Abstract
Interaction of natural killer (NK) cells with autologous immature dendritic cells (DCs) results in reciprocal activation; however, the underlying mechanisms are so far elusive. We show here that NK cells trigger immature DCs to polarize and secrete interleukin 18 (IL-18), a cytokine lacking a secretory leader sequence. This occurs through a Ca2+-dependent and tubulin-mediated recruitment of IL-18-containing secretory lysosomes toward the adhering NK cell. Lysosome exocytosis and IL-18 secretion are restricted at the synaptic cleft, thus allowing activation of the interacting NK cells without spreading of the cytokine. In turn, DC-activated NK cells secrete the proinflammatory cytokine high mobility group B1 (HMGB1), which induces DC maturation and protects DCs from lysis. Also HMGB1 is a leaderless cytokine that undergoes regulated secretion. Differently from IL-18, soluble HMGB1 is consistently detected in NK/DC supernatants. These data point to secretion of leaderless cytokines as a key event for the reciprocal activation of NK cells and DCs. DCs initiate NK cell activation by targeted delivery of IL-18, thus instructing NK cells in the absence of adaptive-type cytokines; in turn, activated NK cells release HMGB1, which promotes inflammation and induces DC maturation, thus favoring the onset of the adaptive immune response. (Blood. 2005;106:609-616)
Introduction
Interaction among cells of the immune system is essential for the onset, progress, and end of immune-inflammatory reactions. Immune cells coordinate reciprocally their responses through signaling or release of appropriate soluble factors or both. A well-known example is given by dendritic cell (DC) priming of naive T cells, which depends on the formation of the immunologic synapse, a complex cluster of molecules organized at the contact area of cell conjugates.1,2 Activation is not only one way, in that T lymphocytes also induce dramatic changes in DCs. This is well evident in mixed lymphocyte reactions, where alloreactive CD8+ T cells trigger Ca2+ rises in DCs, followed by release of interleukin 1-β (IL-1β)at the immunologic synapse.3,4 This polarized secretion of IL-1β may activate locally the interacting target cell, thus controlling immune response without spreading around the potentially dangerous cytokine. Similar results were observed for another member of the IL-1 family, IL-18.5,6 Both IL-1β and IL-18 are leaderless secretory proteins that do not follow the classical exocytotic route to get out of the cell.7 Their secretion involves translocation of cytosolic molecules into secretory lysosomes.4,6,8,9 These are calcium-dependent secretory organelles, abundant in hemopoietic cells, that play the dual function of degradation and regulated secretion of proinflammatory factors.10
Natural killer (NK) cells are lymphocytes active in innate responses against viruses, bacteria, and tumors, due to their potent cytotoxic activity and rapid production of cytokines.11 Interestingly, NK cells have been shown to interact also with immature DCs (iDCs); this interaction seems crucial in the initiation/amplification of the early phases of an immune response, before specific T cells are generated.12-17 iDC-NK crosstalk results in activation of NK cells that, in turn, induces DC maturation or killing (or both). The differential fate of iDCs (death or maturation) may depend on dynamics of the iDCs and NK cells and their respective density.16 However, the mechanisms underlying the reciprocal DC and NK cell activation are largely unknown. Various cytokines produced by DCs, including IL-12 and IL-18, up-regulate NK cell cytotoxicity.18,19 IL-18 is constitutively produced by iDCs,5,20 whereas IL-12 is expressed after DC maturation.21 This may suggest that IL-18 is the first dendrikine that triggers the activation of NK cells on NK/iDC interaction, whereas the involvement of IL-12 is a later event that occurs after the maturation of DCs induced by NK cells. Also, IL-15 may contribute to NK cell activation, but, like IL-12, its expression seems restricted to maturing DCs22 ; furthermore, whether this cytokine is secreted by human DCs or just presented via DC membrane receptors is so far unclear.23
The mechanisms underlying NK cell-mediated DC maturation are also uncertain; in humans, cell-cell interaction seems required15,16 ; a role for tumor necrosis factor α (TNF-α) and interferon γ (IFN-γ) has been proposed but it is still controversial.15,16,24,25
A number of factors released by dying cells, including adenosine triphosphate (ATP),24 uric acid,26 high mobility group B1 (HMGB1),27,28 have been found to promote DC maturation. Interestingly, although most of these “endogenous danger signals” are passively released by necrotic cells,29 the nuclear protein HMGB1 can also be secreted, in the absence of cell death, by inflammatory cells such as activated monocytes.30 In these cells, HMGB1 undergoes hyperacetylation31 that allows its relocation from the nucleus to the cytoplasm, followed by lysosome-mediated regulated secretion.30 Secreted HMGB1 behaves as a powerful proinflammatory cytokine.32
We have investigated the early events occurring after iDC/NK interaction. Our results show that following conjugate formation with autologous NK cells, iDCs undergo a functional polarization, with increase in intracellular free Ca2+ concentration ([Ca2+]i), cytoskeleton rearrangement, accumulation of secretory lysosomes at the NK/DC synapse, and regulated secretion of IL-18 toward the interacting NK cell. In turn, NK cells secrete large amounts of HMGB1, which induces DC maturation and protects them from NK cell cytotoxicity.
Materials and methods
Purification of resting NK cells and generation of activated NK cells
Peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated by Ficoll (Cedarlane Lab, Hornby, ON, Canada) density centrifugation and depleted of plastic-adherent cells. NK cells were enriched by incubating nonadherent cells 30 minutes at 4°C with anti-CD3 (UCHT1), anti-CD4 (Leu3a), and anti-CD8 (Leu2a) monoclonal antibodies (mAbs; all from Becton Dickinson, Milan, Italy), followed by 30 minutes of incubation at 4°C with goat anti-mouse immunoglobulin-coated magnetic beads (Dynabeads, Dynal, Oslo, Norway) and immunomagnetic depletion as described.33 CD3-CD4-CD8- cells were kept as resting NK (rNK) cells or stimulated with 10 μg/mL phytohemagglutinin (Gibco, Paisley, United Kingdom) and cultured in RPMI 1640 medium (Sigma-Aldrich, Milan, Italy) supplemented with 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Euroclone, Milan, Italy), 10% heat-inactivated fetal calf serum (Sigma-Aldrich) in the presence of 500 IU/mL recombinant human (rhu) IL-2 (Proleukin; Chiron, Emeryville, CA) to obtain polyclonal NK cell populations. The percent of NK cells (CD3-CD16+) freshly isolated (rNK cells) or after 7 days of culture (activated NK [aNK] cells) was evaluated by fluorescence-activated cell sorting (FACS) analysis (FACSort, Becton Dickinson, Milan, Italy) using phycoerythrin (PE)-conjugated anti-CD3 and fluorescein isothiocyanate (FITC)-conjugated anti-CD16 mAb (Beckman-Coulter, Milan, Italy) and varied from 85% to 95% in the different experiments. Contamination of myeloid cells was evaluated using PE-conjugated anti-CD14 and FITC-conjugated anti-CD33 (Beckman-Coulter) and was consistently less than 5% in the different experiments.
Derivation of iDCs from adhering cells
iDCs were generated from PBMCs as previously described3,4 by culturing adhering cells for 7 days in RPMI 1640 medium (Sigma-Aldrich) supplemented with l-glutamine, penicillin, streptomycin, 10% heat-inactivated fetal calf serum (see “Purification of resting NK cells and generation of activated NK cells”), and with 40 ng/mL rhu granulocyte-macrophage colony-stimulating factor (Schering-Plough, Milan, Italy) plus 1000 IU/mL rhuIL-4 (Euroclone). The DC populations obtained at the end of the culture were analyzed by flow cytometry using the following mAbs: PE-conjugated anti-CD14, FITC-conjugated anti-HLA-DR (Beckman-Coulter), PE-conjugated anti-CD86, PE-conjugated anti-CD1a (Immunotech-Coulter, Marseille, France).
Culture conditions
iDCs, prepared as described, were incubated alone or with rNK or aNK cells at a different NK/iDC ratio for different periods of time in RPMI medium supplemented with 1% Nutridoma-HU (Sigma-Aldrich). When indicated, rhuTNF-α (50 ng/mL, Genzyme, Cinisello B, Milan, Italy), lipopolysaccharide (LPS; 1 μg/mL, Sigma-Aldrich), CD40L-expressing cells34 (ratio 1:1) plus IFN-γ (100 ng/mL; PeproTech, Milan, Italy), recombinant or purified HMGB1 (kind gift from Dr M. Bianchi, Milan, Italy, used 1 μg/mL), were added during the last 24 hours of culture to induce DC maturation. HMGB1 preparations did not contain LPS activity sufficient to interfere in the in vitro assays.28
For blocking experiments, purified rNK cells were cultured 7 days with suboptimal doses (50 IU/mL) of rhuIL-2, or with 1 ng/mL rhuIL-18 (R&D Systems, Minneapolis, MN), or both, in the absence or presence of 1 μg/mL neutralizing anti-IL-18 mAb (R&D Systems) or of an isotype-matched control mAb. At the end of the culture period, NK cells were washed and cultured for additional 6 hours in RPMI-1% Nutridoma-HU. Supernatants were then collected, centrifuged at 13 000g for 5 minutes, and concentrated with trichloroacetic acid.
In some experiments, spent medium deriving from 24 hours of incubation of rNK or aNK cells or from 4 hours of coculture of aNK/iDC (ratio 5:1) was added for the periods of time indicated. At the end of incubations, supernatants were kept, and cells were processed for immunofluorescence or subcellular fractionation or lysed in Triton X-100.
Cytolytic assay
NK cell cytolytic activity against iDCs or DCs treated with maturation factors as described was tested in a 51Cr-release assay as previously described.35 Briefly, DCs were loaded with 51Cr and cocultured for 4 hours with NK cells used as effector cells, at a different effector-target (E/T) ratio. Results are expressed as percentage of cytotoxicity as described.35
Immunofluorescence
NK/iDC conjugates after 3 hours of coculture were fixed and permeabilized with 3% paraformaldehyde and 0.5% Triton X-100. After washings and saturation with 2% bovine serum albumin (Sigma-Aldrich), cells were labeled with rabbit anti-IL-18 antibody (kind gift from Dr C. A. Dinarello, Denver, CO36 ) and either antiperforin mAb δG9 (Alexis, Lausen, Switzerland) or antitubulin mAb (Sigma-Aldrich) followed by incubation with the appropriate secondary reagents labeled with FITC or cyanine 3 (Cy3; Jackson ImmunoResearch, Soham, United Kingdom). In other experiments cells were labeled with rabbit anti-HMGB1 antibody (BD Biosciences Pharmingen, San Diego, CA) followed by incubation with donkey antirabbit antibody labeled with Cy3 (Jackson ImmunoResearch) and antiperforin mAb δG9 followed by incubation with FITC-labeled secondary reagent. Finally, cells were treated with DAPI (4,6 diamidino-2-phenylindole; 1 μg/mL; Sigma-Aldrich) to stain nuclei and examined under an Olympus AX70 microscope (Milan, Italy) by using a × 100/1.30 oil objective lens. Images were acquired with a Hamamatsu Digital Camera (Milan, Italy).
Subcellular fractionations
Subcellular fractionation by differential ultracentrifugation was carried out as described4,6,8,9 with slight modifications. Briefly, DCs were washed, resuspended in homogenizing buffer (250 mM sucrose, 5 mM EGTA [ethylene glycol tetraacetic acid], 20 mM HEPES [N-2-hydroxyethylpipera-zine-N′-2-ethanesulfonic acid]--KOH, pH 7.2) and broken in a Dounce homogenizer. Postnuclear supernatants were centrifuged at 30 000g for 2 minutes and then to 35 000g for 5 minutes, obtaining 2 pellets enriched in endolysosomes, as confirmed by electron microscopy (not shown). Supernatants were spun again for 40 minutes at 100 000g to obtain soluble cytosol.
Cytoplasmic fractions from rNK cells, aNK cells, iDCs, and mature (mDCs) were prepared as described.37 Briefly, cells (2 × 106 NK cells, 1 × 106 DCs) were washed and resuspended in 1 mL buffer A (10 mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EDTA [ethylenediaminetetraacetic acid], 0.1 mM EGTA, 1 mM dichlorodiphenyltrichloroethane [DTT], and 0.5 mM phenylmethylsulfonyl fluoride) and homogenized. The cells were allowed to swell on ice for 15 minutes, then 50 μL 10% NP40 was added. After vortexing for 10 seconds, the homogenates were centrifuged for 1 minute at 3000g. The supernatants were collected, centrifuged at 13 000g for 5 minutes, and concentrated with trichloroacetic acid.
Western blot analysis
Aliquots of cell lysates and the correspondent trichloroacetic acid-concentrated supernatants, pellets, and cytosolic fractions from subcellular fractionation and cytoplasmic protein fractions were boiled in reducing Laemmli sample buffer, resolved on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred onto a polyvinylidene difluoride (PVDF) membrane (Amersham Biosciences, Milan, Italy) as described.3-6 Filters were hybridized with either one of the following primary antibodies: rabbit anti-human IL-18 antibody or rabbit anti-human HMGB1 antibody (BD Biosciences Pharmingen), anticathepsin D mAb (IgG2a, Calbiochem, Milan, Italy) or anti-IL-1β mAb (3ZD, IgG1, obtained from NCI Biological Resource Branch, Frederick, MD), followed by the appropriated horseradish peroxidase-conjugated secondary reagent (Dako, Milan, Italy), and developed by enhanced chemiluminescence (ECL) Plus (Amersham Biosciences), according to the manufacturer's instructions.
NK cells induce maturation or killing of autologous DCs. (A) Percent of CD86+ DCs (left) or mean fluorescence intensity (MFI) of MHC class II expression (right) after 24 hours of culture with 1 μg/mL LPS or 1 μg/mL HMGB1 or coculture with rNK or aNK cells at an NK/DC ratio of 5:1 or 10:1. Note that the increase in the percentage of CD86+ DCs varies from individual to individual, and requires a higher NK/DC ratio when rNK rather than aNK are used. (B-D) The aNK cells derived from 3 healthy donors were analyzed in a 4-hour 51Cr-release assay for their cytolytic activity against autologous iDCs (B), DCs treated 24 hours with 1 μg/mL LPS (C), or 1 μg/mL HMGB1 (D) at different NK/DC (effector-target [E/T]) ratios, from 10:1 to 2:1.
NK cells induce maturation or killing of autologous DCs. (A) Percent of CD86+ DCs (left) or mean fluorescence intensity (MFI) of MHC class II expression (right) after 24 hours of culture with 1 μg/mL LPS or 1 μg/mL HMGB1 or coculture with rNK or aNK cells at an NK/DC ratio of 5:1 or 10:1. Note that the increase in the percentage of CD86+ DCs varies from individual to individual, and requires a higher NK/DC ratio when rNK rather than aNK are used. (B-D) The aNK cells derived from 3 healthy donors were analyzed in a 4-hour 51Cr-release assay for their cytolytic activity against autologous iDCs (B), DCs treated 24 hours with 1 μg/mL LPS (C), or 1 μg/mL HMGB1 (D) at different NK/DC (effector-target [E/T]) ratios, from 10:1 to 2:1.
Two-dimensional gel electrophoresis
Two-dimensional gel electrophoresis was carried out as described with slight modifications.31 Protein extracts (approximately 50 μg) were solubilized in 7 M urea, 2 M thiourea, 20 mM Tris (tris(hydroxymethyl) aminomethane) HCl, 4% 3[3-cholaminopropyl diethylammonio]1-propane sulfonate (CHAPS), 1% Triton X-100, 1% DTT (all from Sigma-Aldrich), and 1% immobilized pH gradient (IPG) buffer (Amersham Biosciences), in a final volume of 300 μL and loaded onto 13-cm pH 3 to 10 nonlinear (NL) IPG strips (Amersham Biosciences). After 8 hours of rehydration, isoelectric focusing was carried out on an IPGPhor apparatus (Amersham Biosciences) for 80 000 V/h. Focused IPG strips were then equilibrated for 15 minutes in SDS buffer containing 1% DTT and 15 minutes in SDS buffer containing 2.5% iodacetamide (Sigma-Aldrich), then placed on 12% SDS-PAGE gels, and electrotransferred as described (see “Western blot analysis”).
Laser scan confocal microscopy
NK/iDC conjugates were imaged, after immunofluorescence, by laser scanning confocal microscopy (FV5001, Olympus, Milan, Italy) by using a × 60/1.40 oil objective lens. Quantitative evaluation of DC/NK interactions was performed by scanning slides by Normanski imaging.38
Calcium mobilization assay
iDCs were loaded with the acetoxymethyl-ester of Fura-2 (Fura-2-AM, 1 μM, Sigma-Aldrich) for 1 hour and placed under AXIOVERT 10 microscope (Zeiss, Oberkochen, Germany) as described,4,33 and 5 × unlabeled NK cells were added. The cocultures were maintained at 37°C in a microincubator for the time indicated. Fura-2-AM was excited at 340 and 380 nm, and emitted light was filtered at 510 nm. The fluorescence ratio at 340:380 was evaluated by a charged coupled device camera (ATTO Instruments, Rockville, MD). Results are the mean of fluorescence of at least 30 cells in each experiment monitored for 100 minutes. [Ca2+]i increase was calculated as described.4
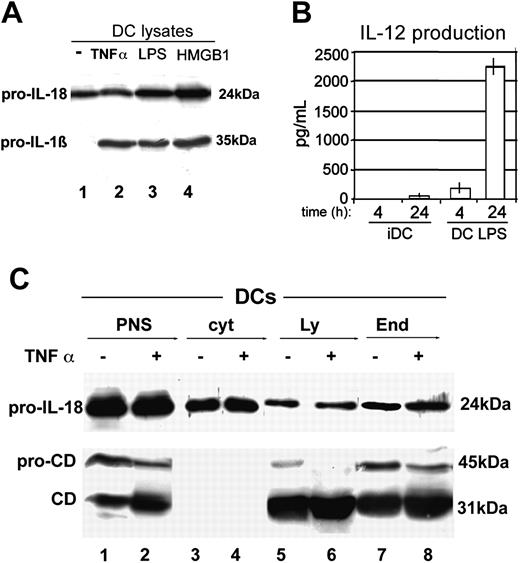
Measurement of IL-12 production
Cell-free supernatants derived from immature or LPS-treated DCs (5 × 105 cells) were collected after 4 or 24 hours of incubation. IL-12 concentrations in culture supernatants were measured following the manufacturer's instructions using the human IL-12 enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems) and results were expressed as picograms per milliliter.
Determination of LDH release
The release of lactate dehydrogenase (LDH) in supernatants as a marker of cell lysis during cocultures of DC and NK cells was determined using the LDH colorimetric assay from Sigma-Aldrich.9
Results
NK cells induce maturation or killing of immature autologous DCs: prevention of DC lysis by HMGB1
Freshly isolated and IL-2-activated NK cells from healthy individuals (both cell populations being > 85% CD3-, CD16+) induce maturation of monocyte-derived autologous iDCs after 24 hours of coculture, as shown by the increased percentage of cells expressing CD86+ (Figure 1A, left panel), which correlates with the intensity of expression of HLA class II (Figure 1A, right panel). As a positive control, LPS strongly increases CD86 and class II expression. In keeping with recent data,27,28 HMGB1 also displays a strong maturation activity (Figure 1A). The aNK cells also display a cytolytic activity against iDCs, of different degrees in the different donors (Figure 1B). Killing was most evident at an aNK/iDC ratio of 10:1, higher than that needed to obtain DC maturation.16 In contrast, the cytotoxic potential was almost absent against DCs induced to maturation with LPS (Figure 1C). Interestingly, consistent with its maturation activity, HMGB1 also prevents DCs from NK cell-mediated lysis (Figure 1D). Differently from aNK cells, rNK cells kill neither iDCs nor mDCs (not shown).
aNK cells from all the donors tested (> 15) displayed both cytolytic and maturation potentials on autologous DCs, although at different extents. However, when the same donors were tested more times after several months, their NK cells gave reproducible results.
Production and subcellular localization of IL-18 in iDCs and mDCs. (A) Western blot analysis of IL-18 (top blot) and IL-1β (bottom blot) in cell lysates from iDCs cultured 24 hours without (-; lane 1) or with 50 ng/mL TNF-α (lane 2), 1 μg/mL LPS (lane 3) or 1 μg/mL HMGB1 (lane 4). (B) Measurement of IL-12 production. IL-12 concentrations in culture supernatants of 4- or 24-hour incubations of iDCs or LPS-treated DCs (5 × 105 cells) were measured using a human IL-12 ELISA kit. Results are expressed as picograms per milliliter. Error bars represent standard error of a single experiment performed in triplicate. (C) Subcellular localization of IL-18 in iDCs and mDCs; iDCs (lanes 1, 3, 5, and 7) and mDCs (treated 24 hours with TNF-α; lanes 2, 4, 6, and 8) were homogenized and postnuclear supernatants (PNS; lanes 1 and 2, 1/20 of total) were subjected to differential ultracentrifugation giving rise to 2 pellets enriched in lysosomes (Ly; lanes 5 and 6) and endosomes (End; lanes 7 and 8), respectively. Lanes 3 and 4 show soluble cytosol (cyt). Fractions were blotted and hybridized with anti-IL-18 (top blot) and anti-cathepsin D (CD; bottom blot). One representative experiment of 4 performed is shown.
Production and subcellular localization of IL-18 in iDCs and mDCs. (A) Western blot analysis of IL-18 (top blot) and IL-1β (bottom blot) in cell lysates from iDCs cultured 24 hours without (-; lane 1) or with 50 ng/mL TNF-α (lane 2), 1 μg/mL LPS (lane 3) or 1 μg/mL HMGB1 (lane 4). (B) Measurement of IL-12 production. IL-12 concentrations in culture supernatants of 4- or 24-hour incubations of iDCs or LPS-treated DCs (5 × 105 cells) were measured using a human IL-12 ELISA kit. Results are expressed as picograms per milliliter. Error bars represent standard error of a single experiment performed in triplicate. (C) Subcellular localization of IL-18 in iDCs and mDCs; iDCs (lanes 1, 3, 5, and 7) and mDCs (treated 24 hours with TNF-α; lanes 2, 4, 6, and 8) were homogenized and postnuclear supernatants (PNS; lanes 1 and 2, 1/20 of total) were subjected to differential ultracentrifugation giving rise to 2 pellets enriched in lysosomes (Ly; lanes 5 and 6) and endosomes (End; lanes 7 and 8), respectively. Lanes 3 and 4 show soluble cytosol (cyt). Fractions were blotted and hybridized with anti-IL-18 (top blot) and anti-cathepsin D (CD; bottom blot). One representative experiment of 4 performed is shown.
NK/iDC interaction results in IL-18 clustering to the immunologic synapse
NK/iDC bidirectional crosstalk is an early event occurring at the interface between innate and adaptive immunity, before an antigen-specific immune response develops.15 For these reasons, among DC cytokines, which may play a role in NK cell activation, we focused on IL-18, that, unlike IL-12,21 is constitutively produced by iDCs, where it accumulates in the cytoplasm as a long-lived protein.5,20 Figure 2A shows that indeed the precursor form of IL-18 (pro-IL-18) is present both in iDCs (upper panel, lane 1) or after maturation induced by TNF-α or LPS (upper panel, lanes 2-4), whereas the proinflammatory cytokine IL-1β, as previously reported,3 is detected only in mDCs (lower panel, lane 1 versus lanes 2 and 3). Remarkably, 24 hours of exposure to HMGB1 triggers DCs to produce pro-IL-1β (lower panel, lane 4), supporting the DC maturation activity of this cytokine. In agreement with previous observations,21 IL-12 is produced by mDCs but not by iDCs (Figure 2B).
DC/IL-18 polarizes toward interacting NK cells. (A) Confocal microscopy analysis of perforin (red) and IL-18 (green) in an iDC/NK conjugate after 3 hours of interaction. Insets shows details of single and double fluorescence at the immunologic synapse. Arrowheads indicate perforin; arrows, IL-18. One representative experiment of 4 performed is shown. (B) Western blot analyses with anti-IL-18 (lanes 1 and 2) or anti-cathepsin D (CD; lanes 3 and 4) of 3-hour supernatants of iDCs cultured alone (iDCs; lane 1 and 3) or with NK cells (NK/iDCs; lanes 2 and 4). Arrows point to the precursors and mature forms of the 2 proteins. One experiment of 10 performed is shown.
DC/IL-18 polarizes toward interacting NK cells. (A) Confocal microscopy analysis of perforin (red) and IL-18 (green) in an iDC/NK conjugate after 3 hours of interaction. Insets shows details of single and double fluorescence at the immunologic synapse. Arrowheads indicate perforin; arrows, IL-18. One representative experiment of 4 performed is shown. (B) Western blot analyses with anti-IL-18 (lanes 1 and 2) or anti-cathepsin D (CD; lanes 3 and 4) of 3-hour supernatants of iDCs cultured alone (iDCs; lane 1 and 3) or with NK cells (NK/iDCs; lanes 2 and 4). Arrows point to the precursors and mature forms of the 2 proteins. One experiment of 10 performed is shown.
Subcellular fractionation experiments revealed that, in DCs, IL-18, like IL-1β,4 accumulates in the soluble cytosol and in 2 particulate fractions enriched in lysosomes and endosomes,6 as confirmed by the presence in these fractions of the lysosomal marker cathepsin D and of its endosomal precursor (Figure 2C).
To investigate whether IL-18 relocates in iDCs after interaction with NK cells, iDCs were cocultured with NK cells for 3 hours and the subcellular localization of IL-18 as well as the extracellular release of the cytokine were evaluated by confocal immunofluorescence and Western blot of supernatants, respectively. Figure 3A shows the double staining of IL-18 and perforin in NK/iDC conjugates. A large iDC interacting with some NK cells displays a diffuse cytoplasmic staining for IL-18, in keeping with its cytosolic location, which obscures the lysosomal one; 3 of the interacting NK cells at the focus layer shown display scattered perforin granules. The fourth NK cell appears polarized (arrow), and perforin arises as a half moon at the focus layer corresponding to the immunologic synapse, suggesting that it is going to be released against the iDC. In the meantime, IL-18 polarizes toward the NK cell and clusters at the synapse. The colocalization of the 2 proteins is not complete (Figure 3A, arrows and arrowheads) because perforin derives from the NK cell and IL-18 from the DC.
Of 50 conjugates that formed tight cell-to-cell contacts under Normarski light microscopy conditions, 20 to 30 in the different subjects analyzed displayed IL-18 polarization at the site of contact.
Western blot analyses of supernatants of NK/iDC cocultures (Figure 3B) showed that soluble IL-18 is detectable in its mature form in about 25% of individuals tested (Figure 3B, lane 2); in the majority of experiments, a faint, if any, band of soluble IL-18 is found (not shown). Detection of secreted IL-18 was independent from the lytic potential of NK cells and was not associated with release of LDH (not shown), ruling out a role for cell lysis in the externalization of the cytokine. The involvement of secretory lysosomes was supported by the observation that secretion of IL-18 in NK/iDC cocultures is paralleled by release of the lysosomal enzyme cathepsin D (Figure 3B, lane 4). In contrast, supernatants of iDCs cultured alone contain dim pro-IL-18 (Figure 3B, lane 1) and cathepsin D bands, the latter being present in its precursor (nonlysosomal) form (Figure 3B, lane 3).
IL-18 polarization in iDCs induced by NK cells is mediated by Ca2+ mobilization and tubulin rearrangement
Lysosome exocytosis is a calcium-dependent process that requires rearrangement of cytoskeletal proteins.10 Remarkably, a Ca2+ rise is observed in iDCs after 2000 seconds from the beginning of NK/iDC cocultures (Figure 4A) and is maintained for 2 hours and more, whereas as a control no modification in [Ca2+]i was detected in iDCs cultured alone (Figure 4A) or in homotypic (NK/NK or DC/DC) conjugates (not shown). The calcium rise was not accompanied by any LDH release in the time frame considered (not shown).
NK cells induce a Ca2+ flux in iDCs followed by translocation of microtubule organizing center and IL-18 at the immunologic synapse. (A) NK cells were added to Fura2-AM-loaded iDCs and Ca2+ waves were monitored. Results are expressed as [Ca2+]i and represent the mean of calcium response of 15 different cells. (B) iDCs or iDC/NK conjugates after 3 hours of culture were stained for tubulin (green) or IL-18 (red). Nuclei are stained in blue by DAPI. Arrows point to the clustering of tubulin and IL-18 at the contact site between NK cells and iDCs.
NK cells induce a Ca2+ flux in iDCs followed by translocation of microtubule organizing center and IL-18 at the immunologic synapse. (A) NK cells were added to Fura2-AM-loaded iDCs and Ca2+ waves were monitored. Results are expressed as [Ca2+]i and represent the mean of calcium response of 15 different cells. (B) iDCs or iDC/NK conjugates after 3 hours of culture were stained for tubulin (green) or IL-18 (red). Nuclei are stained in blue by DAPI. Arrows point to the clustering of tubulin and IL-18 at the contact site between NK cells and iDCs.
Interaction with NK cells also results in tubulin rearrangement (Figure 4B); whereas tubulin is scattered from the microtubule-organizing center in DCs cultured alone (arrowhead), where IL-18 appears dispersed in the cytoplasm, in NK/DCs conjugates it rearranges with IL-18, the 2 proteins colocalizing in a condensed zone at the interface between the 2 cells. Thus, the microtubular cytoskeleton of DCs is likely involved in the polarized movement of IL-18-containing secretory lysosomes toward NK cells.
NK cells secrete HMGB1 that induces DC maturation. (A) Western blot analysis of HMGB1 in supernatants (sups, upper panel) and cell lysates (CL, lower panel) from 4-hour cultures of IL-2-activated NK cells (lane 1), rNK cells (lane 2), coculture of iDC and IL-2-activated NK cells (lane 3), coculture of iDC and rNK cells (lane 4), iDCs (lane 5), and LPS-treated DCs (mDCs; lanes 6). One representative experiment of 4 performed is shown. (B) CD86+ expression on iDCs (▦, percentage; □, MFI) after 24 hours of culture without (iDCs) or with 1 μg/mL purified HMGB1, or with supernatants from iDCs, rNK cells or aNK cells, or from iDC/aNK cocultures, as indicated. One representative experiment of 4 performed is shown. (C) Confocal immunofluorescence analysis of a DC/NK conjugate stained for HMGB1 (left) and perforin (right). In DCs most HMGB1 is nuclear, whereas in NK cells it is almost equally distributed between nucleus and cytoplasm. Arrows point to the cytoplasmic staining of HMGB1 and perforin in NK cell cytoplasm. (D) Western blot analysis of HMGB1 in cytoplasmic fractions from iDCs (lane 1), LPS-treated DCs (mDCs, lane 2), rNK cells (lane 3), and aNK cells (lane 4). (E) Aliquots of iDCs (upper panel) and IL-2-activated NK cells (lower panel) were lysed and 50 μg total protein extract was loaded onto 2-dimensional gels, blotted, and hybridized with anti-HMGB1 antibody. Note the presence of more acidic HMGB1 spots in NK cells.
NK cells secrete HMGB1 that induces DC maturation. (A) Western blot analysis of HMGB1 in supernatants (sups, upper panel) and cell lysates (CL, lower panel) from 4-hour cultures of IL-2-activated NK cells (lane 1), rNK cells (lane 2), coculture of iDC and IL-2-activated NK cells (lane 3), coculture of iDC and rNK cells (lane 4), iDCs (lane 5), and LPS-treated DCs (mDCs; lanes 6). One representative experiment of 4 performed is shown. (B) CD86+ expression on iDCs (▦, percentage; □, MFI) after 24 hours of culture without (iDCs) or with 1 μg/mL purified HMGB1, or with supernatants from iDCs, rNK cells or aNK cells, or from iDC/aNK cocultures, as indicated. One representative experiment of 4 performed is shown. (C) Confocal immunofluorescence analysis of a DC/NK conjugate stained for HMGB1 (left) and perforin (right). In DCs most HMGB1 is nuclear, whereas in NK cells it is almost equally distributed between nucleus and cytoplasm. Arrows point to the cytoplasmic staining of HMGB1 and perforin in NK cell cytoplasm. (D) Western blot analysis of HMGB1 in cytoplasmic fractions from iDCs (lane 1), LPS-treated DCs (mDCs, lane 2), rNK cells (lane 3), and aNK cells (lane 4). (E) Aliquots of iDCs (upper panel) and IL-2-activated NK cells (lower panel) were lysed and 50 μg total protein extract was loaded onto 2-dimensional gels, blotted, and hybridized with anti-HMGB1 antibody. Note the presence of more acidic HMGB1 spots in NK cells.
HMGB1 is released by NK cells: effects on DC maturation
The mechanism underlying NK cell-mediated DC maturation is so far unclear. Among factors proposed to induce DC maturation, we have shown here that HMGB127,28 is a powerful one, evaluated both by phenotypic changes (Figure 1A) and induction of IL-1β synthesis (Figure 2A). Interestingly, in inflammatory cells such as activated monocytes, HMGB1 undergoes regulated secretion.30 We then investigated whether also NK cells are able to secrete HMGB1. Figure 5A shows that IL-2-activated NK cells spontaneously secrete HMGB1 (upper panel, lane 1), whereas only a faint, if any, HMGB1 band is erratically detected in supernatants from resting NK cells (lane 2) and from immature or LPS-treated DCs (lanes 5 and 6). However, abundant HMGB1 is secreted by both aNK/iDCs (lane 3) and rNK/iDCs (lane 4) in 4-hour cocultures. NK cells displayed a negligible contamination of myeloid cells (< 2% CD14+, < 1% CD33+) ruling out the possibility that secreted HMGB1 derives from myeloid rather than NK cells.
The treatment of DCs for 24 hours with supernatants either from rhuIL-2-activated NK cells or from NK/DC cocultures containing HMGB1 increases the surface expression of CD86, similarly to purified HMGB1 (Figure 5B). In contrast, only slight or no signs of DC maturation are observed with supernatants from iDCs or resting NK cells. Supernatants from DCs induced to maturation with different stimuli (LPS, TNF-α, CD40L plus IFN-γ) did not contain detectable HMGB1 and induced a slight increase of CD86 expression, consistently lower than that triggered by NK cell supernatants (not shown).
Together these data indicate that HMGB1 is secreted by NK cells on interaction with DCs and that it induces DC maturation. The possibility that secreted HMGB1 derives from DCs and not from NK cells is ruled out by the following lines of evidence. First, confocal immunofluorescence analysis of HMGB1 in NK/iDC conjugates (Figure 5C) reveals that although in iDCs HMGB1 is mostly in the nucleus, in NK cells it is abundant also in cytoplasm (Figure 5C, left panel, arrows), as confirmed by the presence of the cytoplasmic marker perforin (Figure 5C, right panel). Furthermore, in iDCs and mDCs the amount of cytoplasmic HMGB1 is comparable (Figure 5D, lanes 1 and 2) and less than that present in aNK cells (lane 4), in agreement with the immunofluorescence data shown in Figure 5C; moreover, in aNK cells cytoplasmic HMGB1 is consistently increased with respect to rNK cells (lane 3). This is reminiscent of what happens in monocytes; whereas in resting monocytes HMGB1 is restricted to the nucleus, after activation it moves to cytoplasm, allowing its secretion.32,33 Finally, Figure 5E shows the 2-dimensional gel patterns of HMGB1 extracted from iDCs or from aNK cells; whereas in DCs 3 different HMGB1 spots regularly spaced along the pH gradient are detected, in NK cells the 2 more basic HMGB1 spots are absent, and the most acidic one is strongly increased and 2 still more acidic spots arise. We have previously shown that HMGB1 appears in 2-dimensional gels with various spots displaying a different migration along the pH gradient, corresponding to different acetylation levels of the molecules.31 In activated monocytes, which are able to secrete HMGB1, but not in nonsecreting cells, the protein is hyperacetylated and is detected mostly as acidic spots. On these bases, the prevalence of acidic spots in aNK cells, but not DCs, indicates that aNK cells contain mainly hyperacetylated HMGB1, corresponding to cytoplasmic HMGB1, which is the precursor of the secreted cytokine.31
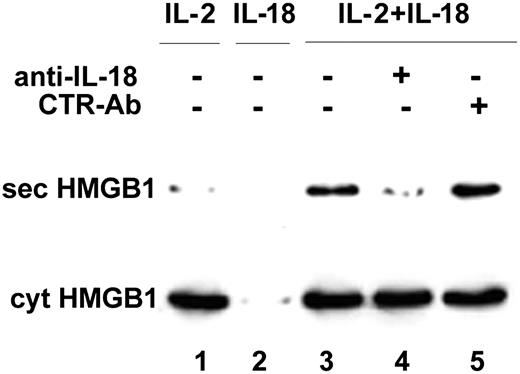
IL-18 induces HMGB1 secretion by NK cells: inhibition by anti-IL-18. rNK cells were cultured for 7 days with 50 IU/mL rhuIL-2 (lane 1) or 1 ng/mL rhuIL-18 (lane 2) or both (lanes 3-5), in the absence (lanes 1-3) or presence of 0.5 μg/mL of a neutralizing anti-IL-18 mAb (lane 4) or of an isotype-matched control mAb (lane 5). At the end of the culture period, culture fluids were replaced and cells were incubated in serum-free medium for an additional 6 hours. These supernatants were then assessed for the presence of HMGB1 by Western blotting (top blot). The bottom blot shows as a control the intracellular content of HMGB1 in all the culture conditions; note that IL-18 is unable to maintain NK cells alive in the absence of IL-2, resulting in an empty lane (lane 2).
IL-18 induces HMGB1 secretion by NK cells: inhibition by anti-IL-18. rNK cells were cultured for 7 days with 50 IU/mL rhuIL-2 (lane 1) or 1 ng/mL rhuIL-18 (lane 2) or both (lanes 3-5), in the absence (lanes 1-3) or presence of 0.5 μg/mL of a neutralizing anti-IL-18 mAb (lane 4) or of an isotype-matched control mAb (lane 5). At the end of the culture period, culture fluids were replaced and cells were incubated in serum-free medium for an additional 6 hours. These supernatants were then assessed for the presence of HMGB1 by Western blotting (top blot). The bottom blot shows as a control the intracellular content of HMGB1 in all the culture conditions; note that IL-18 is unable to maintain NK cells alive in the absence of IL-2, resulting in an empty lane (lane 2).
Secretion of HMGB1 by NK cells is induced by IL-18
These data strongly suggest that IL-18 secreted by iDCs may activate the interacting NK cells to release HMGB1. Blocking experiments with neutralizing anti-IL-18 mAbs, however, were not consistent in preventing HMGB1 secretion by NK cells (not shown), probably because they cannot reach the synaptic cleft, where secretion of IL-18 is confined in most cases.
To verify whether IL-18 is indeed able to induce the release of HMGB1 by NK cells, we investigated the effects of IL-18 exogenously added to NK cells on HMGB1 secretion. Purified NK cells were cultured 1 week with suboptimal doses (50 IU/mL) of rhuIL-2 or with 1 ng/mL rhuIL-18, or both, in the absence or presence of a neutralizing anti-IL-18 mAb or of an isotype-matched control mAb. At the end of the culture period, NK cells were washed and HMGB1 secreted in the following 6 hours was evaluated. As shown in Figure 6, suboptimal doses of rhuIL-2 sustain NK cell proliferation but do not induce HMGB1 secretion (lane 1). rhuIL-18 alone failed to maintain NK cells alive (lane 2). In contrast, NK cells cultured with both rhuIL-2 and rhuIL-18 were activated to secrete HMGB1 (lane 3). The presence of neutralizing anti-IL-18 mAb (lane 4), but not of the control mAb (lane 5), completely prevented HMGB1 secretion.
Discussion
This paper uncovers some molecular events occurring in the first phases of the NK/iDC crosstalk. Interaction with NK cells results in Ca2+ rise and cytoskeleton rearrangement in iDCs with clustering of tubulin and of secretory lysosomes containing IL-18 and cathepsin D at the immunologic synapse. This polarization is followed by secretion of the 2 proteins in the synaptic cleft. In turn, IL-18-activated NK cells secrete HMGB1, which induces DC maturation. Thus, the synapse may not simply be involved in signaling,2 but may play several important roles in effector function, including targeted delivery of cytokines.
Unlike most epithelial or neuronal cells, DCs are nonpolarized cells, and their secretory proteins are neither endowed with sorting motifs nor targeted to different plasma membrane domains.39 Thus, the occurrence of a secretion restricted at the immunologic synapse indicates that DCs undergo a functional polarization, induced by the same interacting NK cell toward which secretion is targeted. The surface molecules involved in this stimulation are so far unknown, but their engagement results in Ca2+ rise and tubulin polarization in DCs, eventually leading to secretion. These data are consistent with previous reports showing that Ca2+ mobilization and tubulin rearrangement are required for exocytosis of secretory lysosomes10 and for lysosome-mediated secretion of IL-1β.4,9 In addition, our findings provide a molecular basis to the observation that a cell-to-cell contact is needed for NK cell activation to occur.15,16
Despite the detection of a high percentage of cell conjugates displaying IL-18 polarization, soluble IL-18 is present infrequently in iDC/NK coculture supernatants, suggesting that the cytokine may be trapped at the synapse by binding sites on target cells. The erratic presence of soluble IL-18 can explain the failure of blocking IL-18 activity in NK/DC coculture with neutralizing antibody, in keeping with previous results.40
From a functional viewpoint, the polarized secretion of IL-18 may activate locally the interacting NK cell without spreading of the cytokine; the meaning of the polarized secretion of cathepsin D is, however, less evident. On the one hand, this may represent a mechanism of negative feedback to limit the inflammatory response. Indeed, killing of DCs by NK cells in infected tissues has been proposed to amplify the stranger/danger signals derived from invading pathogens.16 The release of lysosomal hydrolases could be a defense of DCs against the lytic proteins delivered by NK cells; this defense can be overcome by a high NK/DC ratio, thus explaining why the presence of a large number of NK cells induces killing rather than maturation.16 On the other hand, the degranulation restricted to the immunologic synapse precludes the dissemination of cathepsin D, preventing the excessive extracellular matrix degradation and the tissue damage. In any case, this mechanism of regulated secretion limited to the synaptic cleft between immune cells may provide a molecular explanation to the observation that treatment with anticytokine reagents is sometimes ineffective in chronic inflammation and autoimmune diseases.41,42
NK cells respond to DC triggering by releasing HMGB1. In contrast to the focused secretion of IL-18, HMGB1 is secreted quite abundantly by NK cells after interaction with iDCs. HMGB1 is a chromatin component that belongs to the functional family of endogenous danger factors (for a review, see Erlandsson Harris and Andersson43 ). Unlike the other danger signals, however, HMGB1 in activated monocytes undergoes acetylation-dependent cytoplasmic relocation30,31 followed by regulated secretion.30 Here we have shown that, in activated NK cells, HMGB1 is largely cytoplasmic and highly acetylated, thus fulfilling the requirements for being secreted. In keeping, both resting and activated NK cells secrete HMGB1 following interaction with iDCs. HMGB1 was recently shown to induce maturation of DCs.27,28 Our data confirm and extend these observations by showing that HMGB1 induces in DCs not only surface expression of maturation markers, but also production of the proinflammatory cytokine IL-1β, which is restricted to mDCs.3 Remarkably, according to the resistance to NK cell cytotoxicity displayed by mDCs, exposure to HMGB1 prevents DCs from NK cell-mediated lysis. Thus, our findings strongly suggest that HMGB1, secreted by NK cells, plays a major role on NK cell-induced DC maturation, and that the more or less availability of this cytokine in the DC/NK microenvironment may switch the fate of DCs toward death or maturation. More generally, the regulated secretion of HMGB1 by cells of the innate immune response, monocytes and NK cells, may allow the modulation of inflammation in a “controlled” fashion.
We were unable to block HMGB1 secretion by NK cells in iDC/NK cocultures with anti-IL-18 neutralizing mAbs, most likely because of the restricted secretion of IL-18 at the synaptic cleft, difficult to reach by antibodies. However, we found that rhuIL-18 induces HMGB1 secretion by NK cells cultured with suboptimal doses of IL-2, and that this induction is prevented by anti-IL-18 neutralizing antibodies. These results provide a novel NK cell-activating function to IL-18, that is, induction of HMGB1 secretion, and strongly support that IL-18 released by iDCs plays a major role in NK cell activation and subsequent DC maturation. Moreover, the requirement of IL-2 as a survival factor is in agreement with previous observations that DC-derived IL-2 contributes to activate NK cells.44 Later on, when DCs are matured, other dendrikines such as IL-12 may replace IL-18 in activating NK cells.19
Altogether our results indicate that the crosstalk between iDCs and NK cells leads to reciprocal activation, mediated by preformed soluble factors that undergo regulated, calcium-dependent secretion. The targeted delivery of IL-18 by iDCs restricts activation to the surrounding NK cells. Once activated, NK cells may amplify the response by secreting HMGB1 and inducing DC maturation. These observations contribute to uncovering the mechanism underlying the bidirectional crosstalk between DCs and NK cells and point to local secretion of leaderless cytokines as a key event in the modulation of their response.
Prepublished online as Blood First Edition Paper, March 31, 2005; DOI 10.1182/blood-2004-10-3906.
Supported in part by grants from Associazione Italiana per la Ricerca sul Cancro, Ministero della Salute, MIUR, CIPE (02/07/2004, CBA project).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr M. Bianchi for the kind gift of recombinant and purified HMGB1, Dr C.A. Dinarello and Dr S. Ferrini for anti-IL-18 antibodies, the NCI Biological Resource Branch for 3ZD mAb, Dr P. Lane for CD40L-transfected cells, Dr R. Notaro for helpful discussion, and the Ospedale Galliera of Genoa for kindly providing buffy coats.

![Figure 1. NK cells induce maturation or killing of autologous DCs. (A) Percent of CD86+ DCs (left) or mean fluorescence intensity (MFI) of MHC class II expression (right) after 24 hours of culture with 1 μg/mL LPS or 1 μg/mL HMGB1 or coculture with rNK or aNK cells at an NK/DC ratio of 5:1 or 10:1. Note that the increase in the percentage of CD86+ DCs varies from individual to individual, and requires a higher NK/DC ratio when rNK rather than aNK are used. (B-D) The aNK cells derived from 3 healthy donors were analyzed in a 4-hour 51Cr-release assay for their cytolytic activity against autologous iDCs (B), DCs treated 24 hours with 1 μg/mL LPS (C), or 1 μg/mL HMGB1 (D) at different NK/DC (effector-target [E/T]) ratios, from 10:1 to 2:1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/2/10.1182_blood-2004-10-3906/6/m_zh80140581310001.jpeg?Expires=1763470867&Signature=TNuWV18QZu16~Y5ryJT~cy7TuHcy0XbxOBvAemDbKkS26NGOIrdqZf7BtvOflVaM8XBTRiQ0xC~eDIoBSuHZBq5lLNDHi~U7Kl6ecxKoPwHLzGXXBJHahIJMCDOnsCIMzrXdfgrKLDK7aL~ONRPP5VU9-wF6rk1C73nUbqMY2yZdUEN~x0Ywoxto-~qNoiG6thcDzMNvA8WtsyhzeOnnLAv4TqLbvK9ByRZWHF9yM8q17s1PlINmZCsFVg9hHgjMnOKLcZTou~7MU63MrTgOBCQvxII9l9XLaek0ZElKZgzpg~VvDHTWXhhmVUpvYRIqg2JauuURFPha6jSHbhBXbw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 4. NK cells induce a Ca2+ flux in iDCs followed by translocation of microtubule organizing center and IL-18 at the immunologic synapse. (A) NK cells were added to Fura2-AM-loaded iDCs and Ca2+ waves were monitored. Results are expressed as [Ca2+]i and represent the mean of calcium response of 15 different cells. (B) iDCs or iDC/NK conjugates after 3 hours of culture were stained for tubulin (green) or IL-18 (red). Nuclei are stained in blue by DAPI. Arrows point to the clustering of tubulin and IL-18 at the contact site between NK cells and iDCs.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/2/10.1182_blood-2004-10-3906/6/m_zh80140581310004.jpeg?Expires=1763470867&Signature=z8d6vjwbIzaAwxhmXoI7kFQmNIkrAz3hP1hIbIawliEoY4QXLvoFtIuX6duru4toPVe5aNhjlbHK~p6T5s33hrs6dmWb4rxFYeqU82P-uMO17yZs0R89VMcm-~nKq9pX7e9MWh9FlVkzsKBtiiQpCiymAJDUB7qvYZ4bhRR0kYm~KUDF82NsKl6nnE4IgUc8dF7tXc4t5hZqwT39ExDakC7RSj~FVmiT4TdN71~oIu3v-vUyjz57EU22O8npPQ6x4-pMBhN6~p-Q2e4W-QiwkV-crcAOZuO8q6OPs~f5bg3WCBk46RQBw9IYym4EN9PYVYug9inU-~JewWmyX1Nwyw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal