Abstract
Ethical constraints restrict direct tracking of immune-cell migration throughout the human body in vivo. We, therefore, used deletion of the immunoglobulin M (IgM) heavy-chain constant-gene (Cμ) segment as a marker to provide a dispersal signature of an effector B-cell subset (IgD+IgM-CD38+) induced selectively in human tonsils. By DNA analysis, the Cμ deletion identified dissemination of such blasts and their plasma-cell progeny to peripheral blood, lymph nodes, and bone marrow, as well as to mucosae and glands of the upper airways. Also the endocervix was often positive, while the small intestine was mainly negative, as could be expected from the identified homing-molecule profile of the marker cells, with relatively low levels of integrin α4β7 and CC chemokine receptor 9 (CCR9). Of further importance for vaccine design, the circulating cells expressed abundantly CD62L (L-selectin) and CCR7, which provided a mechanism for integration of respiratory and systemic immunity. Most mucosal vaccines are at present administered perorally, and our results suggested that the nasal route is no alternative for vaccination against rotavirus or other small-intestinal infections in humans. However, immunization of nasopharynx-associated lymphoid tissue clearly appears preferable to target respiratory pathogens and may to some extent also protect against infections of the female genital tract. (Blood. 2005;106:593-600)
Introduction
Most pathogens use mucosae as portals of entry, and mucosal vaccines are therefore desirable,1 particularly to elicit secretory IgA (SIgA) antibodies.2,3 The secretory antibody system represents a major first-line defense and is triggered by antigens targeting mucosa-associated lymphoid tissue (MALT), which samples antigens directly from epithelial surfaces.4 T and B cells activated in MALT home preferentially to mucosal effector sites, where B cells after extravasation undergo terminal differentiation. The local plasma-cell (PC) progeny cooperates with the polymeric Ig receptor (pIgR) to provide SIgA and secretory IgM (SIgM) antibodies.5,6 Thus, locally produced IgA dimers and IgM pentamers are actively exported by the pIgR. For this epithelial transport mechanism to be operative, the Ig polymers depend on a selective structural incorporation of the J chain,6,7 although the cytoplasmic expression of this peptide is a common characteristic of mucosal PCs regardless of their concurrent Ig isotype.8,9
Induction of immunity at various mucosae is clearly an integrated response, but the traditional term “a common mucosal immune system” has detracted from the fact that there may be considerable compartmentalization.4,5 This has been difficult to substantiate, however, because no inherent marker exists for mucosal B cells that unequivocally identify their MALT origin. Also notably, homing studies performed with primed human T cells, traced by 111In labeling or gene marking, have provided conflicting results.10,11 Nevertheless, rational site selection for mucosally applied vaccines would require detailed knowledge of where the activated mucosal B cells home.
Here, we have used a molecular approach to track the dispersal of surface (s)IgD+IgM-CD38+ B cells that are selectively derived from human nasopharynx-associated lymphoid tissue (NALT)4 such as the palatine tonsils.12 Locally, these activated B cells give rise to extrafollicular IgD+ PCs with 80% J-chain positivity in most (∼ 90%) subjects examined.13 Tonsillar germinal centers (GCs) have been determined to generate 2% to 5% sIgD+IgM-CD38+ centroblasts as a result of nonclassical Ig heavy-chain constant (CH)-gene switching; this process involves deletion of the Cμ-gene segment, variably including the switch (Sμ) region, thereby principally prohibiting further downstream class switch.12,14
By a specifically designed nested polymerase chain reaction (PCR) method performed on extracted DNA, we were able to show that the sIgD+IgM-CD38+ subset enters peripheral blood, and we could identify these plasmablasts and/or their IgD+ PC progeny at distant tissue sites. The IgD+ PCs showed a mucosal phenotype by expressing J chain, and we believe that we have established a marker to track the general homing pattern of mucosal B cells activated in human NALT, including the sIgA+ plasmablasts predominantly involved in the generation of secretory immunity. Our notion is supported by circumstantial evidence obtained from previous immunization studies in humans as discussed later in the article. We could thus directly characterize the homing-molecule profile of NALT-derived B-cell blasts and found it to be strikingly different from that imprinted in gut-associated lymphoid tissue (GALT). These novel results obtained by tracking of B-cell migration throughout the human body have to be taken into consideration in future design of mucosal vaccines.
At present, most mucosal vaccines licensed for human use are administered perorally to target GALT.2,15,16 However, there is currently considerable interest also in the nasal route in an effort to design vaccines that can be applied for immunization in a simple and cheap way, without the use of potentially contaminated sharps.1,2 Further, nasal administration that targets NALT avoids the problem with immunogen degradation by gastrointestinal enzymes, and a specially designed application device developed for mass vaccination can protect the lungs from contact with the immunogen and associated adjuvant.17 Our study reveals for the first time at a compartmental tissue level the potentials and limitations of nasal vaccine administration in humans by mapping the in vivo homing pattern of NALT-derived effector B cells.
Materials and methods
FACS sorting of B cells from tonsils and blood
Surgically removed palatine tonsils from adults who had recurrent tonsillitis, were minced and filtered through a 45-μm screen. Mononuclear cells were separated on Lymphoprep (Nycomed Pharma, Oslo, Norway) and immunostained with goat anti-IgD (fluorescein isothiocyanate, FITC) or anti-IgM (phycoerythrin, PE) conjugates (Southern Biotechnology, Birmingham, AL). B-cell subsets were isolated by fluorescence-activated cell sorting (FACS) (FACSVantage; Becton Dickinson, Mountain View, CA). IgD+ blasts were also sorted from peripheral blood of 2 IgA-deficient and 2 healthy adult donors. The samples have been collected and used with approval obtained from the review board of the Faculty Division, Rikshospitalet University Hospital, Oslo. Three regions were defined in the forward light scatter-IgD dot plot (see Figure 4A). Genomic DNA was obtained by Nucleon DNA extraction kit (Amersham, Aylesbury, United Kingdom) and analyzed for Cμ deletion by nested PCR (as described in “PCR analysis”).
Immunohistochemistry
Diseased tissue specimens were obtained during diagnostic workup (celiac disease, ulcerative colitis, and Crohn disease) or during surgery of palatine and nasopharyngeal tonsils (recurrent tonsillitis and adenoid hyperplasia); normal samples were collected bioptically according to guidelines of regional Ethics Committees, or from organ donors (Table 1). Altogether, 199 individual tissue blocks were prepared by cold-ethanol fixation and paraffin wax embedding.9,13 Sections (6 μm) were evaluated histologically, immunostained (2-color immunofluorescence) for IgA, IgM, IgG, IgD, and J chain with polyclonal rabbit IgG antibody conjugates prepared in our laboratory,9,13 or with monoclonal antibody (mAb) against the CC chemokine receptor 10 (CCR10; 1B5, 1 μg/mL; Millennium Pharmaceuticals, Cambridge, MA), and the preparations were analyzed morphometrically.9,13 Stained sections were mounted in polyvinylalcohol (P8136; Sigma Aldrich, Steinheim, Germany), pH 8.7. Fluorescence microscopy was performed with a Nikon microscope (EcLipse E800; Nikon, Tokyo, Japan) equipped with objectives and a Ploem-type beam-splitting device (Nikon). Images were captured with an F-view digital camera controlled by Analysis 3.2 software (Analysis Soft Imaging System, GmbH, Münster, Germany) and transferred to Adobe Photoshop (Adobe, San Jose, CA).
Human tissue samples analyzed by immunohistochemistry and subjected to nested PCR analysis for detection of C μ deletion after DNA extraction
Tissue category and clinicopathologic information . | No. of individual samples . | Subject median age, y (range) . |
|---|---|---|
| Subjects without IgA deficiency | ||
| Palatine tonsils | ||
| Normal controls | 9 | 10 (2-72) |
| Recurrent tonsillitis | 13 | 5 (3-75) |
| Nasopharyngeal tonsil (adenoids) | ||
| Normal controls | 5 | 6 (2-10) |
| Adenoid hyperplasia | 10 | 6.5 (3-60) |
| Lacrimal glands, normal | 6 | 31 (6-65) |
| Salivary glands, normal parotid and submandibular | 8 | 33.5 (25-73) |
| Nasal mucosa, normal or slightly inflamed | 16 | 42 (6-70) |
| Small bowel mucosa | ||
| Normal duodenum | 2 | 21-35 |
| Duodenum with celiac disease | 11 | 49 (29-64) |
| Normal ileum | 6 | 16 (7-39) |
| Ileum with Crohn disease | 3 | 36 (27-47) |
| Large bowel mucosa | ||
| Normal | 8 | 45 (7-70) |
| Ulcerative colitis or Crohn disease | 7 | 29 (24-43) |
| Peyer patches, normal | 7 | 20 (7-39) |
| Cervical lymph nodes, slight reactive changes | 8 | 14 (1-30) |
| Inguinal lymph nodes, slight reactive changes | 5 | 38 (26-45) |
| Mesenteric lymph nodes, slight reactive changes | 7 | 20 (7-39) |
| Bone marrow, normal | 6 | 6 (5-7) |
| Uterine cervix mucosa, normal or slightly inflamed | 5 | 43 (27-45) |
| Subjects with selective IgA deficiency | ||
| Adenoids, hyperplastic | 1 | 5 |
| Palatine tonsil, recurrent tonsillitis | 2 | 5-20 |
| Lacrimal gland, normal | 1 | 53 |
| Salivary (parotid) gland, normal | 1 | 25 |
| Nasal mucosa, normal or slightly inflamed | 26 | 31.5 (5-65) |
| Small bowel mucosa | ||
| Normal duodenum | 22 | 33 (2-67) |
| Duodenum with celiac disease | 1 | 12 |
| Large bowel mucosa, normal | 3 | 11 (1-35) |
Tissue category and clinicopathologic information . | No. of individual samples . | Subject median age, y (range) . |
|---|---|---|
| Subjects without IgA deficiency | ||
| Palatine tonsils | ||
| Normal controls | 9 | 10 (2-72) |
| Recurrent tonsillitis | 13 | 5 (3-75) |
| Nasopharyngeal tonsil (adenoids) | ||
| Normal controls | 5 | 6 (2-10) |
| Adenoid hyperplasia | 10 | 6.5 (3-60) |
| Lacrimal glands, normal | 6 | 31 (6-65) |
| Salivary glands, normal parotid and submandibular | 8 | 33.5 (25-73) |
| Nasal mucosa, normal or slightly inflamed | 16 | 42 (6-70) |
| Small bowel mucosa | ||
| Normal duodenum | 2 | 21-35 |
| Duodenum with celiac disease | 11 | 49 (29-64) |
| Normal ileum | 6 | 16 (7-39) |
| Ileum with Crohn disease | 3 | 36 (27-47) |
| Large bowel mucosa | ||
| Normal | 8 | 45 (7-70) |
| Ulcerative colitis or Crohn disease | 7 | 29 (24-43) |
| Peyer patches, normal | 7 | 20 (7-39) |
| Cervical lymph nodes, slight reactive changes | 8 | 14 (1-30) |
| Inguinal lymph nodes, slight reactive changes | 5 | 38 (26-45) |
| Mesenteric lymph nodes, slight reactive changes | 7 | 20 (7-39) |
| Bone marrow, normal | 6 | 6 (5-7) |
| Uterine cervix mucosa, normal or slightly inflamed | 5 | 43 (27-45) |
| Subjects with selective IgA deficiency | ||
| Adenoids, hyperplastic | 1 | 5 |
| Palatine tonsil, recurrent tonsillitis | 2 | 5-20 |
| Lacrimal gland, normal | 1 | 53 |
| Salivary (parotid) gland, normal | 1 | 25 |
| Nasal mucosa, normal or slightly inflamed | 26 | 31.5 (5-65) |
| Small bowel mucosa | ||
| Normal duodenum | 22 | 33 (2-67) |
| Duodenum with celiac disease | 1 | 12 |
| Large bowel mucosa, normal | 3 | 11 (1-35) |
PCR analysis
Genomic DNA was isolated from the same 199 dewaxed tissue blocks by proteinase K digestion and organic extraction, or with the Nucleon method (Amersham). Nested PCR was performed to detect Cμ and Sμ deletion (Figure 1A). The quality of extracted DNA was verified, and the PCR was performed as follows: 100 ng DNA was subjected to first-round PCR with 2 forward (5′-TGGGGTATCAAGTAGAGGGAG; 5′-AGAGGGAGACAAAAGATGGAAG) and 2 reverse (5′-TTACAAAGGTCCACAGATGGAG; 5′-TCACCCAGAATGAATCTCACC) primers for 25 cycles (95°C for 1 minute, 62°C for 1 minute, and 72°C for 3 minutes) with a mix of Taq2000:Pfu (7:1) (Stratagene, La Jolla, CA) in Taq2000 buffer; 0.1-μL product was used as template for the nested PCR (20 cycles; 95°C for 1 minute, 60°C for 1 minute, and 72°C for 3 minutes) with forward and reverse primers. The PCR products were analyzed by agarose gel electrophoresis in a blinded manner. Retesting of 20 random positive samples revealed 30% false negatives. All negative samples were therefore repeated twice, and all samples noted to yield a PCR product at least once were considered positive.
Phenotyping of B cells
Blood mononuclear cells from 10 healthy adult volunteers were incubated with appropriately diluted, unconjugated mouse IgG or rat IgM mAbs directed against human L-selectin (SK11; Becton Dickinson), integrin α4β7 (Act-1; courtesy I. A. Lazarovits, London, ON, Canada) or β1 (4B4; Coulter Clone, Miami, FL), CCR7 (MAB197; R&D Systems, Abingdon, United Kingdom), CCR9 (3C3 or 96-1; Millennium), CCR4 (1G1) or CCR10 (1B5) (Millennium), CD27 (3A12; courtesy R. A. W. Van Lier, Amsterdam, The Netherlands), CD38 (HB7; Becton Dickinson), and cutaneous lymphocyte antigen (CLA) (HECA-452; courtesy L. Picker, Dallas, TX). Secondary conjugates were goat anti-mouse IgG phycoerythrin (PE) or anti-rat IgM (indocarbocyanine 3, Cy3) (Jackson Immunoresearch Laboratories, West Grove, PA), followed by FITC-labeled goat anti-human IgD or IgA (Southern Biotechnology) together with allophycocyanin (APC)-labeled anti-CD14 (MφP9) and peridin chlorophyll protein (PerCP)-labeled anti-CD19 (4B7) (Becton Dickinson). Controls were isotype- and concentration-matched irrelevant mAbs or conjugates. Samples were analyzed on a FACSCalibur with the CellQUEST software (Becton Dickinson).
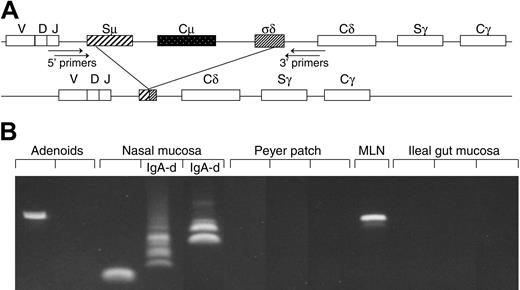
Method and performance of PCR used to detect B cells with Cμ deletion. (A) Schematic representation of the Cμ-Cδ switch region of the CH gene. Recombination between donor and acceptor switch regions (S-S recombination) is the basis for Ig class switch. Because no authentic Sδ region exists, IgD expression depends on recombination between Sμ and an intronic Cμ-Cδ sequence (σδ), thereby deleting Cμ and a variable part of the Sμ region. Amplification of Sμ-σδ recombination was obtained by nested PCR with primer positions as indicated. (B) DNA samples from 12 consecutive tissue samples analyzed as shown above. Variable break points give rise to dissimilar size of the PCR products, thereby revealing several clones depending on the frequency of their local occurrence. MLN indicates mesenteric lymph node; IgA-d, IgA-deficient samples.
Method and performance of PCR used to detect B cells with Cμ deletion. (A) Schematic representation of the Cμ-Cδ switch region of the CH gene. Recombination between donor and acceptor switch regions (S-S recombination) is the basis for Ig class switch. Because no authentic Sδ region exists, IgD expression depends on recombination between Sμ and an intronic Cμ-Cδ sequence (σδ), thereby deleting Cμ and a variable part of the Sμ region. Amplification of Sμ-σδ recombination was obtained by nested PCR with primer positions as indicated. (B) DNA samples from 12 consecutive tissue samples analyzed as shown above. Variable break points give rise to dissimilar size of the PCR products, thereby revealing several clones depending on the frequency of their local occurrence. MLN indicates mesenteric lymph node; IgA-d, IgA-deficient samples.
Results
Regional lymphoid tissue, peripheral blood, and selected secretory effector sites are seeded by NALT-derived B cells
Our nested PCR performed on extracted DNA to detect the NALT-derived sIgD+IgM-CD38+ marker cells (Figure 1A) had limited sensitivity due to repetitive Cμ and Sμ sequences and a variable product size caused by heterogeneous deletion break points.12,14 Nevertheless, we were able to identify the actual marker cells and/or their IgD+ PC progeny in peripheral blood and various tissues, although at different levels (Figure 1B). The Cμ deletion was revealed not only in recurrent tonsillitis specimens as previously reported12,14 but also in hyperplastic adenoids and, at somewhat lower levels, in normal NALT biopsies from children down to the age of 1 to 2 years as well as in lymph nodes, bone marrow, and Peyer patches (Figures 1B and 2, left).
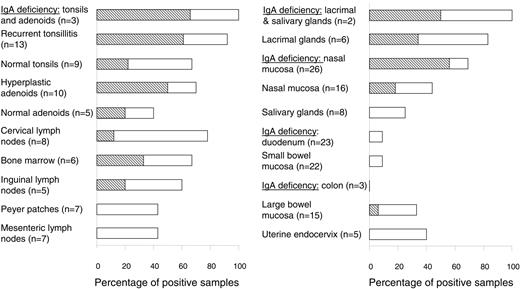
Tracking of B cells with Cμ deletion in various tissues by PCR. The frequency (%) of positive samples is represented by horizontal columns and the numbers (n) of analyzed individual samples are indicated on the left. Detection of a single or multiple clones was graded according to the number of PCR products revealed (≥ 2 electrophoretic bands, indicated by ▧; see Figure 1B). Of the 4 positive samples (9%) of small bowel mucosa, 3 were histologically normal and 1 had celiac disease. Of the 5 positive samples (28%) of large bowel mucosa, 3 were histologically normal, 1 had Crohn colitis, and 1 had ulcerative colitis.
Tracking of B cells with Cμ deletion in various tissues by PCR. The frequency (%) of positive samples is represented by horizontal columns and the numbers (n) of analyzed individual samples are indicated on the left. Detection of a single or multiple clones was graded according to the number of PCR products revealed (≥ 2 electrophoretic bands, indicated by ▧; see Figure 1B). Of the 4 positive samples (9%) of small bowel mucosa, 3 were histologically normal and 1 had celiac disease. Of the 5 positive samples (28%) of large bowel mucosa, 3 were histologically normal, 1 had Crohn colitis, and 1 had ulcerative colitis.
It was interesting to note that the 3 positive Peyer patches were from the upper age range (20-39 years), while no age dependency was indicated for the other lymphoid tissues (data not shown). A similar DNA-based approach was recently used to track NALT-derived human B cells with latent Epstein-Barr virus (EBV) infection; they were shown to be dispersed to blood and less so to lymphoid organs such as mesenteric lymph nodes and spleen.18
The most striking finding in our study was that the Cμ deletion of the NALT-derived B-cell subset frequently occurred in normal secretory tissues from the upper aerodigestive tract, but in only 4 (9%) of 45 samples from small-bowel mucosa (Figure 2, right). This relative exclusion of the marker cells from the duodenal and ileal lamina propria was maintained even in celiac disease, with only 1 of 11 cases being positive. Also notably, it was not possible to exclude that the few positive samples contained GALT structures, particularly organized isolated lymphoid follicles (ILFs). Such contamination could in fact be the reason for the higher percentage of positive samples seen in large-bowel mucosa (Figure 2, right), where the frequency of ILFs is known to be increased.4 This possibility was supported by the observation that the 2 positive diseased colon samples (one ulcerative colitis and one Crohn disease) showed high-grade inflammation, which is often associated with lymphoid neogenesis.19
Compartmentalized dispersal of sIgD+IgM-CD38+ cells agrees with the fact that IgD+ PCs often occur in the upper aerodigestive tract but rarely in the gut.5,9 This is highlighted in IgA deficiency whereby 30% to 60% of the PCs in the former body region are IgD+; the remainders are of the IgM and IgG classes, which by contrast make up at least 95% of the compensatory gut PCs.5,9 Accordingly, in the upper airways, but not in the gut, the frequency of samples positive for Cμ deletion increased strikingly when IgA was lacking, eg, in nasal mucosa the proportion rose from 44% to 69% (Figure 2, right), and multiple Cμ-deficient B-cell clones were often detectable (Figure 1B). Finally, the mucosal phenotype of the IgD+ PCs was signified by their J-chain positivity (80%-100%), both normally and in IgA deficiency (Figure 3A).
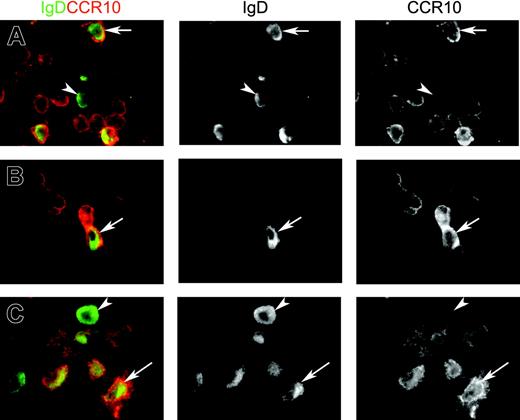
IgD+ plasma cells examined in situ for J chain, Cμ deletion, and CCR10 expression. (A) Paired immunofluorescence staining for IgD and J chain in normal lacrimal gland from an IgA-deficient individual. The merge shows that virtually all IgD+ plasmablasts and plasma cells are J chain+ (yellow cytoplasm) and, thus, of a mucosal phenotype. Epithelial elements (acini) are devoid of IgD, which is not a secretory antibody. IgD-J chain+ cells (purely red in merge) were either IgM+ (20%) or IgG+ (9.5%) as revealed in adjacent sections (not shown) (× 80). (B) Correlation (Spearman rank test) between the percentage of IgD+ plasmablasts and plasma cells relative to other isotypes as determined by immunohistochemistry (logarithmic scale), and the parallel frequency of samples containing B-cell clones with Cμ deletion graded according to the number of PCR products revealed as electrophoretic bands: 1, +; 2-3, ++; and > 3, +++ (see Figure 2B). Open symbols represent IgA-deficient samples. (C) Paired immunofluorescence staining for IgD and CCR10 on sections of 3 tissues as indicated. Merges show many IgD-CCR10+ plasmablasts or plasma cells (presumably mainly IgA+) but also several IgD+ cells with (arrows) or without (arrowheads) peripheral CCR10 expression. Two CCR10+ plasmablasts, one being distinctly double-positive, are seen in tonsillar germinal center (GC) at the border to the mantle zone (MZ), which consists mainly of naive sIgD+ B lymphocytes (left, × 60). Extrafollicular area of adenoids (middle, × 100) and glandular area of nasal mucosa (right, × 100) contain many CCR10+ large cells.
IgD+ plasma cells examined in situ for J chain, Cμ deletion, and CCR10 expression. (A) Paired immunofluorescence staining for IgD and J chain in normal lacrimal gland from an IgA-deficient individual. The merge shows that virtually all IgD+ plasmablasts and plasma cells are J chain+ (yellow cytoplasm) and, thus, of a mucosal phenotype. Epithelial elements (acini) are devoid of IgD, which is not a secretory antibody. IgD-J chain+ cells (purely red in merge) were either IgM+ (20%) or IgG+ (9.5%) as revealed in adjacent sections (not shown) (× 80). (B) Correlation (Spearman rank test) between the percentage of IgD+ plasmablasts and plasma cells relative to other isotypes as determined by immunohistochemistry (logarithmic scale), and the parallel frequency of samples containing B-cell clones with Cμ deletion graded according to the number of PCR products revealed as electrophoretic bands: 1, +; 2-3, ++; and > 3, +++ (see Figure 2B). Open symbols represent IgA-deficient samples. (C) Paired immunofluorescence staining for IgD and CCR10 on sections of 3 tissues as indicated. Merges show many IgD-CCR10+ plasmablasts or plasma cells (presumably mainly IgA+) but also several IgD+ cells with (arrows) or without (arrowheads) peripheral CCR10 expression. Two CCR10+ plasmablasts, one being distinctly double-positive, are seen in tonsillar germinal center (GC) at the border to the mantle zone (MZ), which consists mainly of naive sIgD+ B lymphocytes (left, × 60). Extrafollicular area of adenoids (middle, × 100) and glandular area of nasal mucosa (right, × 100) contain many CCR10+ large cells.
At secretory sites of the upper aerodigestive tract (and also in the bone marrow), a significant correlation did indeed exist between the frequency of IgD+ PCs and the appearance of multiple clones with Cμ deletion (Figure 3B). Conversely, in NALT (palatine tonsils and adenoids) where GCs generate sIgD+IgM-CD38+ blasts, the detectability of Cμ deletion was unrelated to the number of IgD+ PCs (Figure 3B). The same was true in cervical lymph nodes, which displayed the highest frequency of positive lymphoid samples beyond NALT (Figure 2, left). When B cells leave NALT via draining lymph, they have to pass through the cervical nodes where oligoclonal expansion driven by microbial products from the nasopharynx could promote Cμ deletion like in the tonsils.12 Concurrently, antigen-presenting DCs might imprint the B cells with homing molecules that make them targeted to regional effector sites as reported for T cells stimulated either in murine Peyer patches or draining mesenteric lymph nodes.20,21
The homing-molecule profile of NALT-derived mucosal B cells does not favor extravasation in intestinal lamina propria
The marker cell specificity was established by performing the nested PCR on DNA extracted from sorted tonsillar sIgD+IgM- cells and circulating B-cell blasts of the same phenotype (Figure 4A). To explore the molecular mechanisms behind the compartmentalized extravasation of the marker cells, we performed FACS analysis for homing receptors. A function in mucosal seeding of human sIgA+ blast cells was recently proposed for CCR10.22 Its ligand, the mucosae-associated epithelial chemokine (MEC, CCL28), is produced by secretory epithelia all over the body but, notably, at only low levels in the small bowel both in humans23 and mice.24 We found a substantial fraction (∼ 22%) of circulating sIgD+ blasts to be CCR10+ (Figure 4B), whereas only a few of them (∼ 8%) expressed the skin-homing molecule CCR4 (Figure 4B). They were also virtually negative for CLA.
Kunkel et al22 demonstrated in vitro that CCL28 attracts CCR10+ tonsillar IgA+ plasmablasts but not IgA+ PCs. It was, therefore, interesting to find that CCR10+ cells with cytoplasmic IgD occasionally appeared in tonsillar GCs and more often in extrafollicular NALT areas and secretory tissues of the upper aerodigestive tract (Figure 3C; Table 2). Plasmablasts rather than mature PCs were most clearly positive (Figure 5).
Percentage of IgD-producing cells expressing CCR10
Tissue type . | Median (range), % . |
|---|---|
| Adenoids, extrafollicular | 17 (4-17) |
| Palatine tonsils, extrafollicular | 33 (4-33) |
| Nasal mucosa | 32 (22-36) |
| Salivary glands | 65 (39-100) |
| Lacrimal glands | 64 (59-75) |
Tissue type . | Median (range), % . |
|---|---|
| Adenoids, extrafollicular | 17 (4-17) |
| Palatine tonsils, extrafollicular | 33 (4-33) |
| Nasal mucosa | 32 (22-36) |
| Salivary glands | 65 (39-100) |
| Lacrimal glands | 64 (59-75) |
Paired staining for IgD and CCR10 was performed on sections of the indicated tissue categories. Between 10 and 164 (median, 27) plasmablasts or plasma cells with cytoplasmic IgD were evaluated for distinct peripheral expression of CCR10 (percentage of positivity) in 3 individual samples of each category (median and observed range provided).
Because of low local CCL28 levels,23,24 B-cell homing to the small-intestinal lamina propria may depend on CCR9 as reported in mice.24,25 CCR9 interacts with the thymus-expressed chemokine (TECK; CCL25) selectively produced by the epithelium in that part of the gut.23,24,26 Our FACS analyses showed that circulating sIgD+ blasts expressed relatively little CCR9, although the results differed somewhat with the 2 antibody reagents tested (mAb 3C3, 10%-15% for sIgD+ blasts versus 35%-45% for the sIgA+ counterparts; mAb 96-1, < 5% of sIgD+ blasts versus 30%-35% for the sIgA+ counterparts). More importantly, the sIgD+ blasts expressed only low to intermediate levels of the classical gut homing receptor α4β7 (Figure 4C), thereby being prohibited from successful interactions with mucosal addressin cell adhesion molecule-1 (MAdCAM-1) on intestinal venules.26 In this respect they were similar to small naive sIgD+ lymphocytes and contrasted sharply with sIgA+ blasts that bore either very high or rather low levels of α4β7 (Figure 4C). The sIgA+α4β7hi cells most likely home to the gut,26 while the α4β7lo cells presumably seed extraintestinal effector sites,27 like the dispersal of sIgD+IgM- blasts demonstrated by tracking of their Cμ deletion (Figure 2). Notably, these marker cells were positive for CD27 similarly to the sIgA+ blasts (Figure 4C), thus confirming their GC derivation,28 and they expressed abundantly the plasmablast marker CD38hi (median, 71%; range, 52%-75%).
Potential molecules involved in selective homing of NALT-derived mucosal B cells
Adhesion molecules involved in mucosal homing beyond the gut remain elusive. One candidate is vascular cell adhesion molecule-1 (VCAM-1; CD106) that interacts with α4β1 (very late antigen-4 [VLA-4], CD49d/CD29), and fractions of the circulating sIgD+ blasts expressed either high (12%-18%) or intermediate (82%-88%) levels of integrin β1. By contrast, sIgA+ blasts expressed either high (36%-51%) or low to intermediate levels (data not shown). Although VCAM-1 occurs on 25% to 30% of vessels in normal human nasal mucosa,29 we were unable to detect it in normal salivary glands (Takeshi Yamanaka and P.B., unpublished observations, March 2001), and its expression is reportedly scant in the human cervix.30
Flow cytometry and Cμ-deletion analysis of circulating sIgD+ B cells. (A) After FACS sorting of sIgD+ and sIgD- subsets (left), the PCR products obtained (right) from extracted DNA showed Cμ deletion in the fraction of large sIgD+ (R2) but not sIgD- (R3) cells (lane 1, 1000 R2 cells [∼ 2.5 kb]; lane 2, 3000 R3 cells; lane 3, 3000 unsorted mononuclear cells; lane 4, 30 sIgD+IgM- tonsillar cells [∼ 0.7 kb]; and lane 5, H2O control). The fraction of naive sIgD+IgM+ cells (R1) was not tested. Sample equivalent to 2 million unsorted mononuclear cells was positive (not shown). (B) Top row shows the gating performed to select large sIgA+ and sIgD+ cells (encircled) with relatively low levels of CD19 and sIg for further analysis (CD14+ monocytes excluded). CCR10 was variably expressed by both categories of blasts, while CCR4 and particularly CCR7 were mainly expressed by the large sIgD+ cells. One of 3 representative experiments is displayed with mean ± SD. (C) Whereas large sIgA+ cells were strongly positive for α4β7, large sIgD+ cells expressed mainly intermediate to low levels of this integrin, like naive sIgD+ small B lymphocytes. Both large sIgD+ and large sIgA+ cells expressed abundant levels of the postgerminal center marker CD27 (right). One of 3 representative experiments is displayed. Cursors were set on the basis of isotype- and concentration-matched control Abs, and in Figure 4C (left) to discriminate 3 levels of α4β7 expression.
Flow cytometry and Cμ-deletion analysis of circulating sIgD+ B cells. (A) After FACS sorting of sIgD+ and sIgD- subsets (left), the PCR products obtained (right) from extracted DNA showed Cμ deletion in the fraction of large sIgD+ (R2) but not sIgD- (R3) cells (lane 1, 1000 R2 cells [∼ 2.5 kb]; lane 2, 3000 R3 cells; lane 3, 3000 unsorted mononuclear cells; lane 4, 30 sIgD+IgM- tonsillar cells [∼ 0.7 kb]; and lane 5, H2O control). The fraction of naive sIgD+IgM+ cells (R1) was not tested. Sample equivalent to 2 million unsorted mononuclear cells was positive (not shown). (B) Top row shows the gating performed to select large sIgA+ and sIgD+ cells (encircled) with relatively low levels of CD19 and sIg for further analysis (CD14+ monocytes excluded). CCR10 was variably expressed by both categories of blasts, while CCR4 and particularly CCR7 were mainly expressed by the large sIgD+ cells. One of 3 representative experiments is displayed with mean ± SD. (C) Whereas large sIgA+ cells were strongly positive for α4β7, large sIgD+ cells expressed mainly intermediate to low levels of this integrin, like naive sIgD+ small B lymphocytes. Both large sIgD+ and large sIgA+ cells expressed abundant levels of the postgerminal center marker CD27 (right). One of 3 representative experiments is displayed. Cursors were set on the basis of isotype- and concentration-matched control Abs, and in Figure 4C (left) to discriminate 3 levels of α4β7 expression.
Other extraintestinal vascular adhesion candidates are mucin-like ligands corresponding to peripheral lymph node addressin (PNAd), principally expressed on high endothelial venules (HEVs) for interaction with CD62L (L-selectin). Even without showing HEV morphology, venules in normal human nasal mucosa are often reactive with mAb MECA-79 (directed against PNAd).31 Notably, more sIgD+ (79%-90%) than the sIgA+ (50%-65%) blasts expressed CD62L, and murine studies have supported its possible role in B-cell homing to extraintestinal mucosal sites.32 A similar role for CCR7 has moreover been suggested in the airways of mice.33 It was, therefore, interesting to observe that most sIgD+ blasts (∼ 85%), in contrast to only few of the sIgA+ counterparts (∼ 6%), did indeed express CCR7 (Figure 4B). The ligands for CCR7 are the secondary lymphoid tissue chemokine (SLC, CCL21) and EBV-induced molecule 1 ligand chemokine (ELC, CCL19), both particularly well known for recruiting CCR7+ immune cells via HEVs to organized lymphoid tissue.34,35
Merges and single-color separations of paired immunofluorescence staining for IgD (green) and CCR10 (red). The 3 depicted samples represent (A) adenoids (extrafollicular area), (B) salivary gland (normal parotid), and (C) nasal mucosa (glandular area). Many cells with variable peripheral CCR10 expression are seen, being particularly intense when showing a large plasmablast appearance. Some of these contain cytoplasmic IgD (arrows), but occasional clearly mature IgD+ plasma cells are completely negative for CCR10 (arrowheads). Magnification ×100.
Merges and single-color separations of paired immunofluorescence staining for IgD (green) and CCR10 (red). The 3 depicted samples represent (A) adenoids (extrafollicular area), (B) salivary gland (normal parotid), and (C) nasal mucosa (glandular area). Many cells with variable peripheral CCR10 expression are seen, being particularly intense when showing a large plasmablast appearance. Some of these contain cytoplasmic IgD (arrows), but occasional clearly mature IgD+ plasma cells are completely negative for CCR10 (arrowheads). Magnification ×100.
Discussion
Nasal vaccine administration as an alternative route for immunization against intestinal pathogens has come to recent attention because of intussusception in 15 of 800 000 American children vaccinated orally with a highly efficient attenuated live rotavirus vaccine (RotaShield, Wyeth-Ayerst Research Laboratories, Philadelphia, PA). This complication, perhaps caused by hypertrophy of GALT structures such as Peyer patches, led to immediate withdrawal of RotaShield from the market. If no substitute for the lack of such an effective mucosal vaccine can be offered, a global toll amounting to 2.5 to 4.5 million rotavirus-related deaths may occur over the next 5 to 8 years.16 It has remained unclear whether B cells stimulated in human NALT can home efficiently to the intestinal lamina propria. Regrettably, the migration pattern revealed for the tonsillar mucosal marker cells in our study suggested that this indeed is not the case. Thus, the nasal route appears to be no alternative for vaccination against rotavirus or other small-intestinal pathogens in humans, although being preferable to target respiratory pathogens and probably to some extent also infections of the female genital tract.
Our results confirmed and extended the study of Laichalk et al18 based on latent EBV infection as a marker for memory/effector B cells dispersed from palatine tonsils; they showed such cells in peripheral blood at a frequency similar to that in tonsils, but at 20-fold and 10-fold lower frequency in spleen and mesenteric lymph nodes, respectively. This difference between diseased tonsils and mesenteric lymph nodes was in harmony with the difference in detectability of our sIgD+IgM- marker cells in the same lymphoid tissues. Our result also supported the notion of Arpin et al12 that IgD myelomas are derived from the circulating sIgD+IgM- subset in that we showed a relatively high frequency of the Cμ deletion in normal bone marrow, which notably was significantly correlated with the local development of IgD+ PCs.
The sIgD+IgM- marker cells represent a minor fraction (2%-5%) of tonsillar B cells.12,36 This subset had previously been identified only in a small number of investigated samples of diseased palatine tonsils,12,14,36 whereas we documented its frequent presence also in clinically normal palatine tonsils as well as in samples of the normal or diseased nasopharyngeal tonsil (adenoids). These organized lymphoid structures make up most of Waldeyer ring, which is considered to represent NALT in humans.4
The upper airway microbiota might contribute to the Cμ deletion in human NALT because local colonizers such as Haemophilus influenzae and Moraxella catarrhalis produce an IgD-binding factor; by cross-linking of the B-cell receptor (BCR), this factor can stimulate sIgD+ cells to proliferate excessively,37 thereby perhaps driving Ig variable (V)-gene hypermutation and Cμ deletion.12,36 A microbial influence on this process was supported by our observation that the deletion is more frequently detectable in diseased than in clinically normal NALT samples (Figure 2, left). IgD-deficient mice are sensitive to tolerance induction,38 and sIgM in contrast to sIgD is associated with prohibitin and a prohibitin-related protein that transduce negative signals.39 Therefore, sIgD+IgM- cells could have a particular proliferative advantage when stimulated through their BCR. In this context, it is of relevance to note that we have previously observed more IgD+ PCs in recurrent tonsillitis of adults,40 and in adenoid hyperplasia of children,41 than in the normal organ counterparts; molecular evidence strongly suggests that such PCs are indeed derived from the tonsillar sIgD+IgM-CD38+ centroblast subset.12
It is interesting that sIgD+IgM- B cells generated by nonclassical switching appear to express predominantly Ig V-gene repertoires that may allow considerable cross-reactivity, including autoimmunity, but understanding the biologic significance of this observation requires further studies.42 Although numerous IgD antimicrobial and other antibody activities have been measured both in mouse and human serum, the protective or pathogenic role of circulating IgD remains elusive.43 Because IgD does not activate the classical complement pathway, it is likely that such antibodies can block defense functions of IgG and IgM within the mucosae and reduce the immune-exclusion efficiency of secretory antibodies in the upper airways in the face of bacterial infections that drive regional IgD production.44,45 However, there is no obvious reason to believe that the homing-molecule profile of the induced sIgD+IgM-CD38+ centroblast subset should be imprinted differently from that of other mucosal B cells activated in NALT and associated lymph nodes.4,20,21
In addition to explaining the compartmentalized homing revealed for the NALT-derived sIgD+IgM- marker cells, their unique profile of adhesion molecules and chemokine receptors (α4β7int/loCCR7hiCCR9loCCR10+CD62Lhi) probably also provides a mechanism for their joint tropism for the upper aerodigestive tract and organized lymphoid tissue. Because the circulating B-cell fraction analyzed by flow cytometry on average was 85% CCR7+ and 71% CD38hi, it clearly consisted mainly of plasmablasts with both CCL21/CCL19- and CD62L-binding properties. The homing-molecule profile of the sIgD+IgM- B cells might thus allow their access to the large bowel via HEVs in its numerous ILFs,19 which likely was the reason for detectable Cμ deletion in more colonic than small-intestinal samples (Figure 2, right). Moreover, in severe inflammatory bowel disease lesions the microvasculature can express ligands both for CCR7 and CD62L46 which, according to some human and murine studies, are also expressed at mucosal effector sites of the upper airways.31-33 Of further note, a recent report on gene expression profiles of microvascular endothelium of putative relevance for B-cell homing, suggested a relationship of the uterus with the nose rather than with the gut,47 which might explain detection of the NALT-derived marker cells in many samples of the endocervix (Figure 2, right). Intranasal immunization in mice, monkeys, and humans has suggested an inconsistent immune-response link between NALT and the cervix mucosa.48-52
In the normal situation, a common hallmark of mucosal B cells is a remarkably dominating local production of dimeric IgA with J chain, constituting the main basis of pIgR-dependent secretory immunity both in mice and humans.2-6 However, a notable species difference is the fact that hepatocytes of rodents, in contrast to humans, express pIgR.6 The consequence of this is that mice obtain most of their proximal intestinal SIgA content from hepatobiliary transfer of circulating IgA dimers, whereas in humans nearly all SIgA (∼ 98%) in the gut lumen is normally derived from mucosal IgA+ PCs.5,6 This striking difference has masked compartmentalization of secretory immunity in many murine immunization studies. Nevertheless, a B-cell homing dichotomy between the upper aerodigestive tract and the small bowel has been suggested by examination of local mucosal IgA+ PC responses obtained after immunization via the nose in humans.52 It is also important that, compared with peroral immunization, nasal vaccination has been shown to induce better systemic immunity concurrently with SIgA antibody production both in rodents,48 humans,51-55 and nonhuman primates.56
Such circumstantial evidence is in line with our notion that the NALT-derived sIgD+IgM- marker cells in fact revealed the homing pattern also of NALT-derived sIgA+ plasmablasts (Figure 6). Although not directly proven, Rott et al27 suggested that this putative fraction of circulating sIgA+ B cells does express homing molecules matching our sIgD+IgM- marker cells, thus being distinctly different from the presumably GALT-derived majority of α4β7hi sIgA+ plasmablasts (Figure 4). We believe that graded tissue site-dependent CCR10-CCL28 interactions, together with insufficient levels of classic gut-homing molecules, most likely explain the observed dispersion dichotomy for effector B cells derived from Waldeyer ring versus GALT (Figure 6). Our data thus appear to explain for the first time why nasal vaccination in humans can elicit SIgA antibody defense preferentially in the upper airways and endocervix and further provide a molecular basis for an integrated induction of respiratory and systemic immunity. For survival in the face of most invasive infections, a systemic antibody response is crucial, whereas inhibition of initial epithelial colonization with pathogens depends on SIgA antibodies.2 These complementary defense roles have recently been documented in mice exhibiting pIgR deficiency57 or being injected with either IgG or dimeric IgA mAbs against a respiratory pathogen.58 On a special note, however, vaccines targeting NALT cannot be expected to generate efficient gut immunity in humans, particularly not in the small intestine.
Putative scheme for mucosal B-cell homing in humans. Depicted are graded pathways (arrows) presumably followed by mucosal B cells of any isotype activated in nasopharynx-associated lymphoid tissue (NALT) represented by human Waldeyer lymphoid ring (including palatine tonsils and adenoids), versus gut-associated lymphoid tissue (GALT) represented by Peyer patches and isolated lymphoid follicles (ILFs) scattered along the intestinal tract, particularly in the large bowel. The principal homing-molecule profiles of the respective B-cell populations (top compartments), and the adhesion/chemokine cues believed to direct the extravasation of effector B cells into various tissue compartments (boxes), are indicated.
Putative scheme for mucosal B-cell homing in humans. Depicted are graded pathways (arrows) presumably followed by mucosal B cells of any isotype activated in nasopharynx-associated lymphoid tissue (NALT) represented by human Waldeyer lymphoid ring (including palatine tonsils and adenoids), versus gut-associated lymphoid tissue (GALT) represented by Peyer patches and isolated lymphoid follicles (ILFs) scattered along the intestinal tract, particularly in the large bowel. The principal homing-molecule profiles of the respective B-cell populations (top compartments), and the adhesion/chemokine cues believed to direct the extravasation of effector B cells into various tissue compartments (boxes), are indicated.
Prepublished online as Blood First Edition Paper, April 12, 2005; DOI 10.1182/blood-2004-12-4630.
Supported by the University of Oslo, the Research Council of Norway, the Norwegian Cancer Society, and Anders Jahre's Fund.
F.-E.J., E.S.B., H.S.C., and I.N.F. contributed equally to this study. F.-E.J. was responsible for the PCR analyses; E.S.B. and I.N.F. performed flow cytometric analyses; H.S.C. and P.B. were responsible for immunohistochemistry; D.S. provided important new reagents; and P.B. designed the study and wrote the manuscript which was reviewed by all the other coauthors and approved for submission after being finalized.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the clinical departments at Rikshospitalet University Hospital, Oslo, and the Department of ENT (Dr G. Karlsson), Sahlgren's Hospital, University of Göteborg, Sweden, for providing tissue material. We thank Hege Eliassen and Erik K. Hagen for excellent assistance with the manuscript. We also thank Dr Y. L. Liu for sharing unpublished base sequences with us, enabling the preparation of primary primers for PCR detection of the Cμ deletion.


![Figure 4. Flow cytometry and Cμ-deletion analysis of circulating sIgD+ B cells. (A) After FACS sorting of sIgD+ and sIgD- subsets (left), the PCR products obtained (right) from extracted DNA showed Cμ deletion in the fraction of large sIgD+ (R2) but not sIgD- (R3) cells (lane 1, 1000 R2 cells [∼ 2.5 kb]; lane 2, 3000 R3 cells; lane 3, 3000 unsorted mononuclear cells; lane 4, 30 sIgD+IgM- tonsillar cells [∼ 0.7 kb]; and lane 5, H2O control). The fraction of naive sIgD+IgM+ cells (R1) was not tested. Sample equivalent to 2 million unsorted mononuclear cells was positive (not shown). (B) Top row shows the gating performed to select large sIgA+ and sIgD+ cells (encircled) with relatively low levels of CD19 and sIg for further analysis (CD14+ monocytes excluded). CCR10 was variably expressed by both categories of blasts, while CCR4 and particularly CCR7 were mainly expressed by the large sIgD+ cells. One of 3 representative experiments is displayed with mean ± SD. (C) Whereas large sIgA+ cells were strongly positive for α4β7, large sIgD+ cells expressed mainly intermediate to low levels of this integrin, like naive sIgD+ small B lymphocytes. Both large sIgD+ and large sIgA+ cells expressed abundant levels of the postgerminal center marker CD27 (right). One of 3 representative experiments is displayed. Cursors were set on the basis of isotype- and concentration-matched control Abs, and in Figure 4C (left) to discriminate 3 levels of α4β7 expression.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/2/10.1182_blood-2004-12-4630/6/m_zh80140581040004.jpeg?Expires=1763636433&Signature=Om1T0lH9OOVVYTwy1MZmuwbCWI343cgJiU1h9Ta6ltrIYK4qlJ7-sBQhACudcS6fYDJdkI57VrYbdXDLv3JSIx90lWgLkbPsG0tYc3pN80rnidZejTxZluO1T-BBi3cuW1sEAYUdh44rNEg-xnM-KIyoSD84-yRQqRiSvTZzAhJbaVnHvSh2hXn2UB3WbknU1eN9WkPI~DljAjhybdrYfA0nIbeOj5STzW93IOyBLNwsz6zJKYs5Nf6L-paUAihLPzLvW8L6deHTN1SmaQmx0jWT3f3ObXFhXh658Ps7QsCS7IPJyuFEfXooR4kuv-MEFBtu3IaBNhOyoocq4PI0yA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal