The homeobox gene Hoxa-9 is normally expressed in primitive bone marrow cells, and overexpression of Hoxa-9 markedly expands hematopoietic stem cells, suggesting a function in early hematopoiesis. We present evidence for major functional defects in Hoxa-9-/- hematopoietic stem cells. Hoxa-9-/- marrow cells have normal numbers of immunophenotypic stem cells (Lin-c-kit+flk-2-Sca-1+ [KLFS] cells). However, sublethally irradiated Hoxa-9-/- mice develop persistent pancytopenia, indicating unusual sensitivity to ionizing irradiation. In competitive transplantation assays, Hoxa-9-/- cells showed an 8-fold reduction in multilineage long-term repopulating ability, a defect not seen in marrow cells deficient for the adjacent Hoxa-10 gene. Single-cell cultures of KLFS cells showed a 4-fold reduction in large high-proliferation potential colonies. In liquid cultures, Hoxa-9-deficient Lin-Sca-1+ cells showed slowed proliferation (a 5-fold reduction in cell numbers at day 8) and delayed emergence of committed progenitors (a 5-fold decrease in colony-forming cells). Slowing of proliferation was accompanied by a delay in myeloid maturation, with a decrease in Gr-1hiMac-1hi cells at the end of the culture. Retroviral transduction with a Hoxa-9 expression vector dramatically enhanced the cytokine-driven proliferation and in vivo engraftment of Hoxa-9-/- marrow cells. Hoxa-9 appears to be specifically required for normal hematopoietic stem cell function both in vitro and in vivo.
Introduction
A role for Hox homeobox genes in normal hematopoietic development is becoming increasing evident.1 Several members of the Hoxa, including Hoxa-9, and Hoxb gene clusters are expressed in primitive hematopoietic cells of human and murine origin, and that expression is down-regulated as blood cells differentiate, suggesting a function in early hematopoiesis.2,3 In addition, retrovirally enforced expression of several Hox genes in murine and human marrow cells enhances in vitro proliferation of hematopoietic cells in short-term in vitro cultures,4-7 and, in the case of Hoxb-4 and Hoxa-9 proteins, results in a marked expansion of hematopoietic stem cells when transplanted in vivo.4,5 While these studies confirm a dramatic ability of Hox proteins to expand stem cells under these nonphysiologic conditions, it is not clear that they serve a function in normal stem-cell biology. Other investigators have reported a modest stem-cell defect in mice deficient in Hoxb-48 or deficient in both Hoxb-3 and Hoxb-4,9 and stem-cell abnormalities were not specifically analyzed in other Hox mutant animals.10
We have previously reported that Hoxa-9-deficient mice exhibit a variety of hematopoietic defects, including mild leukopenia, reduced numbers of committed myeloid and B-cell progenitors, a blunted neutrophilic response to granulocyte colony-stimulating factor (G-CSF), and aberrant fetal thymic development, demonstrating a role for this gene in intermediate stages of normal hematopoietic differentiation.11,12 However, previous assays of earlier pluripotent progenitors, such as day-12 colony-forming units-spleen (CFU-Ss) and long-term culture-initiating cells (LTC-ICs), in Hoxa-9-/- marrow cells revealed no significant defects, suggesting that Hoxa-9 might not influence earlier stages of hematopoietic development.11 To examine this question in more detail, we specifically evaluated hematopoietic stem cell (HSC) function in Hoxa-9-/- animals. In these studies, we have uncovered a markedly defective in vivo repopulating ability in Hoxa-9-/- marrow cells, and a severely impaired proliferative response of primitive hematopoietic cells to cytokines in vitro, indicating a significant functional defect in the Hoxa-9-/- HSC.
Materials and methods
Mice
Animals bearing a targeted disruption of the Hoxa-9 gene were originally provided by Dr Mario Capecchi (University of Utah, Salt Lake City).13 Hoxa-10-deficient mice were provided by Dr Robert Maas (Harvard Medical School).14 The construction of the targeting constructs, the generation of mutant mice, and the polymerase chain reaction (PCR)-based genotyping strategies have been previously described in detail.11,14 The Hoxa-9 and Hoxa-10 mutant strains were back bred against a C57/BL6 background for at least 4, and in some experiments up to 10, generations. Furthermore, in all experiments, mutant animals were matched with age- and sex-matched wild-type siblings as controls. For all transplantation experiments, recipients were 8- to 12-week-old female congenic C57BL/6 mice from Charles River Laboratories (Wilmington, MA). Donor and recipient mice are distinguishable based on allelic polymorphism at the CD45 locus. All bone marrow recipients were irradiated with x-ray irradiation at a total dose of 800 cGy given at a rate of 100 cGy/min; sublethally irradiated mice received a total of 500 cGy. All irradiated mice were maintained in microisolator cages with sterile food and antibiotic-treated acidified water for the first 2 weeks after treatment. Mice that were treated with 5-fluorouracil received a dose of 150 mg/kg by intraperitoneal injection.
Nonlimiting-dilution competitive repopulating assays
Nonlimiting-dilution competitive repopulating assays were performed as described by Harrison et al.15 Marrow cells (donor cells) from either CD45.2+Hoxa-9-/- or wild-type sibling mice were injected together with CD45.1+ normal marrow cells (competitor cells) in various ratios into cohorts of lethally irradiated C57/BL6 (CD45.1+). In each cohort the number of competitor cells was held at 500 000; the range of donor cells was 500 000 (1:1 ratio), 1 500 000 (1:3 ratio), and 4 500 000 (1:9 ratio). The percentage of CD45.2- and CD45.2+ granulocytes and lymphocytes in the peripheral blood of recipients was determined by fluorescence-activated cell sorting (FACS) analysis 12 to 20 weeks later. Competition assays using Hoxa-10-/- cells were performed in an identical manner. The relative repopulating ability of mutant and wild-type test cells was calculated by comparing the observed and expected contributions of test cells at various ratios of test cells-competitor cells.
Limiting-dilution CRU assays
Limiting-dilution competitive repopulating unit (CRU) assays were performed as previously described.4 Marrow cells from either Hoxa-9-/- mice or wild-type sibling controls bearing the CD45.2 antigen were injected at different doses into cohorts of lethally irradiated C57/BL6 (CD45.1+) mice together with a fixed rescue dose of 2 × 105 normal competitor cells from C57/BL6 (CD45.1+) animals. The level of lymphomyeloid repopulation provided by CD45.2+ donor cells was measured 12 to 26 weeks after transplantation by performing FACS analysis of the peripheral blood of each recipient.16 Recipients with more than 1% donor (CD45.2+) contribution to both lymphoid and myeloid leukocytes were considered to have been repopulated with at least one donor CRU. CRU frequency was then calculated by applying Poisson statistics and the method of maximum likelihood to the proportion of negative recipients at different dilutions as previously described.17
FACS analysis
For the competitive repopulating assays and for the analysis of myeloid differentiation in cultured marrow cells, cells from peripheral blood or in vitro culture were stained with appropriately diluted fluorochrome-conjugated antibodies and analyzed on a Becton Dickinson FACScan using Cellquest software (Becton Dickinson, San Jose, CA). Antibodies used for the peripheral-blood studies included anti-CD45.2 and anti-CD45.1 (Pharmingen, San Diego, CA). For CD45.2 and CD45.1 staining of peripheral-blood leukocytes, granulocytes and lymphocytes in the peripheral blood were distinguished by standard forward- and side-scatter characteristics. Antibodies used for the cultured cells included phycoerythrin (PE)-conjugated Mac-1 and fluorescein isothiocyanate (FITC)-conjugated Gr-1 (Caltag, Burlingame, CA).
For the immunophenotyping of primitive marrow populations, the femurs and tibia of donor mice were flushed with Hanks Balanced Salt Solution (Life Technologies, Bethesda, MD) supplemented with 2% fetal calf serum (FCS) and 2 mM EDTA (ethylenediaminetetraacetic acid). Red blood cells were lysed in a 0.15-M ammonium chloride, 0.01-M potassium bicarbonate solution on ice. For HSC isolation, c-Kit+ cells were enriched by positive selection using magnetic activated cell sorted (MACS; Miltenyi Biotec, Auburn, CA) streptavidin-conjugated magnetic beads and an AutoMACs cell separator according to the manufacturer's instructions. c-Kit-enriched cells were stained with directly labeled antibodies. Prior to FACS analysis, cells were resuspended in 1 μg/mL propidium iodine to detect and exclude dead cells.
The monoclonal antibodies used in immunofluorescence staining for HSC and progenitor isolation/analysis were prepared from hybridomas that included 2B8 (anti-c-kit, allophycocyanin [APC] conjugate), E13-161 (anti-Sca-1, Ly6A/E, Texas Red conjugate), A2F10 (anti-Flk-2/Flt3, CD135, PE conjugate; eBioscience, San Diego, CA), RAM34 (anti-CD34, FITC conjugate; Pharmingen), 2.4G2 (CD16/CD32, PE conjugate; Pharmingen), and A7R34 (CD127, PE conjugate; eBioscience). Lineage marker antibodies included 6B2 (anti-B220), KT31.1 (anti-CD3), GK1.5 (anti-CD4), 53-7.3 (anti-CD5), 53-6.7 (anti-CD8), Ter119 (anti-erythrocyte-specific antigen), 8C5 (anti-Gr-1), and M1/70 (anti-Mac-1). Unconjugated lineage antibodies were used in conjunction with anti-rat immunoglobulin G (IgG) cyanin5 (Cy5)-PE (Caltag). Directly labeled PE, FITC, and biotinylated lineage antibodies were also used. Biotinylated 3C11 (anti-c-kit) was used for c-kit enrichment (3C11 and 2B8 recognize distinct, nonoverlapping epitopes of c-Kit).
HSCs were analyzed as c-Kit+Flk-2-lineage-Sca-1+.18 Myeloid progenitor populations were analyzed as previously reported19 : common myeloid progenitors (CMPs) such as c-Kit+lineage-/loSca-1-CD34+CD16lo, granulocyte-monocyte progenitors (GMPs) such as c-Kit+lineage-/loSca-1-CD34+CD16+, and megakaryocyte-erythroid progenitors (MEPs) such as c-Kit+lineage-/lo Sca-1-CD34-CD16-. Common lymphoid progenitors (CLPs) were analyzed as previously reported: lineage-Sca-1loc-kitloCD127+.20 Cell sorting was performed using a modified FACSVantage (Becton Dickinson) or a modified 3-laser cytometer (Cytomation [Fort Collins, CO] and Becton Dickinson) maintained by the Stanford University Shared FACS Facility, Stanford, CA. Cells used in this study were double sorted to ensure purity. Data were analyzed using FloJo software (Tree Star, San Carlos, CA).
Progenitor homing assays
Homing assays were performed as previously described.21 Briefly, marrow cells harvested from Hoxa-9-/- and wild-type siblings were injected into lethally irradiated mice at doses of 5 000 000 or 10 000 000 cells per recipient. For each experiment, a control animal was irradiated and did not undergo transplantation. Recipients were killed 16 to 17 hours after transplantation, and marrow cells in their femurs were enumerated and plated in clonogenic assays. Based on those colony counts, the number of myeloid progenitors per femur was determined, and the total number of progenitors in the entire marrow was calculated, based on the presumption that marrow cells from one femur represent 5.9% of the total marrow.22 The number of progenitors in the control that did not undergo transplantation was subtracted from this total, as a measure of surviving endogenous progenitors (< 0.2% of the estimated number of endogenous progenitors before irradiation). The calculated number of exogenous progenitors was divided by the number of progenitors injected, as determined by clonogenic assays of donor marrow prior to transplantation, to give the proportion of injected progenitors that had homed to the marrow.
For in situ visualization of transplanted hematopoietic cells, adult bone marrow cells were subjected to negative selection for lineage-specific markers (Lin-1) and positive selection of Sca-1, using the MACS Miltenyi (Miltenyi Biotec) streptavidin-conjugated magnetic beads and an AutoMACs cell separator according to the manufacturer's instructions. Lin-Sca-1+ cells were then labeled ex vivo with carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR).23 CFSE-labeled cells (500 000 cells/animal) were injected into nonirradiated syngeneic recipients, 3 animals receiving Hoxa-9-/- cells, and 3 animals receiving wild-type cells. Fifteen hours later, recipient mice underwent in vivo formalin fixation under anesthesia and were immediately killed. Both femurs from each animal were sectioned and examined under a fluorescent microscope. Fluorescent cells were scored according to their proximity to the endosteal layer of the bone, with cells within 12 layers of the endosteum scored as residing in the endosteal region, whereas cells more distant were scored as residing in the central vascular region. A total of 194 Hoxa-9-/- fluorescent cells and 265 wild-type fluorescent cells were scored.
Assays for myeloid progenitors
Standard clonogenic assays for myeloid progenitors of marrow, spleen, and blood from adult mice were performed in 0.8% methylcellulose in alpha medium containing 30% FCS and 2% spleen-cell-conditioned medium (StemCell Technologies, Vancouver, BC, Canada) as previously described.11
For single-cell cultures, stem and progenitor cells were sorted at 1 cell/well, with an automated cell-deposition unit (ACDU) device (Becton Dickinson), into 96-well plates containing Methylcult M3231 (StemCell Technologies) supplemented with the following cytokines: stem-cell factor (SCF), Flt-3, interleukin-6 (IL-6), IL-11, granulocyte-macrophage (GM)-CSF, IL-3, human thrombopoietin (TPO), and human erythropoietin (EPO) (R&D Systems, Minneapolis, MN). The final concentration of growth factors was 10 ng/mL, except for EPO, which had a final concentration of 4 U/mL.
Retroviral transfections
The MIG vector used in the transfection experiments is a murine stem-cell virus (MSCV)-based vector24 containing an internal ribosomal re-entry sequence (IRES) and enhanced green fluorescent protein (EGFP) cDNA.25 The Hoxa-9 vector was constructed by inserting a cDNA encoding a full-length murine Hoxa-9 protein fused to an N-terminal FLAG epitope. High-titer retrovirus was produced by transient transfection of 293-T cells with the appropriate retroviral vector and the pCL Ecotropic packaging plasmid using Metafectene transfection reagent (Biontex Laboratories, Munich, Germany). Retroviral supernatants were harvested at 48 and 72 hours after transfection. Donor bone marrow (CD45.2+) was harvested from mice 5 days after intraperitoneal injection with 150 mg/kg 5-fluorouracil and cultured in prestimulatory media consisting of StemSpan serum-free expansion medium (SFEM) supplemented with 15% heat-inactivated FCS, 100 ng/mL recombinant murine (rm) SCF, 50 ng/mL rm IL-6, and 10 ng/mL rm IL-3 (StemCell Technologies) for 48 hours. Marrow cells were infected with retrovirus on 2 consecutive days by spinoculation in the presence of 4 μg/mL polybrene (Sigma, St Louis, MO). Twenty-four hours after the second spinoculation, bone marrow cells were analyzed by FACS analysis for transduction efficiency (GFP+).26
Statistical analysis
All statistical comparisons of blood-cell counts and FACS analysis results were performed using a 2-tailed t test, using the Primer of Biostatistics Version 3.0 software for Apple Macintosh by Dr S. A. Glantz (McGraw-Hill, New York, NY). Standard error bars are indicated in all figures.
Results
Hoxa-9-/- marrow cells show blunted recovery after hematopoietic stress
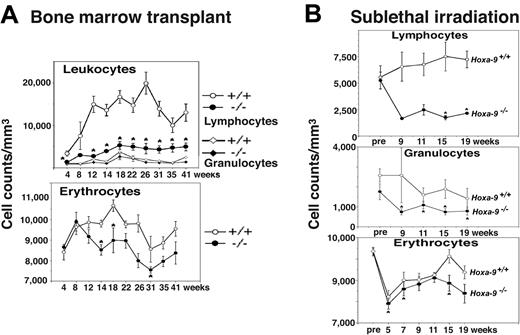
Preliminary evidence for a stem-cell lesion in Hoxa-9-/- hematopoietic cells came from bone marrow transplantation experiments. In order to establish that the hematopoietic defects in these animals were intrinsic to the blood cells and not due to microenvironmental problems in the marrow, we transplanted 500 000 unfractionated Hoxa-9-/- marrow cells in lethally irradiated wild-type congenic mice. Recipient mice recovered with mild hematopoietic defects indistinguishable from those seen in the primary animals (data not shown). However, in experiments in which recipients received a lower dose of 200 000 marrow cells, recipients of Hoxa-9-/- marrow suffered persistent cytopenias that were more severe than in the primary animals (Figure 1A). This included a severe depression in lymphocyte counts, moderate anemia, and mild reduction in granulocytes, compared with recipients of wild-type marrow, which displayed nearly complete recovery in their peripheral blood counts.
As a second indirect measure of stem-cell function, mutant and wild-type mice were assessed for hematopoietic recovery following total body irradiation. Following a sublethal dose of 500 cGy x-ray irradiation, Hoxa-9-/- mice developed severe persistent lymphopenia, as well as significant neutropenia and anemia (Figure 1B). FACS analysis of peripheral-blood lymphocyte subsets showed reductions in both T and B cells; however, the reduction in B lymphocytes was particularly profound (data not shown). Sublethally irradiated Hoxa-9+/- animals showed white blood cell counts intermediate between Hoxa-9-/- and wild-type mice, with a statistically insignificant reduction in lymphocyte subpopulations (data not shown). The persistent multilineage cytopenias seen following the hematopoietic stresses of either marrow transplantation or sublethal irradiation suggested a defect at the level of the pluripotent stem cell.
Hoxa-9-/- marrow cells have impaired recovery following hematopoietic stress. (A) Peripheral-blood counts in recipient animals following transplantation of either 200 000 Hoxa-9-/- or 200 000 wild-type unfractionated nucleated bone marrow cells. Recipient mice were pretreated with 800 cGy x-ray irradiation prior to transplantation, and then monitored with serial peripheral-blood counts beginning 4 weeks after transplantation and then every 2 to 6 weeks for up to 41 weeks. Graphs represent composite data from 2 separate experiments, involving a total of 4 Hoxa-9-/- and 3 wild-type donors, and 10 recipients each of Hoxa-9-/- and wild-type marrow cells. *P < .05 for Hoxa-9-/- versus wild-type value. (B) Blood counts in primary animals following sublethal irradiation. Groups of Hoxa-9-/- and wild-type mice were exposed to 500 cGy x-ray irradiation, and peripheral-blood counts were monitored every 2 to 4 weeks, starting 9 weeks after irradiation and continuing to 19 weeks. Graphs represent a composite of 3 separate experiments involving 13 Hoxa-9-/- animals and 7 wild-type animals. *P < .05 for Hoxa-9-/- versus wild-type value.
Hoxa-9-/- marrow cells have impaired recovery following hematopoietic stress. (A) Peripheral-blood counts in recipient animals following transplantation of either 200 000 Hoxa-9-/- or 200 000 wild-type unfractionated nucleated bone marrow cells. Recipient mice were pretreated with 800 cGy x-ray irradiation prior to transplantation, and then monitored with serial peripheral-blood counts beginning 4 weeks after transplantation and then every 2 to 6 weeks for up to 41 weeks. Graphs represent composite data from 2 separate experiments, involving a total of 4 Hoxa-9-/- and 3 wild-type donors, and 10 recipients each of Hoxa-9-/- and wild-type marrow cells. *P < .05 for Hoxa-9-/- versus wild-type value. (B) Blood counts in primary animals following sublethal irradiation. Groups of Hoxa-9-/- and wild-type mice were exposed to 500 cGy x-ray irradiation, and peripheral-blood counts were monitored every 2 to 4 weeks, starting 9 weeks after irradiation and continuing to 19 weeks. Graphs represent a composite of 3 separate experiments involving 13 Hoxa-9-/- animals and 7 wild-type animals. *P < .05 for Hoxa-9-/- versus wild-type value.
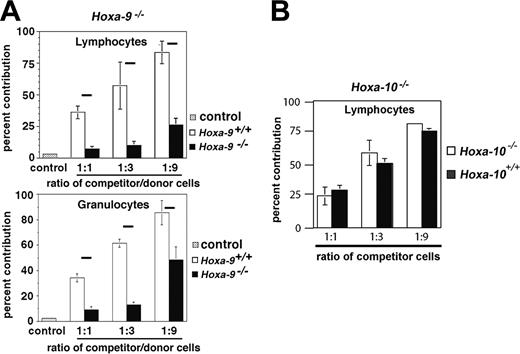
Hoxa-9-/- marrow cells have diminished repopulating ability in competitive transplantation assays
In order to measure the long-term repopulating ability of Hoxa-9-/- marrow cells in vivo, a standard competition assay was used in which lethally irradiated mice received transplants with various numbers of either Hoxa-9-/- or sibling wild-type marrow cells along with a fixed dose of competitor cells, distinguished by the presence or absence of the surface marker CD45.2 on peripheral-blood leukocytes. As shown in Figure 2A, the percentage contribution of Hoxa-9-/- cells to the granulocyte (lower panel) and lymphocyte (upper panel) compartment was markedly diminished compared with Hoxa-9+/+ cells and well below the predicted ratios (eg, 50%, 75%, and 90%), showing a 4- to 12-fold reduction in relative repopulating ability. We also analyzed the repopulating ability of marrow cells from mice deficient in Hoxa-10, a neighboring gene in the Hoxa cluster. Hoxa-10-/- mice have no discernible abnormalities in their hematopoietic system, or clonogenic assays for myeloid or B-cell progenitors (data not shown). In contrast to Hoxa-9-/- marrow cells, Hoxa-10-deficient marrow cells were as competitive in repopulating assays as wild-type cells (Figure 2B).
To measure stem-cell frequency, competitive repopulating assays of Hoxa-9-deficient marrow cells were repeated at limiting dilution. FACS analysis of CD45.2 and CD45.1 expression in the peripheral-blood leukocytes of recipient mice was performed 12 to 20 weeks after transplantation to determine the contribution of test cells to the lymphoid and myeloid compartments. In 3 separate experiments, the calculated frequency of Hoxa-9-/- competitive repopulating units (CRUs) was reduced 7-fold compared with wild-type marrow (0.61 vs 4.38 per 105 transplanted cells at 12 weeks, and 0.64 vs 4.00 at 20 weeks; Table 1). This difference in CRU was consistent from experiment to experiment, each of which was statistically significant at P < .05 individually, and P = .001 in composite. Subsets of these mice were re-evaluated at later time points up to 1 year after transplantation and showed no significant change in the percentage of donor contribution, indicating neither delayed appearance nor disappearance of Hoxa-9-/--derived cells over time (data not shown). Thus in this functional in vivo assay, Hoxa-9-deficient marrow had a substantial stem-cell defect, raising the question as to whether Hoxa-9-/- stem cells were reduced in number or had a qualitative defect.
Assessment of competitive repopulating ability limiting dilution analysis
No. cells injected . | Wk 12 . | Wk 20 to 26 . |
|---|---|---|
| Hoxa-9-/- | ||
| 240 000 | 6/6 | 6/6 |
| 125 000 | 3/5 | 3/5 |
| 120 000 | 3/4 | 2/3 |
| 100 000 | 1/4 | 1/4 |
| 60 000 | 0/5 | 0/4 |
| 50 000 | 1/5 | 1/5 |
| 30 000 | 1/7 | 1/4 |
| 20 000 | 0/5 | 0/5 |
| 15 000 | ND | ND |
| 10 000 | 0/5 | ND |
| CRU frequency per 105 cells, ± 1 SE* (95% CI) | 0.61 (0.47-0.79) | 0.64 (0.49-0.84) |
| Wild type | ||
| 30 000 | 9/11 | 8/11 |
| 20 000 | ND | ND |
| 15 000 | 6/10 | 5/10 |
| 10 000 | 0/2 | 0/2 |
| 7 500 | 2/10 | 2/9 |
| 4 000 | 0/5 | ND |
| 3 750 | 0/4 | ND |
| CRU frequency per 105 cells, ± 1 SE* (95% CI) | 4.38 (3.42-5.60) | 4.00 (3.07-5.22) |
No. cells injected . | Wk 12 . | Wk 20 to 26 . |
|---|---|---|
| Hoxa-9-/- | ||
| 240 000 | 6/6 | 6/6 |
| 125 000 | 3/5 | 3/5 |
| 120 000 | 3/4 | 2/3 |
| 100 000 | 1/4 | 1/4 |
| 60 000 | 0/5 | 0/4 |
| 50 000 | 1/5 | 1/5 |
| 30 000 | 1/7 | 1/4 |
| 20 000 | 0/5 | 0/5 |
| 15 000 | ND | ND |
| 10 000 | 0/5 | ND |
| CRU frequency per 105 cells, ± 1 SE* (95% CI) | 0.61 (0.47-0.79) | 0.64 (0.49-0.84) |
| Wild type | ||
| 30 000 | 9/11 | 8/11 |
| 20 000 | ND | ND |
| 15 000 | 6/10 | 5/10 |
| 10 000 | 0/2 | 0/2 |
| 7 500 | 2/10 | 2/9 |
| 4 000 | 0/5 | ND |
| 3 750 | 0/4 | ND |
| CRU frequency per 105 cells, ± 1 SE* (95% CI) | 4.38 (3.42-5.60) | 4.00 (3.07-5.22) |
Results are expressed as number of mice repopulated with donor-derived cells (CD45.2+)/total mice in the cohort as measured by FACS analysis 12 to 20 weeks after transplantation. CRU frequency was calculated using limiting-dilution analysis (see “Materials and methods”). The results are a composite of 3 separate experiments involving 6 Hoxa-9-/- and 7 wild-type sibling control mice as donors.
ND indicates not determined; CI, confidence interval.
P = .001
Hoxa-9-/- marrow cells have decreased competitive repopulating ability in vivo. Cohorts of lethally irradiated mice were injected with a fixed dose of normal competitor marrow (500 000 cells) and 1 of 3 doses of test cells (either Hoxa-9-/- vs sibling wild type or Hoxa-10-/- vs sibling wild type): 500 000 cells (1:1 ratio), 1 500 000 cells (3:1), or 4 500 000 cells (9:1). After 12 to 20 weeks, the percentage of test marrow contribution was determined by the percentage of CD45.2 cells as measured by FACS analysis of peripheral-blood lymphocytes and granulocytes. The fractional contribution of Hoxa-9-/- cells was markedly below the predicted value (horizontal black bars) at all three cell ratios tested—50%, 75%, and 90% (A), whereas the contribution of Hoxa-10-/- cells closely approximated the values for Hoxa-10+/+ cells, which were all close to the predicted values (B).
Hoxa-9-/- marrow cells have decreased competitive repopulating ability in vivo. Cohorts of lethally irradiated mice were injected with a fixed dose of normal competitor marrow (500 000 cells) and 1 of 3 doses of test cells (either Hoxa-9-/- vs sibling wild type or Hoxa-10-/- vs sibling wild type): 500 000 cells (1:1 ratio), 1 500 000 cells (3:1), or 4 500 000 cells (9:1). After 12 to 20 weeks, the percentage of test marrow contribution was determined by the percentage of CD45.2 cells as measured by FACS analysis of peripheral-blood lymphocytes and granulocytes. The fractional contribution of Hoxa-9-/- cells was markedly below the predicted value (horizontal black bars) at all three cell ratios tested—50%, 75%, and 90% (A), whereas the contribution of Hoxa-10-/- cells closely approximated the values for Hoxa-10+/+ cells, which were all close to the predicted values (B).
Hoxa-9-/- marrow shows a normal frequency of immunophenotypically defined stem cells and normal homing of primitive progenitors to the marrow
Previous studies have identified a series of surface markers that, in combination, correlate with functional stem cells as measured in in vivo transplantation assays.18,19 To quantify stem cells by immunophenotype, we undertook a detailed FACS characterization of early lineage-negative (Lin-) hematopoietic compartments in Hoxa-9-deficient marrow using these markers. The frequency of Lin-c-kit+flk-2-Sca-1+ (KLFS) cells was determined to be 1 in 10 000 nucleated cells, a frequency identical to that of normal bone marrow (Figure 3). In addition, there was an approximate 2-fold reduction in CMPs, consistent with the reductions seen in myeloid colony assays.11 These observations, which have been confirmed in another study,27 indicate that there is no apparent reduction in the number of immunophenotypically defined stem cells, despite a dramatic impairment in functional repopulating ability. These results suggested that Hoxa-9-/- hematopoietic stem cells have a qualitative defect.
We explored the possibility that the defective in vivo repopulating ability of Hoxa-9-deficient cells might be due to impairment in their ability to home to the marrow after transplantation using 2 different assays. In the first assay, Hoxa-9-/- and wild-type marrow cells with known myeloid progenitor content were injected into lethally irradiated mice. The recipient animals were killed 15 hours later, and their marrows were assessed for myeloid progenitor numbers by standard clonogenic assay, permitting a determination of the percentage of progenitors reaching the marrow during this early time period. In these experiments (Supplemental Figure S1A, available at the Blood website; click on the Supplemental Figure link at the top of the online article), the percentage of Hoxa-9-/- progenitors reaching the marrow (9%) was virtually identical to wild-type marrow (8%). Although a normal percentage of Hoxa-9-/- progenitors had reached the marrow within 15 hours, we reasoned that the mutant cells might be defective in reaching the endosteal region of the marrow, where HSCs are thought to reside.28 To test this possibility, Lin-Sca-1+ marrow cells were labeled ex vivo with CFSE and injected into nonirradiated recipients. These animals were killed 15 hours later, and their femurs were fixed and sectioned and then examined under a fluorescent microscope to determine the percentage of fluorescent cells residing in the endosteal region and the central zone of the marrow. As with the progenitor assays, the frequency of Hoxa-9-/- cells in the endosteal region was essentially identical to that of wild-type marrow (approximately 50%; Supplemental Figure S1B).
Hoxa-9-/- marrow has a normal frequency of immunophenotypic HSCs. Normal and Hoxa-9-/--deficient marrow cells were analyzed by FACS for expression of lineage markers, flk-2, Sca-1, and c-kit. Marrow cells were initially gated for Lin-flk-2- cells (left-hand panels). Lin-flk-2- cells were then analyzed for expression of Sca-1 and c-kit (middle- and right-hand panels). The frequency of c-kit+flk-2-Lin-Sca-1+ (KFLS) cells in mutant (A) and wild-type (B) marrow was essentially identical (1 in 10 000 nucleated cells).
Hoxa-9-/- marrow has a normal frequency of immunophenotypic HSCs. Normal and Hoxa-9-/--deficient marrow cells were analyzed by FACS for expression of lineage markers, flk-2, Sca-1, and c-kit. Marrow cells were initially gated for Lin-flk-2- cells (left-hand panels). Lin-flk-2- cells were then analyzed for expression of Sca-1 and c-kit (middle- and right-hand panels). The frequency of c-kit+flk-2-Lin-Sca-1+ (KFLS) cells in mutant (A) and wild-type (B) marrow was essentially identical (1 in 10 000 nucleated cells).
KFLS cells from Hoxa-9-deficient marrow have a reduced number of high-proliferative potential (HPP) colonies. Single KFLS cells (Figure 3) were sorted into individual wells of a 96-well plate containing media with a combination of cytokines (see “Materials and methods”) and cultured for 10 days. (A) Colonies were enumerated in each well according to type (GM [granulocyte-monocyte], mixed, BFU-E, and HPP). (B) Typical colony formed by Hoxa-9-/- KFLS cell. (C) HPP colony formed by wild-type KFLS cell. Colony images were obtained using an Axiovert 25 inverted microscope equipped with an Axioplan 32 ×/0.40 objective lens (Carl Zeiss, Thornwood, NY). Images were captured with a Nikon Coolpix 4500 digital camera (Nikon USA, Torrance, CA) and enhanced using Nikon Photoimpression 4.0 software.
KFLS cells from Hoxa-9-deficient marrow have a reduced number of high-proliferative potential (HPP) colonies. Single KFLS cells (Figure 3) were sorted into individual wells of a 96-well plate containing media with a combination of cytokines (see “Materials and methods”) and cultured for 10 days. (A) Colonies were enumerated in each well according to type (GM [granulocyte-monocyte], mixed, BFU-E, and HPP). (B) Typical colony formed by Hoxa-9-/- KFLS cell. (C) HPP colony formed by wild-type KFLS cell. Colony images were obtained using an Axiovert 25 inverted microscope equipped with an Axioplan 32 ×/0.40 objective lens (Carl Zeiss, Thornwood, NY). Images were captured with a Nikon Coolpix 4500 digital camera (Nikon USA, Torrance, CA) and enhanced using Nikon Photoimpression 4.0 software.
Lin-Sca-1+ cells from Hoxa-9-/- marrow have diminished proliferative capacity in vitro
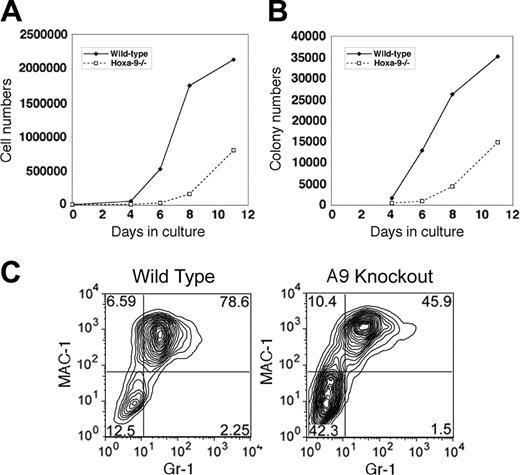
An alternative explanation for the reduced contribution of Hoxa-9-/- cells to the hematopoietic compartment in competitive transplantation experiments was an impairment in their proliferative response to cytokines. To assess the proliferative capacity of early hematopoietic cells in Hoxa-9-/- marrow, individual wild-type and Hoxa-9-deficient KFLS marrow cells were sorted singly into wells and cultured for 10 days, when colony types were enumerated. As shown in Figure 4A, wells seeded with wild-type cells contained colonies in virtually every well, whereas the Hoxa-9-/- cells showed a small and statistically insignificant reduction of approximately 30%. Furthermore, the frequencies of mixed and CFU-GM colonies were similar for normal and Hoxa-9-/- KFLS cells, and there was a statistically insignificant reduction in blast-forming units-erythroid (BFU-Es) in the Hoxa-9-/- cultures. However, when very large high-proliferative potential (HPP) colonies were enumerated, there was a significant 4-fold reduction in their frequency in Hoxa-9-/- KFLS cells. Normal HPP colonies were composed of thousands of cells covering the bottom of the well (Figure 4C), whereas colonies in the Hoxa-9-/- cultures were much smaller and less dense (Figure 4B).
To further confirm this proliferative defect, Lin-Sca-1+ cells were placed in bulk liquid cultures with the same mix of cytokines. Cell counts during a period of 11 days in culture revealed a marked reduction in the number of cells produced in the Hoxa-9-/- cultures, such that on day 8, there was a 5-fold reduction of total cell numbers (Figure 5A). In addition, aliquots of cells were removed at various time points of culture and placed in semisolid media to measure the frequency of myeloid progenitors. In these secondary cultures, the Hoxa-9-/- cells displayed a delayed emergence of clonogenic cells with a 5-fold reduction in colony numbers on day 8 (Figure 5B). To analyze the maturation status of cells in these bulk cultures, the coexpression of the myeloid differentiation markers Mac-1 and Gr-1 was measured by FACS after 8 days. As shown in Figure 5B, the percentage of Mac-1hiGr-1hi cells was significantly reduced in the Hoxa-9-/- cultures, with a concomitant increase in the Mac-1-/loGr-1-/lo population (Figure 5C), consistent with a delayed output of mature myeloid cells.
Reintroduction of Hoxa-9 expression corrects the proliferative and repopulating defects of Hoxa-9-/- marrow cells
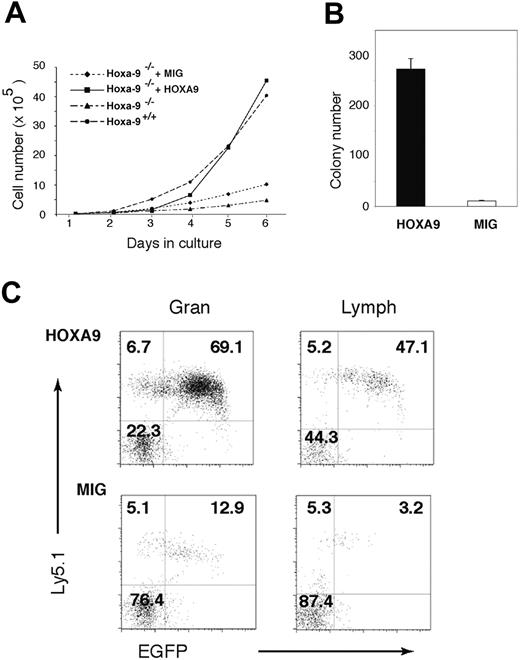
Marrow cells from Hoxa-9-deficient mice were transduced with a vector expressing either Hoxa-9 and GFP or GFP alone, and then placed in liquid culture. As shown in Figure 6A, cells transduced with GFP alone show modestly enhanced growth when compared with untransduced Hoxa-9-/- cells, whereas cells expressing exogenous Hoxa-9 showed a proliferative rate identical to that of wild-type cells (Figure 6A). This improvement in cell growth was associated with a greatly enhanced emergence of myeloid progenitors at day 8 of culture (Figure 6B), showing that restoration of Hoxa-9 expression is sufficient to correct the defects in in vitro cell growth. Transduced CD45.2+Hoxa-9-/- cells were injected with equal numbers of untransduced CD45.1+ wild-type cells into lethally irradiated CD45.1+ mice. The contribution of transduced cells to the peripheral-blood granulocytes and lymphocytes in the recipients was assessed by FACS analysis for expression of GFP and CD45.2 4 weeks after transplantation. As shown in Figure 6C (upper panels), the contribution of Hoxa-9-transduced cells to the granulocyte and lymphocyte populations in recipient peripheral blood was robust (-73.6% ± 2.3% in the granulocyte pool and 36.5% ± 7.3% in the lymphocyte pool). In contrast, cells transduced with the MIG control vector (Figure 6C, lower panels) competed very poorly against wild-type (CD45.2-) cells (8.1% ± 3.5% in the granulocyte pool and 1.8% ± 0.8% in the lymphocyte pool), reflecting the repopulating defect of the parental Hoxa-9-/- marrow cells. This defect is borne out by the poor repopulating ability of untransduced cells (CD45.2+GFP-) seen in all 4 panels in Figure 6C. Thus, enforced expression of HOXA-9 restores the repopulating ability of Hoxa-9-/- cells.
Hoxa-9-/- primitive cells have a proliferative defect in vitro and delayed emergence of committed progenitors and mature myeloid cells, defects that are corrected by re-expressing Hoxa-9. Lin-Sca-1+ marrow cells were plated in bulk culture with the same combination of cytokines as Figure 4 and grown for up to 11 days. (A) Total cell numbers in the cultures over time. (B) The number of myeloid progenitors as assayed in secondary clonogenic assays in semisolid medium. (C) FACS analysis of cultured cells for Mac-1 and Gr-1 expression after 8 days in culture.
Hoxa-9-/- primitive cells have a proliferative defect in vitro and delayed emergence of committed progenitors and mature myeloid cells, defects that are corrected by re-expressing Hoxa-9. Lin-Sca-1+ marrow cells were plated in bulk culture with the same combination of cytokines as Figure 4 and grown for up to 11 days. (A) Total cell numbers in the cultures over time. (B) The number of myeloid progenitors as assayed in secondary clonogenic assays in semisolid medium. (C) FACS analysis of cultured cells for Mac-1 and Gr-1 expression after 8 days in culture.
Restoration of Hoxa-9 expression corrects the in vitro proliferative defects and in vivo repopulating ability of Hoxa-9-/- marrow cells. Marrow cells from 5-fluorouracil-treated Hoxa-9-/- mice were transduced either with a retroviral vector expressing Hoxa-9-/- together with GFP (HOXA9 vector) or a control vector expressing GFP alone (MIG). The transduction efficiency of the HOXA9 vector was 53% and the MIG vector 71%, as measured by the percentage of GFP-positive cells by FACS 3 days after transduction. Unsorted cells (ie, mixtures of transduced and untransduced cells) were placed in liquid cultures, as described in panel A, or transplanted into cohorts of lethally irradiated CD45.1+ recipients (3 animals each) together with equal numbers (500 000 cells) of unfractionated CD45.1+ competitor cells. (A) Cell-growth curves of cultured Hoxa-9-/- marrow cells transduced with either the Hoxa-9 or MIG vector, compared with untransduced control wild-type and Hoxa-9-/- cells. (B) The number of myeloid progenitors on day 8 of liquid culture as assayed in clonogenic assays in semisolid medium. An aliquot of cells was removed from the cultures on day 8 and plated directly into methylcellulose cultures and scored for colony formation. (C) FACS analysis of peripheral blood of mice that received transplants 4 weeks previously with either Hoxa-9-transduced (top panels) or MIG-transduced (bottom panels) Hoxa-9-/- marrow cells, gated for the granulocyte (Gran, left panels) and lymphocyte (Lymph, right panels) populations. In each panel, the transduced Hoxa-9-/- cells (CD45.2+GFP+) are represented in the top-right quadrant; untransduced Hoxa-9-/- cells (CD45.2+GFP-), in the top-left quadrant; and wild-type competitor cells (CD45.2-GFP-), in the bottom-left quadrant.
Restoration of Hoxa-9 expression corrects the in vitro proliferative defects and in vivo repopulating ability of Hoxa-9-/- marrow cells. Marrow cells from 5-fluorouracil-treated Hoxa-9-/- mice were transduced either with a retroviral vector expressing Hoxa-9-/- together with GFP (HOXA9 vector) or a control vector expressing GFP alone (MIG). The transduction efficiency of the HOXA9 vector was 53% and the MIG vector 71%, as measured by the percentage of GFP-positive cells by FACS 3 days after transduction. Unsorted cells (ie, mixtures of transduced and untransduced cells) were placed in liquid cultures, as described in panel A, or transplanted into cohorts of lethally irradiated CD45.1+ recipients (3 animals each) together with equal numbers (500 000 cells) of unfractionated CD45.1+ competitor cells. (A) Cell-growth curves of cultured Hoxa-9-/- marrow cells transduced with either the Hoxa-9 or MIG vector, compared with untransduced control wild-type and Hoxa-9-/- cells. (B) The number of myeloid progenitors on day 8 of liquid culture as assayed in clonogenic assays in semisolid medium. An aliquot of cells was removed from the cultures on day 8 and plated directly into methylcellulose cultures and scored for colony formation. (C) FACS analysis of peripheral blood of mice that received transplants 4 weeks previously with either Hoxa-9-transduced (top panels) or MIG-transduced (bottom panels) Hoxa-9-/- marrow cells, gated for the granulocyte (Gran, left panels) and lymphocyte (Lymph, right panels) populations. In each panel, the transduced Hoxa-9-/- cells (CD45.2+GFP+) are represented in the top-right quadrant; untransduced Hoxa-9-/- cells (CD45.2+GFP-), in the top-left quadrant; and wild-type competitor cells (CD45.2-GFP-), in the bottom-left quadrant.
Discussion
Although there are abundant data that enforced expression of a number of Hox genes in hematopoietic cells results in increased proliferation and expansion of hematopoietic stem cells, the results of the current study demonstrate the converse finding that the loss of a Hox gene results in an apparent defect in HSC function. This conclusion is tempered by the fact that the biologic assays used to assess stem-cell function are also dependent on progenitor-cell function. Hoxa-9-/- marrow cells do display reductions in myeloid and pre-B-cell progenitors, but the magnitude of those defects is much smaller than the repopulating defect we report here. Hoxa-9-deficient HSCs exhibit a markedly diminished capacity to repopulate recipient mice, despite the presence of normal numbers of putative stem cells based on standard immunophenotypic markers.18,29 One interpretation of this disparity is that assessing HSCs based on immunophenotype alone may not correlate with measuring stem cells in a functional assay. This apparent lack of congruence between functional HSCs and immunophenotypic HSCs does not appear to be due to a difference in the homing capacity of the Hoxa-9-/- cells. However, Hoxa-9-/- HSCs exhibit a substantial loss of proliferative capacity in response to cytokines and a dramatic decrease of HPP colonies when grown in vitro, an observation that is consistent with our previously reported in vivo analysis that showed a very blunted neutrophilic response to G-CSF administration in Hoxa-9-/- animals.11 Finally, restoration of Hoxa-9 by retroviral transfection completely corrected all of the in vitro and in vivo functional defects.
The major HSC defect resulting from the loss of Hoxa-9 stands in contrast to both the apparently normal HSC function in mice deficient in the neighboring gene, Hoxa-10, as well as the mild HSC defects previously reported for cells lacking Hoxb-4 alone or a combination of Hoxb-4 and Hox-b3. Loss of Hoxb-4 was also associated with an insignificant reduction in the absolute number of primitive cells (Lin-Sca1+c-kit+CD34lo—LSK), which showed a normal ratio of cycling cells by 7-aminoactinomycin D (7AAD) and Ki67 staining.8 Nonetheless, when individual LSK cells were grown in vitro with a combination of cytokines, they showed a small reduction in high-proliferative (HPP) clones. A modest but statistically significant decrease in in vivo repopulating ability was seen when Hoxb-4-/--deficient marrow and fetal liver cells were transplanted into lethally irradiated recipients; however, this defect, which was seen at 3 to 6 weeks after transplantation, was largely resolved by 12 weeks.
The hematopoietic phenotype of mice engineered to be deficient in both Hoxb-3 and Hoxb-4 has also been described.9 As observed in this study of Hoxa-9, Hoxb-3/Hoxb-4-deficient marrow cells showed no defects in homing to the marrow but a blunted proliferative response to cytokine stimulation in vitro, with a small but significant decrease in HPP colonies. Hoxb-3/Hoxb-4-deficient marrow showed reduced repopulating ability in transplantation studies, which again was transient. Thus loss of Hoxb-4, or Hoxb-3 and Hoxb-4 together, is associated with very similar phenotypes as we have described here for Hoxa-9, albeit much milder.
Until recently, it was not clear why Hoxa-9 had such a predominant effect on hematopoiesis when compared with other Hox genes, at least in the knock-out models studied to date. A recent study by Thompson et al measured RNA levels of Hox genes in Lin-c-kit+ cells from murine fetal liver and showed that Hoxa genes were more highly expressed than genes of the Hoxb, Hoxc, and Hoxd cluster, and that Hoxa-9 was one of the mostly highly expressed in primitive hematopoietic cells.30 Thus while overexpression of a number of individual Hox genes can result in stem-cell expansion, Hoxa-9 may be a major determinant of stem-cell growth and self renewal under physiologic conditions.
A key question emerging from this study is what molecular pathways mediate the effects of Hoxa-9 in the proliferation of primitive hematopoietic cells. Clues as to the transcriptional target of the Hoxa-9 protein come from our recent gene expression study of hematopoietic cell lines engineered to overexpress this protein.31 Of the genes showing a significant 2-fold or greater up-regulation after the introduction of Hoxa-9, a number encode proteins that regulated cell proliferation. Those genes include genes involved with the cell cycle, such as cdc6 and CDK7, kinases such as pim-1 and nemolike kinase (NLK), the oncogene Myb, and signaling molecules such as epidermal growth factor receptor (EGFR) substrate 8 (EPS8). Conversely, the gene junB, which appears to suppress myeloid proliferation,32 was significantly down-regulated by Hoxa-9. That some of these changes in gene expression represent direct transcriptional effects was suggested by transient transcription assays of promoter-reporter constructs for selected genes. Notably, 78 of the genes that were modulated by Hoxa-9 have been previously reported to be part of the transcriptome, or gene expression signature, of normal CD34+ hematopoietic cells, suggesting that Hoxa-9 regulates a number of molecular pathways involved with stem-cell function. Establishing which of these genes mediated the biologic effect of Hoxa-9 and other Hox proteins will be an important area of future study.
In the study by Bjornsson et al,9 it was shown that Hoxb-3/Hoxb-4-/- cells were more resistant to 5-fluorouracil treatment and entered into cell cycle in a delayed fashion after exposure to cyclophosphamide. A recurring observation in all of these knock-out models, including Hoxa-9, is a blunted response to hematopoietic stress, whether it be myelosuppression (chemotherapy, irradiation), transplantation, in vivo treatment with G-CSF, or cultivation in the presence of multiple cytokines. It is perhaps noteworthy that, in the case of Hoxa-9-/-, the blunted response to cytokines appears to be a delay or a pause in the proliferative response, rather than an absolute decrease in growth rate, since examination of the growth curves (Figure 6) shows that the proliferative rate of Hoxa-9-/--deficient cells begins to parallel that of wild-type cells after several days. This finding is also consistent with our observation of delayed T-cell development in Hoxa-9-/--deficient mice.12 The fetal thymuses in the mutant animals were 10-fold reduced in size compared with their wild-type littermates, whereas adult Hoxa-9-/- thymuses were reduced only by half.
These findings suggest that Hox genes function downstream of hematopoietic cytokines to mediate, at least in part, their biologic effects. Recent support for such a model comes from 2 recent studies on the interaction between the cytokine TPO and Hox proteins. Kirito et al recently reported that TPO, which in addition to stimulating platelet production also enhances HSC self renewal, can stimulate the expression of Hoxb-4 at the transcriptional level, through a p38 mitogen-activated protein kinase (MAPK)/upstream stimulatory factor 1 (USF-1) pathway.33 They further showed that TPO-deficient hematopoietic cells, which have diminished repopulating ability, exhibited reduced levels of Hoxb-4. This same group more recently reported that TPO, while not stimulating the transcription of the Hoxa-9 gene, nonetheless enhanced the nuclear localization of Hoxa-9 protein in the nucleus, and presumably thereby enhanced its transcriptional activity.34 These findings suggest that proliferative effects of TPO are mediated in part by Hoxb-4 and Hoxa-9, and perhaps other Hox genes, and open the possibility that other blood-specific cytokines and transcription factors, such as GATA-1, act through the Hox pathway.35
Prepublished online as Blood First Edition Paper, August 9, 2005; DOI 10.1182/blood-2005-05-2003.
Supported in part by grant no. DK48642 from the National Institutes of Health (H.J.L.) and by grants from the Department of Veteran Affairs (H.J.L. and C.L.). H.J.L. is a recipient of a VA Career Development Award.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Figure 4. KFLS cells from Hoxa-9-deficient marrow have a reduced number of high-proliferative potential (HPP) colonies. Single KFLS cells (Figure 3) were sorted into individual wells of a 96-well plate containing media with a combination of cytokines (see “Materials and methods”) and cultured for 10 days. (A) Colonies were enumerated in each well according to type (GM [granulocyte-monocyte], mixed, BFU-E, and HPP). (B) Typical colony formed by Hoxa-9-/- KFLS cell. (C) HPP colony formed by wild-type KFLS cell. Colony images were obtained using an Axiovert 25 inverted microscope equipped with an Axioplan 32 ×/0.40 objective lens (Carl Zeiss, Thornwood, NY). Images were captured with a Nikon Coolpix 4500 digital camera (Nikon USA, Torrance, CA) and enhanced using Nikon Photoimpression 4.0 software.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/12/10.1182_blood-2005-05-2003/2/m_zh80240587350004.jpeg?Expires=1765926498&Signature=qeOe7qUc9lnt6LbndIUH7U47dNAhbO3RQxDZg33srBbvN4qbz-d3dToOTYSbneOOrRu4bNG5R3i~Q9jYjrMEyxZ7~ln6oKZbS3fxaJvBp-YLOxc3tU-dr6btm8UiIXxuwX0-Xi~rS~-E4Qgaz54XHWWiYzyetywsaXMwZhBQmd9cdsXPr56OscZD5nlTwUSy~UFbmFt5PS8~VJfNG1fVTttGMznNqUA-7AHLIBNr7QmCXnbXGo6mZjK8jKFNooYbljBjxw~8B429lsTr-aDpTvk0sCDr35B38jePlFG90Zy-6Ni8639MkyGSe48EY0xfdzGbGI9ddXuPNvZ-Xwslyw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal