells that adhered to the dishes (▦) were enumerated. (C) TF-1-fms-Nef-ER cells were seeded as in panel B. Cells were cultured for 12 hours in the presence of M-CSF, TPA, and increasing concentrations of 4-HT. Cells adhering to the dishes (•) or remaining in suspension (○) were enumerated. (B-C) Error bars from triplicate assays are shown and represent SD. Results are representative of 3 independent experiments.
Inhibitory effect of Nef on M-CSF bioactivity is not mediated by soluble factors or down-regulation of M-CSF receptor
The inhibitory effects of Nef on M-CSF-induced cell proliferation (Figure 1) and macrophage differentiation (Figure 2) suggested that Nef affected signaling pathways transduced by M-CSF. However, because Nef has been shown to induce the expression of a number of soluble factors (chemokines and cytokines) in monocytes/macrophages,9,10,35-37 the inhibitory effects of Nef observed in our culture systems might be mediated through the secretion of inhibitory molecule(s) by Nef rather than through a direct effect on the M-CSF receptor signaling pathways. To address this issue, we conducted a coculture experiment with parental TF-1-fms cells and TF-1-fms-Nef-ER cells. Parental cells were engineered to express EGFP protein, allowing us to distinguish parental cells from Nef-ER cells in the cocultures through flow cytometric analysis. As shown in Figure 3A, the relative cell number of Nef-ER cells was comparable to that of the parental cells in the absence of 4-HT (left panel) but was markedly low in the presence of 4-HT (right panel). In fact, the absolute cell number of Nef-ER cells in 4-HT-containing cocultures was reduced to approximately 10% when compared with that in 4-HT-free cocultures (Figure 3B). However, and of importance, the absolute cell numbers of the parental cells remained unchanged between 4-HT-containing cocultures and 4-HT-free cocultures (Figure 3B). These results suggested that soluble factors from Nef-active cells, if any, were not involved in the proliferation inhibitory effect of Nef observed in our culture system.
Secretion of soluble factors is not involved in the impaired proliferation of TF-1-fms-Nef-ER cells induced by Nef activation. Parental TF-1-fms cells, but not TF-1-fms-Nef-ER cells, were engineered to express EGFP protein by the retroviral infection system. The cell density of the TF-1-fms-EGFP (parent-EGFP) and TF-1-fms-Nef-ER (Nef-ER) cells was adjusted to 1 × 104 cells/mL, and the cells were cultured after an equal volume of the cell suspensions was combined. These cultures were incubated for 3 days with M-CSF in the presence or the absence of 4-HT. (A) After the cocultures, cells were subjected to flow cytometric analysis to determine the relative cell numbers. (B) Absolute cell numbers of the parental cells and Nef-ER cells were calculated from the relative cell numbers (A) and the total cell numbers in the cocultures. Error bars from triplicate assays are shown and represent SD. These results are representative of 3 independent experiments.
Secretion of soluble factors is not involved in the impaired proliferation of TF-1-fms-Nef-ER cells induced by Nef activation. Parental TF-1-fms cells, but not TF-1-fms-Nef-ER cells, were engineered to express EGFP protein by the retroviral infection system. The cell density of the TF-1-fms-EGFP (parent-EGFP) and TF-1-fms-Nef-ER (Nef-ER) cells was adjusted to 1 × 104 cells/mL, and the cells were cultured after an equal volume of the cell suspensions was combined. These cultures were incubated for 3 days with M-CSF in the presence or the absence of 4-HT. (A) After the cocultures, cells were subjected to flow cytometric analysis to determine the relative cell numbers. (B) Absolute cell numbers of the parental cells and Nef-ER cells were calculated from the relative cell numbers (A) and the total cell numbers in the cocultures. Error bars from triplicate assays are shown and represent SD. These results are representative of 3 independent experiments.
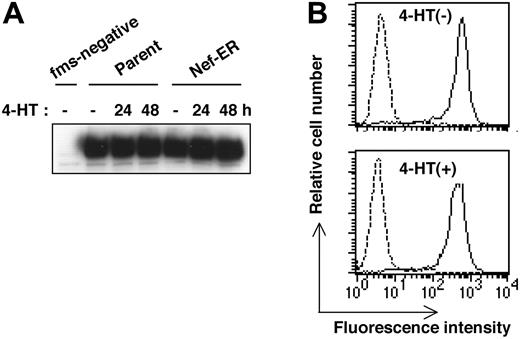
Another well-known function of Nef is the down-regulation of cell surface molecules such as CD4 and MHC class I.7,8 Therefore, we next examined whether Nef activation caused the down-regulation of the receptor for M-CSF in TF-1-fms-Nef-ER cells. As shown by the culture in Figure 4A, on which Western blotting analysis was performed, we did not find any change in the expression level of M-CSF receptor, even when TF-1-fms-Nef-ER cells were cultured for up to 2 days in the presence of 4-HT. Flow cytometric analysis with the Flag-tagged M-CSF also revealed that the level of cell surface expression of M-CSF receptor in TF-1-fms-Nef-ER cells treated for 24 hours with 4-HT was comparable with that in Nef-inactive cells (Figure 4B). Taken together with the result of the coculture experiment (Figure 3), these results strongly suggested that Nef affected intrinsic M-CSF receptor signaling pathways and thereby inhibited M-CSF activities.
Down-regulation of M-CSF receptor is not involved in the impaired proliferation of TF-1-fms-Nef-ER cells induced by Nef activation. (A) Parental TF-1-fms and TF-1-fms-Nef-ER cells were cultured with 4-HT for the indicated periods, and the levels of M-CSF receptor proteins were analyzed by Western blotting. Total cell lysates from TF-1 cells, but not from TF-1-fms cells, in which the M-CSF receptor gene had not been introduced, were included in the analysis as a negative control (fms-negative). (B) TF-1-fms-Nef-ER cells were left untreated (top) or were treated with 4-HT for 24 hours (bottom), and the level of cell surface M-CSF receptor expression was analyzed by flow cytometry with Flag-tagged M-CSF (solid lines). Profiles of cells incubated with a Flag-tagged protein,30 unrelated to M-CSF, are also shown as a control (broken lines).
Down-regulation of M-CSF receptor is not involved in the impaired proliferation of TF-1-fms-Nef-ER cells induced by Nef activation. (A) Parental TF-1-fms and TF-1-fms-Nef-ER cells were cultured with 4-HT for the indicated periods, and the levels of M-CSF receptor proteins were analyzed by Western blotting. Total cell lysates from TF-1 cells, but not from TF-1-fms cells, in which the M-CSF receptor gene had not been introduced, were included in the analysis as a negative control (fms-negative). (B) TF-1-fms-Nef-ER cells were left untreated (top) or were treated with 4-HT for 24 hours (bottom), and the level of cell surface M-CSF receptor expression was analyzed by flow cytometry with Flag-tagged M-CSF (solid lines). Profiles of cells incubated with a Flag-tagged protein,30 unrelated to M-CSF, are also shown as a control (broken lines).
Mechanism by which Nef inhibits M-CSF receptor signaling
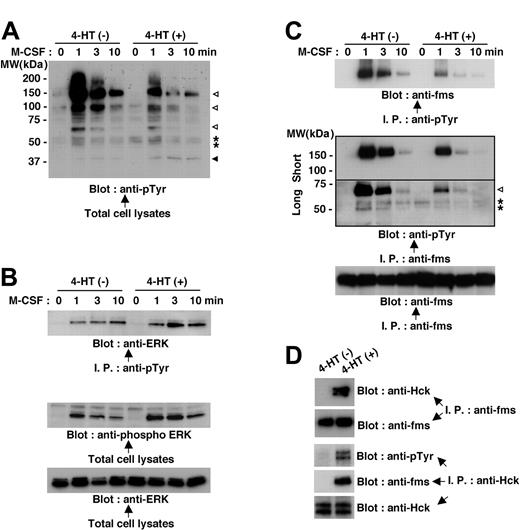
Because M-CSF receptor is a receptor tyrosine kinase,14,16 we initially compared the M-CSF-induced protein tyrosine phosphorylation in Nef-active TF-1-fms-Nef-ER cells with that in Nefinactive cells. On M-CSF stimulation, a number of signaling molecules, such as proteins, with molecular weights of 150 kDa to 160 kDa, 100 kDa, and 60 kDa were shown to be rapidly tyrosine phosphorylated in Nef-inactive cells (Figure 5A, open arrowheads). However, the tyrosine phosphorylation of these proteins in response to M-CSF stimulation was markedly diminished in Nef-active cells (Figure 5A). Yet, the blot also showed that the effect of Nef was not an overall reduction in tyrosine phosphorylation. The proteins, seen as a closely spaced doublet at approximately 55 kDa, were phosphorylated only on M-CSF stimulation in Nef-inactive cells but were phosphorylated before stimulation in Nef-active cells (Figure 5A, asterisks). Moreover, the tyrosine phosphorylation of protein, seen at 40 to 45 kDa, was elevated in Nef-active cells (Figure 5A, filled arrowhead). Judging by its molecular weight, the latter protein (40-45 kDa, filled arrowhead) seemed to be extracellular signal-related protein kinase (ERK).17 This was confirmed by further analyses in which total cell lysates were immunoprecipitated with antiphosphotyrosine antibody and then were probed with anti-ERK antibody or in which total cell lysates were directly probed with phosphorylated ERK-specific antibody (Figure 5B). Using a similar approach, we identified the protein seen at 150 to 160 kDa, the tyrosine phosphorylation of which was induced by M-CSF stimulation but was diminished in Nef-active cells (Figure 5A, top open arrowhead) as M-CSF receptor (Figure 5C, top and middle panels). The blot shown in the middle panel also revealed that the 60-kDa protein (Figure 5A, bottom open arrowhead) was a component of M-CSF receptor complex, and its association with the complex was markedly diminished in Nef-active cells (Figure 5C, open arrowhead). These results indicated that Nef activation caused inhibition in the tyrosine phosphorylation/activation of M-CSF receptor and the molecular association with the activated receptor, which was the earliest signaling event induced by M-CSF.
Nef activation causes perturbations in M-CSF receptor signaling in TF-1-fms-Nef-ER cells. (A-C) TF-1-fms-Nef-ER cells were deprived of M-CSF for 14 hours and restimulated with M-CSF for the indicated periods. For the activation of Nef, 4-HT was added to the culture at the initiation of M-CSF deprivation. Total cell lysates or immunoprecipitates were analyzed by Western blotting. (A) Total cell lysates from untreated or 4-HT-treated cells were analyzed with antiphosphotyrosine (pTyr) antibody. See “Mechanism by which Nef inhibits M-CSF receptor signaling” for explanations of the symbols in detail. (B) Immunoprecipitates with anti-pTyr antibody were analyzed with anti-ERK antibody (top). Alternatively, total cell lysates were analyzed with antibody specific for phosphorylated ERK (middle). The blot shown in the bottom panel, in which the total cell lysates were analyzed with -ERK antibody, verified that comparable amounts of proteins were loaded in the top panel. (C) Immunoprecipitates with anti-pTyr antibody were analyzed with anti-M-CSF receptor (c-fms) antibody (top). Alternatively, the immunoprecipitates with anti-M-CSF receptor antibody were analyzed with anti-pTyr antibody (middle). The blot was exposed to autoradiography film for 10 seconds (short) or 1 minute (long). See “Mechanism by which Nef inhibits M-CSF receptor signaling” for explanations of the symbols in detail. The blot shown in the bottom panel, in which the immunoprecipitates were analyzed with anti-M-CSF receptor antibody, is a loading control experiment for the upper panel. (D) TF-1-fms-Nef-ER cells were cultured with or without 4-HT for 14 hours under M-CSF-free conditions. Total cell lysates were prepared and subjected to immunoprecipitation with anti-M-CSF receptor (c-fms) antibody or anti-Hck antibody. Anti-M-CSF receptor immunoprecipitates were analyzed with anti-Hck antibody or anti-M-CSF receptor antibody. Anti-Hck immunoprecipitates were analyzed with anti-pTyr antibody, anti-M-CSF receptor antibody, or anti-Hck antibody.
Nef activation causes perturbations in M-CSF receptor signaling in TF-1-fms-Nef-ER cells. (A-C) TF-1-fms-Nef-ER cells were deprived of M-CSF for 14 hours and restimulated with M-CSF for the indicated periods. For the activation of Nef, 4-HT was added to the culture at the initiation of M-CSF deprivation. Total cell lysates or immunoprecipitates were analyzed by Western blotting. (A) Total cell lysates from untreated or 4-HT-treated cells were analyzed with antiphosphotyrosine (pTyr) antibody. See “Mechanism by which Nef inhibits M-CSF receptor signaling” for explanations of the symbols in detail. (B) Immunoprecipitates with anti-pTyr antibody were analyzed with anti-ERK antibody (top). Alternatively, total cell lysates were analyzed with antibody specific for phosphorylated ERK (middle). The blot shown in the bottom panel, in which the total cell lysates were analyzed with -ERK antibody, verified that comparable amounts of proteins were loaded in the top panel. (C) Immunoprecipitates with anti-pTyr antibody were analyzed with anti-M-CSF receptor (c-fms) antibody (top). Alternatively, the immunoprecipitates with anti-M-CSF receptor antibody were analyzed with anti-pTyr antibody (middle). The blot was exposed to autoradiography film for 10 seconds (short) or 1 minute (long). See “Mechanism by which Nef inhibits M-CSF receptor signaling” for explanations of the symbols in detail. The blot shown in the bottom panel, in which the immunoprecipitates were analyzed with anti-M-CSF receptor antibody, is a loading control experiment for the upper panel. (D) TF-1-fms-Nef-ER cells were cultured with or without 4-HT for 14 hours under M-CSF-free conditions. Total cell lysates were prepared and subjected to immunoprecipitation with anti-M-CSF receptor (c-fms) antibody or anti-Hck antibody. Anti-M-CSF receptor immunoprecipitates were analyzed with anti-Hck antibody or anti-M-CSF receptor antibody. Anti-Hck immunoprecipitates were analyzed with anti-pTyr antibody, anti-M-CSF receptor antibody, or anti-Hck antibody.
We next examined whether the activation of Hck by Nef was involved in the inhibitory effect of Nef on M-CSF receptor signaling. We focused attention on the proteins seen as a closely spaced doublet at approximately 55 kDa. The pattern in tyrosine phosphorylation was different between Nef-inactive and Nef-active cells—the proteins were tyrosine phosphorylated in response to M-CSF stimulation in Nef-inactive cells but were phosphorylated before stimulation in Nef-active cells (Figure 5A, asterisks). Moreover, and of interest, the proteins were recruited to the complex of M-CSF receptor only after M-CSF stimulation in Nef-inactive cells but pre-existed in the complex in Nef-active cells irrespective of M-CSF stimulation (Figure 5C, asterisks). We confirmed that the proteins were Hck. When total cell lysates were prepared from cells that were cultured without M-CSF, immunoprecipitated with anti-M-CSF receptor antibody, and analyzed for the presence of Hck protein in the M-CSF receptor complex, we found that Hck was present in the complex in Nef-active cells but not in Nef-inactive cells (Figure 5D). Analyses of anti-Hck immunoprecipitates further revealed the tyrosine phosphorylation of Hck and its association with M-CSF receptor complex in Nef-active cells (Figure 5D). Given that Src family kinases, including Hck, should associate with M-CSF receptor only if the receptor is activated by its ligand,18-20 the constitutive association of Hck with M-CSF receptor complex that occurred in Nef-active TF-1-fms-Nef-ER cells was an aberrant interaction.
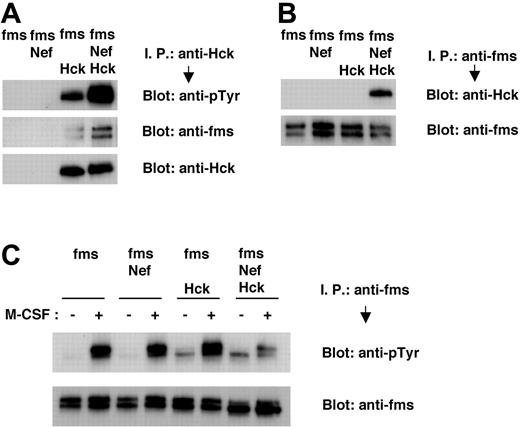
Finally, we performed transient expression experiments with 293T cells and confirmed that Nef induced the activation of Hck, the aberrant molecular interaction between Hck and M-CSF receptor, and the impaired M-CSF receptor activation. Tyrosine phosphorylation of Hck in 293T cells was enhanced by cotransfection with Nef (Figure 6A, top panel), possibly reflecting the finding that Nef stimulated the autophosphorylation of Hck.12,33,34,38 The association between Hck and M-CSF receptor, in the absence of M-CSF stimulation, was stimulated by cotransfection with Nef (Figure 6A, middle panel; Figure 6B, upper panel). The tyrosine phosphorylation of M-CSF receptor in response to M-CSF was impaired in 293T cells coexpressing M-CSF receptor, Nef, and Hck (Figure 6C, upper panel). Such impairment was not observed in 293T cells coexpressing M-CSF receptor and Nef or M-CSF receptor and Hck (Figure 6C, upper panel). In summary, our data strongly suggested that HIV-1 Nef interfered with the activation of M-CSF receptor in response to its ligand by means of the activation of Hck and the subsequent induction of an aberrant molecular association between Hck and M-CSF receptor complex, thereby inhibiting M-CSF bioactivities.
Nef causes tyrosine phosphorylation of Hck, constitutive association of Hck with M-CSF receptor complex, and inhibition in tyrosine phosphorylation of M-CSF receptor in 293T cells. (A-C) 293T cells were transfected with M-CSF receptor (c-fms) expression plasmid, alone or in combination with Nef or Hck expression plasmids. Total cell lysates were prepared and subjected to immunoprecipitation/Western blotting analyses. (C) Transfected 293T cells were treated in the absence or presence of M-CSF for 2 minutes before lysis. (A) Immunoprecipitates with anti-Hck antibody were analyzed with antiphosphotyrosine (pTyr) antibody (top) or anti-M-CSF receptor antibody (middle). The blot shown in the bottom panel, in which the immunoprecipitates were analyzed with anti-Hck antibody, is a loading control experiment for upper panels. (B) Immunoprecipitates with anti-M-CSF receptor antibody were analyzed with anti-Hck antibody (top). The blot shown in the bottom panel, in which the immunoprecipitates were analyzed with anti-M-CSF receptor antibody, is a loading control experiment for the top panel. (C) Immunoprecipitates with anti-M-CSF receptor antibody were analyzed with anti-pTyr antibody (top). The blot shown in the bottom panel, in which the immunoprecipitates were analyzed with anti-M-CSF receptor antibody, is a loading control experiment for the top panel.
Nef causes tyrosine phosphorylation of Hck, constitutive association of Hck with M-CSF receptor complex, and inhibition in tyrosine phosphorylation of M-CSF receptor in 293T cells. (A-C) 293T cells were transfected with M-CSF receptor (c-fms) expression plasmid, alone or in combination with Nef or Hck expression plasmids. Total cell lysates were prepared and subjected to immunoprecipitation/Western blotting analyses. (C) Transfected 293T cells were treated in the absence or presence of M-CSF for 2 minutes before lysis. (A) Immunoprecipitates with anti-Hck antibody were analyzed with antiphosphotyrosine (pTyr) antibody (top) or anti-M-CSF receptor antibody (middle). The blot shown in the bottom panel, in which the immunoprecipitates were analyzed with anti-Hck antibody, is a loading control experiment for upper panels. (B) Immunoprecipitates with anti-M-CSF receptor antibody were analyzed with anti-Hck antibody (top). The blot shown in the bottom panel, in which the immunoprecipitates were analyzed with anti-M-CSF receptor antibody, is a loading control experiment for the top panel. (C) Immunoprecipitates with anti-M-CSF receptor antibody were analyzed with anti-pTyr antibody (top). The blot shown in the bottom panel, in which the immunoprecipitates were analyzed with anti-M-CSF receptor antibody, is a loading control experiment for the top panel.
Discussion
The present study identified for the first time a dysregulated signaling event in monocytes/macrophages induced by the interaction between HIV-1 Nef and the cellular kinase Hck. We have demonstrated that Nef activates Hck and recruits the activated Hck to the M-CSF receptor complex. By means of the induction of the unphysiologic signaling event, Nef interferes with M-CSF receptor signaling pathways, thereby inhibiting M-CSF bioactivities and possibly causing dysregulated functions of HIV-1-infected monocytes/macrophages.
Since Hanna et al13 reported the importance of the interaction between Nef and Hck for the development of AIDS-like disease through studies with HIV-1 transgenic mice and Hck knockout mice, efforts have been made to identify the signaling molecules for which activation is affected by Nef-Hck interaction in monocytes/macrophages. Signal transducer and activator of transcription (STAT) family molecules were considered possible candidates for the Nef-Hck complex. A recent study reported that Nef induced the activation of STAT3 in a myeloid cell line.34 However, it was not established whether the Nef-Hck interaction directly led to STAT3 activation.34 Similarly, another recent study reported that Nef activated STAT-1α and -1β in human macrophages derived from peripheral blood monocytes, but the activation occurred through the secretion of unidentified soluble factors.36 Thus, the STAT family molecules may not be direct targets for the Nef-Hck complex. The secretion of soluble factors, such as cytokines and chemokines, from monocytes/macrophages is a well-established function of Nef,9,10,35-37 and macrophage inflammatory proteins 1α and 1β are well-known examples, as reported by Swingler et al.9 Interestingly, the possibility that the Nef-Hck interaction did not participate in the secretion of these chemokines was raised by the authors.9 After all, in spite of its importance in disease development, the role of the Nef-Hck interaction in the modulation of monocyte/macrophage signaling remains to be determined.
Our present study strongly suggests that M-CSF receptor is a target for the Nef-Hck complex. We demonstrated that Nef caused the inhibition of the activation of M-CSF receptor in response to M-CSF and the molecular association with the activated receptor, which were the earliest signaling events for M-CSF receptor pathways (Figures 5A,C, 6C). As an inevitable consequence, Nef caused the inhibition of biologic activities of M-CSF, such as cell proliferation (Figure 1) and macrophage differentiation (Figure 2) of TF-1-fms cells. We observed a similar effect of Nef in primary myeloid cells. When cord blood CD34+ cells were retrovirally transduced with the Nef gene and then cultured with M-CSF, we found a marked decrease in the proportion of Nef-positive cells during the culture with M-CSF (data not shown). Although further analyses are necessary, the finding raises the possibility that Nef does inhibit M-CSF receptor signaling in primary myeloid cells. The proliferation inhibitory effect of Nef was not mediated by the secretion of inhibitory molecules (Figure 3) or by the down-regulation of M-CSF receptor (Figure 4), further supporting the conclusion that Nef affected intrinsic M-CSF receptor signaling. The most likely mechanism by which Nef interferes with M-CSF receptor signaling is the unphysiologic behavior of Hck. The Hck proteins seem to be constitutively phosphorylated in Nef-active TF-1-fms-Nef-ER cells (Figure 5A, C). The induction of tyrosine phosphorylation of Hck by Nef was confirmed by transient expression experiments in 293T cells (Figure 6A). More important, Hck constitutively formed the complex with M-CSF receptor in active Nef-expressing cells (Figures 5D, 6A,B). In contrast, Hck associates with M-CSF receptor only if the receptor is activated by its ligand (Figure 5C,D).18-20 It is reasonable to expect that the aberrant constitutive association of Nef-activated Hck to M-CSF receptor complex would inhibit the conformational change of the receptor that would be induced by its ligand and would be required for initiating signaling cascades. Therefore, we concluded that Nef interfered with M-CSF receptor signaling through the activation of Hck and its recruitment to M-CSF receptor complex.
Given that Nef activates the kinase activity of Hck11,12 and that the activation of Src kinases, including Hck, is required for biologic effects of M-CSF such as mitogenic signal and differentiation-inducing signal,17-21 one may speculate that Nef mimics the effect of M-CSF on monocytes/macrophages. However, this is not the case. Nef caused the inhibition of biologic activities of M-CSF such as cell proliferation (Figure 1) and macrophage differentiation (Figure 2). In addition, the activation of Nef did not cause M-CSF-independent proliferation of TF-1-fms-Nef-ER cells (data not shown). The reason Nef-activated Hck does not mimic M-CSF functions might be explained by the difference in the mode of Hck activation. The activity of Src family kinases, including Hck, is suppressed by 2 intramolecular interactions, SH2-tail and SH3-linker.39,40 Nef has been shown to activate Hck by binding to its SH3 domain, thus preventing the SH3-linker interaction.12,38 In contrast, the ligand-activated M-CSF receptor has been shown to associate with the SH2 domain but not with the SH3 domain of Src kinases.19 It is reasonable to expect that the different modes of Hck activation might generate distinct output signals. That Nef-activated Hck formed an unphysiologic molecular association with M-CSF receptor complex supported this idea.
Nef activation did not necessarily cause the overall reduction of M-CSF receptor pathways. Tyrosine phosphorylation/activation of ERK in response to M-CSF was enhanced by Nef activation (Figure 5B). This finding raises the possibility that Nef-activated Hck inhibits the process of “full activation” of M-CSF receptor. The activation process of M-CSF receptor is considered to involve the ligand-induced dimerization and subsequent autophosphorylation of specific tyrosine residues in their cytoplasmic region.16 Phosphorylated tyrosine residues then serve as docking sites for SH2 domain-containing molecules, each initiating signaling cascades along specific pathways.16 Future studies in which we determine whether Nef-activated Hck directly binds to M-CSF receptor and identify site(s) within the cytoplasmic region of the receptor to which Nef-activated Hck binds will clarify the mechanism for the selective inhibition of Nef on the M-CSF receptor pathways.
Our finding that Nef inhibits the process of macrophage differentiation mediated by M-CSF (Figure 2) explains the mechanism for dysfunction, such as defective phagocytosis of opportunistic pathogens, of monocytes/macrophages in HIV-1-infected persons. It is well documented that the phagocytosis of pathogens such as Mycobacterium avium complex, Pneumocystis carinii, Toxoplasma gondii, and Candida albicans by peripheral blood monocytes, tissue macrophages, and monocyte-derived macrophages is impaired by in vitro and in vivo HIV-1 infection (reviewed in Kedzierska et al41 ). M-CSF is an important cytokine for macrophage differentiation15 and has been shown to stimulate various effector functions of macrophages.14 In particular, a number of in vitro and in vivo studies have shown that M-CSF activates antimicrobial activities of macrophages.42-45 Moreover, the clinical efficacy of M-CSF has been proven in patients with invasive fungal infections.46,47
We showed that the percentage of apoptotic cells in M-CSF-containing culture of Nef-active TF-1-fms-Nef-ER cells was significantly higher than that of Nef-inactive cells (Figure 1D, E). In contrast, the degree of apoptotic cell death induced by M-CSF withdrawal in Nef-active TF-1-fms-Nef-ER cells was similar to that in Nef-inactive cells (data not shown). These results indicate that Nef does not actively induce apoptotic cell death in the cells but inhibits the signaling from M-CSF. On the other hand, a recent study has revealed that Nef does not inhibit, but that it mimics signaling initiated by granulocyte/macrophage-CSF (GM-CSF),34 another cytokine that stimulates the proliferation and differentiation of monocytes/macrophages. That HIV-1 Nef selectively inhibits the M-CSF/M-CSF receptor system may address how monocytes/macrophages contribute to the development and progression of AIDS in vivo. Monocytes/macrophages are considered to be involved in the disease progression process by their secretion of soluble factors;9,10 the profile of gene expression of M-CSF-derived macrophages differs from that of GM-CSF-derived macrophages.48 It is possible that the selective inhibition of Nef on cytokine signaling results in the modification of macrophage functions, such as cytokine/chemokine production, in HIV-1-infected persons. This may create an immunologic environment favorable for HIV-1 virus production. Alternatively, this may lead to abnormal tissue distribution of cells of the lineage, thereby facilitating the transfer of HIV-1 virus to bystander CD4+ T cells. Our findings provide a novel clue to understanding how monocytes/macrophages contribute to the development and progression of AIDS and to clarifying the role of the Nef-Hck interaction in the disease process.
Prepublished online as Blood First Edition Paper, December 30, 2004; DOI 10.1182/blood-2004-06-2084.
Supported in part by a grant from Uehara Memorial Foundation and by Health and Labour Sciences Research Grants from the Ministry of Health, Labour and Welfare of Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank K. Tabata and S. Okamura for research support. M-CSF was kindly provided by Morinaga Milk Industry. Nef antiserum and pEBB-Nef-ER-IRES-puro were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program (Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD).



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal