Abstract
Fanconi anemia (FA) is a genetically heterogeneous chromosomal instability syndrome associated with multiple congenital abnormalities, aplastic anemia, and cancer. We report that a deletion mutation in the FANCG gene (c.637_643delTACCGCC) was present in 82% of FA patients in the black populations of Southern Africa. These patients originated from South Africa, Swaziland, Mozambique, and Malawi. The mutation was found on the same haplotype and was present in 1% of controls from the black South African population. These data indicate that the birth incidence of FA in this population is higher than 1 in 40 000, which is much higher than previously supposed, and suggest that the FANCG deletion is an ancient founder mutation in Bantu-speaking populations of sub-Saharan Africa. Diagnostic screening is now possible by means of a simple DNA test.
Introduction
Fanconi anemia (FA) is rare in most populations, with a prevalence of about 1 in 300 000, but carrier frequencies of 1 in 90 have been observed in Ashkenazi Jews1,2 and in the Afrikaner population of South Africa.3 FA is genetically heterogeneous, arising from mutations in 1 of at least 11 different genes (FANCA, FANCB, FANCC, FANCD1/BRCA2, FANCD2, FANCE, FANCF, FANCG, FANCI, FANCJ, and FANCL). Almost all Ashkenazi FA cases are caused by the c.711 + 4A > T (IVS4 + 4A > T) mutation in FANCC,1 whereas 80% of Afrikaner cases have 1 of 2 large deletions in FANCA.4 Clinical studies indicated that FA is rare in black South Africans, with a homozygote prevalence of 1 in 476 000.5 We investigated the molecular basis of FA in this population to inform future patient management strategies.
Study design
FA patients were ascertained by the Departments of Hematology/Oncology, Pediatrics, and Human Genetics at hospitals attached to the Universities of the Witwatersrand, Free State, and Pretoria. Approval was obtained from the Witwatersrand University Faculty of Health Sciences Committee for Research on Human Subjects. Clinical diagnoses were confirmed by demonstrating increased chromosome breakage with diepoxybutane or mitomycin C as described previously.6 Blood samples were taken from informed, consenting FA patients, and clinical data on age of onset, severity of hematological symptoms, survival, incidence of acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), and somatic abnormalities6 obtained. Controls were anonymized paternity specimens.
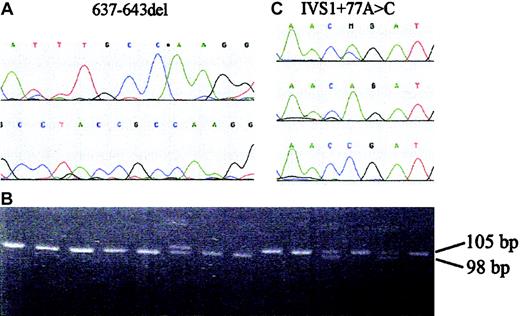
Lymphoblastoid cell lines were established from 4 black South African FA patients and screened for the presence of the known FA proteins by Western blotting.6 The 14 exons of the FANCG gene (OMIM: 602 956; mRNA: AJ007 669.1; genomic: NT_008 413) were amplified and sequenced from forward and reverse strands on an ABI 377 automated DNA sequencer.6 A polymerase chain reaction (PCR) assay was designed to detect the common 7–base pair (bp) deletion in exon 5 of the FANCG gene. Amplification was carried out with primers 637-643delF CCC AGG GAT TGA AGG ATG TC and 637-643delR GCA TGA GAC TGG AGG ACC AC, with 30 cycles of 94°C (1 minute), 60°C (1 minute), and 72°C (1 minute). Products were sized by electrophoresis on a 12.5% polyacrylamide gel. A single nucleotide polymorphism (SNP) in the FANCG gene, c.84 + 77A > C, was genotyped by amplification with primers IVS1 + 77A > C/F: GGT TTG CAG TGG ATT TAC CCC ATC (mismatch in bold) and FANCG 1R: CTT TGG TCA AGC TCA GTC CC, followed by DNA sequencing or by digestion with Fok1 and sizing of the 53-bp and 46-bp alleles by electrophoresis.
Results and discussion
Western blot analysis of known FA proteins in extracts from cell lines from 4 black South African FA patients was carried out for an initial assessment of complementation groups. This established that the FANCG protein was undetectable in all 4 cell lines tested (data not shown). DNA sequencing of the exons of the FANCG gene revealed that all 4 patients were homozygous for a 7-bp deletion in exon 5 (Figure 1A), c.637_643delTACCGCC, that is predicted to produce a truncated protein of 217 amino acid residues (Y213K + 4X). A simple PCR mutation assay was devised, which detects a 105-bp fragment in the wild-type gene and a 98-bp fragment from the deleted allele (Figure 1B). DNA samples from a total of 40 unrelated black FA patients were then screened for the FANCG deletion. The mutation was present in 33 (82.5%) of 40 patients (all except 2 being homozygotes) and in 64 of 80 mutant alleles (80%). Most mutation-carrying patients were from the Sotho, Tswana, Venda, and Zulu tribes from South Africa, but 2 were from Swaziland, 2 from Mozambique, and 1 from the Tumbuka tribe of Malawi. The same mutation was independently detected in a Portuguese FA patient (EUFA939) who was homozygous for the deletion and whose parents originated from the black population of Mozambique, formerly a Portuguese colony.
FANCG mutation and SNP. (A) Sequence of part of exon 5 of the FANCG gene showing the c.637_643delTACCGCC mutation (637-643del). (B) PCR assay for the FANCG deletion. Samples from African FA patients; the final 2 lanes are a heterozygote and a normal control, respectively. The normal allele is 105 bp in length and the mutant allele 98 bp. (C) Sequence of part of intron 1 of the FANCG gene showing the c.84 + 77A > C SNP (IVS1 + 77A > C).
FANCG mutation and SNP. (A) Sequence of part of exon 5 of the FANCG gene showing the c.637_643delTACCGCC mutation (637-643del). (B) PCR assay for the FANCG deletion. Samples from African FA patients; the final 2 lanes are a heterozygote and a normal control, respectively. The normal allele is 105 bp in length and the mutant allele 98 bp. (C) Sequence of part of intron 1 of the FANCG gene showing the c.84 + 77A > C SNP (IVS1 + 77A > C).
These diverse geographic and tribal origins suggest that the mutation predated the arrival of Bantu-speakers in Southern Africa around 400 AD.7 If so, we would expect it to occur on the same haplotype defined by DNA polymorphisms within or very close to FANCG. All patients homozygous for c.637_643delTACCGCC also were homozygous for the C allele of a single nucleotide polymorphism in intron 1 of FANCG, c.84 + 77A > C (Figure 1C), whereas the frequency of the C allele in black South African controls was 0.22. This is consistent with a single origin for the FANCG mutation. In view of the prevalence of the deletion in African FA patients, we then determined its carrier frequency in unrelated black South African controls. The deletion was present in 3 (1%) in 300 of controls (95% CI, 1 in 35 to 1 in 475), indicating an expected birth incidence of 1 in 40 000. The true incidence of FA would be higher, since this mutation accounts for 80% of FA alleles in the population and is therefore likely to be about 10-fold higher than that reported from clinical ascertainment of FA in black school children and estimates of population size from a population census.5
We also investigated the clinical phenotype of black South African patients homozygous for the FANCG deletion. Detailed clinical information was available from 20 patients, and this was compared to data from 20 European FA-G patients and from all 245 European FA patients described previously.6 There was no significant difference in age of diagnosis (mean age, 6.51 years), survival, or rates of AML and MDS (1 MDS and 1 AML in 20 patients) in black FA-G patients compared to either European FA-G or all European FA groups. However, the mean number of severe hematological indices (2.53) was significantly greater in black FA-G patients than in all European FA patients (1.55, P = .0009), although not significantly different from European FA-G patients (2.07, P = .42). Differences in socioeconomic conditions and healthcare provision also may influence hematological status. Black FA-G patients had a significantly higher frequency of distal radial ray abnormalities, growth retardation, and eye abnormalities than all European FA patients, but similar rates of somatic abnormalities to European FA-G patients (Table 1).
Frequency of somatic abnormalities in black South African patients homozygous for the FANCG deletion (c.637-643delTACCGCC) compared with FA patients in the European study
Site of abnormality . | South African FANCG del, % . | European FA-G, % (P) . | European all FA, % (P) . |
|---|---|---|---|
| Skin | 85 | 70 | 70.4 |
| Radial ray | 85 | 50 (.04)* | 46.9 (.002)* |
| Growth retardation | 90 | 75 | 58.0 (.01)* |
| Kidney | 15 | 20 | 21.6 |
| Urogenital | 5 | 5 | 11.6 |
| Cardiac | 10 | 5 | 9.5 |
| Gastrointestinal | 0 | 15 | 12.9 |
| CNS | 0 | 0 | 4.6 |
| Deafness | 5 | 5 | 10.3 |
| Eye | 85 | 65 | 55.3 (.002)* |
Site of abnormality . | South African FANCG del, % . | European FA-G, % (P) . | European all FA, % (P) . |
|---|---|---|---|
| Skin | 85 | 70 | 70.4 |
| Radial ray | 85 | 50 (.04)* | 46.9 (.002)* |
| Growth retardation | 90 | 75 | 58.0 (.01)* |
| Kidney | 15 | 20 | 21.6 |
| Urogenital | 5 | 5 | 11.6 |
| Cardiac | 10 | 5 | 9.5 |
| Gastrointestinal | 0 | 15 | 12.9 |
| CNS | 0 | 0 | 4.6 |
| Deafness | 5 | 5 | 10.3 |
| Eye | 85 | 65 | 55.3 (.002)* |
For South African FANCG del, n = 20; for European FA-G, n = 20; and for European all FA, n = 241.
CNS indicates central nervous system.
South African FA-G patients were compared with FA-G patients in the European study using Fisher exact test, and with all FA patients in the European study using the χ2 test
The presence of the c.637_643delTACCGCC mutation in different tribal groups from South Africa and in Malawi and Mozambique suggests that it represents a founder mutation in the Bantu-speaking peoples of sub-Saharan Africa, consistent with the genetic homogeneity of many Bantu-speaking populations.7 It may therefore contribute to FA in other African countries such as the Congo, Angola, Tanzania, Zambia, and Zimbabwe. Founder mutations in FANCG also have been described for European, South American, and Japanese populations.8-10 The high carrier frequency of the deletion in the black South African population suggests that FA is much more common than previously estimated and may be underdiagnosed in Africa because of a combination of lack of specialist healthcare in rural areas and other more prevalent health problems. A relatively sophisticated chromosomal breakage test is required to confirm the diagnosis of FA, but since the mutation accounts for the majority of FA cases in this population, a simple DNA assay may now permit more widespread testing for FA in Southern Africa.
Prepublished online as Blood First Edition Paper, January 18, 2005; DOI 10.1182/blood-2004-10-3968.
Supported by the Daniel Ayling Fanconi Anaemia Trust, Fanconi Anaemia Breakthrough UK, the South African National Health Laboratory Service, the University of the Witwatersrand Medical Faculty Research Endowment Fund, the HE Griffin Charitable Trust, and the Fritz Thyssen Stiftung.
N.V.M. and F.E. contributed equally to the work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal