Abstract
The addition of rituximab to cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy results in significant improvement in clinical outcome for individuals with non–HIV-associated aggressive B-cell lymphoma. To assess the potential risks and benefits of the addition of rituximab to CHOP for HIV-associated non-Hodgkin lymphoma (HIV-NHL) 150 patients receiving CHOP for HIV-NHL were randomized (2:1) to receive 375 mg/m2 rituximab with each chemotherapy cycle (n = 99) or no immunotherapy (n = 50) in a multicenter phase 3 trial. The complete response rate (CR + CRu) was 57.6% for R-CHOP and 47% for CHOP (P = .147). With a median follow-up of 137 weeks, time to progression, progression-free survival, and overall survival times were 125, 45, and 139 weeks, respectively, for R-CHOP and 85, 38, and 110 weeks, respectively, for CHOP (P = not significant, all comparisons). Treatment-related infectious deaths occurred in 14% of patients receiving R-CHOP compared with 2% in the chemotherapy-alone group (P = .035). Of these deaths, 60% occurred in patients with CD4 counts less than 50/mm3. Progression-free survival was significantly influenced by CD4+ count (P < .001) and International Prognostic Index score (P = .022), but not bcl-2 status. The addition of rituximab to CHOP in patients with HIV-NHL may be associated with improved tumor responses. However, these benefits may be offset by an increase in infectious deaths, particularly in those individuals with CD4+ lymphocyte counts less than 50/mm3.
Introduction
The incidence of aggressive B-cell non-Hodgkin lymphoma is significantly increased in HIV-seropositive individuals1,2 and the risk of systemic lymphoma remains high despite the widespread use of highly active antiretroviral therapy (HAART).3,4
Treatment of HIV-associated non-Hodgkin lymphoma (HIV-NHL) prior to the advent of HAART was frequently complicated by opportunistic infections. Complete remission rates in the 16% to 56% range and median survival times of 5 to 8 months were reported regardless of chemotherapy regimen or dose intensity.5,6 Poor clinical outcome has been associated with a total CD4+ lymphocyte count less than 100/mm3, stage III or IV disease, age greater than 35 years, a history of intravenous drug use, and International Prognostic Index (IPI) score.7 Since the introduction of HAART, studies have suggested better tolerance of chemotherapy and significantly better survival.8,9 Pharmacokinetic interactions between HAART and intermittent chemotherapy have been demonstrated to be modest.8
Rituximab, a chimeric anti-CD20 monoclonal antibody comprised of a human immunoglobulin G1 (IgG1) with a mouse CD20 binding region,10 is present on mature B cells and nearly all B-cell lymphomas.10 Clinical trials of rituximab have documented efficacy in previously untreated as well as refractory low-grade B-cell lymphomas and in refractory aggressive B-cell lymphomas.11-13 Rituximab has been well-tolerated and has not been associated with an increase in infectious complications in any randomized controlled trial. This National Cancer Institute–sponsored AIDS-Malignancies Consortium (AMC) study was designed to determine if the addition of rituximab to CHOP chemotherapy would improve clinical outcome in individuals with HIV-associated aggressive B-cell lymphoma and whether the use of this agent in immunodeficient patients might pose safety issues not previously encountered in immunocompetent populations.
Patients, materials, and methods
Eligibility
Patients were eligible to participate if they were HIV-positive and had previously untreated histologically or cytologically documented CD20+ (50% of cells express CD20) B-cell non-Hodgkin lymphoma of any stage with measurable or assessable disease and Karnofsky performance score greater than or equal to 70%. Adequate hematologic function (absolute neutrophil count [ANC] > 1000 cells/mm3, platelets > 75 000/mm3) was required except in cases where lymphomatous bone marrow involvement was documented. Creatinine less than 176.8 μM/L (2.0 mg/dL), bilirubin less than 34.2 μM/L (2.0 mg/dL), and hepatic transaminases less than 7 times the upper limit of normal were also required at baseline. Patients with parenchymal brain or spinal cord lymphoma or acute HIV-associated opportunistic infection requiring treatment were excluded.
Permuted block randomization was performed centrally by the AMC Operations Office at the time of patient registration. Patients were stratified by extent of disease (stage I/II versus stage III/IV) and within each stratum, randomization was 2 to 1, with 2 patients assigned to chemo-immunotherapy for every one assigned to chemotherapy alone.
Treatment
Both treatment groups received standard-dose cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy on day 1 of a 21-day cycle with cyclophosphamide, 750 mg/m2 intravenously; doxorubicin, 50 mg/m2 intravenously; vincristine, 1.4 mg/m2 (maximum of 2.0 mg); and prednisone, 100 mg by mouth daily for 5 days. Patients assigned to R-CHOP received rituximab (Genentech, supplied by Cancer Treatment Evaluation Program, National Cancer Institute [NCI], Bethesda, MD) at 375 mg/m2 intravenously 2 days prior to each chemotherapy cycle. Partial or complete responders to R-CHOP received 3 monthly maintenance doses of rituximab at 375 mg/m2 by slow intravenous infusion.
Patients with stage I, stage IE (including bulky disease), or nonbulky stage II were treated with 3 cycles of CHOP followed by involved field radiotherapy to a total dose of at least 4000 cGy beginning 3 weeks after the third cycle of chemotherapy. The 2 treatment arms were stratified for these individuals. Patients with more advanced disease received a minimum of 6 cycles of chemotherapy or 2 cycles beyond documentation of complete remission.
Combination antiretroviral therapy was required and could be modified during the course of protocol treatment as necessitated by the occurrence of drug-induced toxicities or based on measurement of HIV-viral load. All patients received pneumocystis cariini prophylaxis with either cotrimoxizole, dapsone, or inhaled pentamidine. Antibiotic prophylaxis for enteric organisms was not required.
Meningeal prophylaxis was performed at the discretion of the local investigator for individuals felt to be at high risk for meningeal recurrence. Prophylaxis was recommended for individuals with small noncleaved histology, bone marrow involvement, paranasal sinus involvement, testicular involvement, or the presence of epidural disease. This was generally accomplished using either 4 weekly intrathecal doses of cytosine arabinoside or 4 intrathecal doses of methotrexate. Individuals with active meningeal lymphoma were treated with intrathecal cytosine arabinoside, and whole-brain radiotherapy was recommended for patients with neurologic signs and symptoms.
All patients received granulocyte–colony-stimulating factor (G-CSF; at a dose of 5 mcg/kg subcutaneously daily beginning on day 4 and continuing through day 13 of each chemotherapy cycle or until the absolute neutrophil count had recovered to 10 000 cells/mm3.
All patients underwent complete staging evaluation with computed tomography (CT) or magnetic resonance imaging (MRI) scan of the chest, abdomen, and pelvis; bone marrow biopsy; and lumbar puncture. Restaging evaluation of all initial sites of measurable or evaluable disease was performed every 2 cycles. CT scans were repeated 8 weeks after the last dose of chemotherapy and then every 8 weeks for 1 year. Standard, international workshop criteria were used to categorize responses.14 Evaluation of response was based on intent-to-treat. Unevaluable patients were considered to be nonresponders.
Quantitative immunoglobulins and circulating B cells as measured by CD19 and CD20 were obtained at baseline, day 1 of chemotherapy cycle 3, 1 and 4 months after chemotherapy, and then monthly for 1 year or until immunoglobulin levels normalized.
Pathology review
Central pathology review was required and was performed after enrollment.
BCL-2 immunostaining
Immunophenotypic analysis for BCL-2 (clone 124; 1:40; DakoCytomation, Glostrup, Denmark) expression was performed on paraffin tissue sections following pressure cooker antigen retrieval, using a TechMate 500TM automated immunostainer (Ventana Medical Systems, Tucson, AZ). Immunostaining was carried out according to a modified monoimmunoperoxidase panel protocol (Ventana Medical Systems) using the ChemMate ABC peroxidase secondary detection system, and employing diaminobenzidine as the chromogen (Ventana Medical Systems) to detect antigen expression.15 As the tissues were processed in a variety of laboratories using various fixatives (formalin, B5, etc) and procedures, which may compromise antigen detection, a case was considered positive for BCL-2 if more than 10% of the malignant cells exhibited definite expression of this protein. In most cases the number of positive cells constituted the majority of the cells. All cases were stained with a section of formalin-fixed tonsil, which served as a positive control.
Statistical considerations
This was an intent-to-treat analysis. The sample size of 120 assessable patients, 80 with rituximab and 40 without rituximab, was sufficient to detect an improvement in complete response rate, the primary study endpoint, from 50% with CHOP alone to 75% with R-CHOP at the one-sided significance level of .05 with a power of .81, and to detect a 50% increase in the median time to progression from 35 weeks on CHOP alone to 53 weeks with R-CHOP at the one-sided .05 significance level with power of .67. The planned enrollment of 150 patients was to ensure 120 evaluable patients for analysis. Secondary endpoints included time to progression, progression-free survival, and overall survival.14 Progression-free survival endpoints include treatment failure or death from any cause. Infectious deaths were considered treatment-related if they occurred during therapy or within 6 months of completion of protocol therapy.
Binomial proportions and their 95% confidence intervals were used to estimate response rates to CHOP and R-CHOP. Fisher exact test was used to compare the 2 groups with respect to response rate, the incidence of specific adverse events, and the incidence of infection-related death. Logistic regression analyses were used to evaluate baseline characteristics and other covariants with response. Time-to-event measures were evaluated with Kaplan-Meier analyses, group comparisons made with the log-rank test, and multivariate analyses performed using the Cox proportional hazards model.
The Data Safety and Monitoring Board convened by the Cancer Therapy and Evaluation Program, National Cancer Institute, in conjunction with the AIDS Malignancies Consortium reviewed the interim analyses performed after 30 and 60 patients had completed chemotherapy and monitored safety on an ongoing basis.
The protocol was reviewed and approved by institutional review boards at each of the participating sites and all patients signed informed consent prior to enrollment.
Results
Patient characteristics
A total of 150 patients were enrolled between December 1998 and May 2002 at the 22 participating AMC sites, with 99 assigned to R-CHOP and 51 to CHOP. Pretreatment characteristics are shown in Table 1. There were no statistically significant differences between the 2 groups with respect to any pretreatment characteristics. A total of 112 specimens underwent central pathologic review. For these cases the reviewed pathologic diagnosis is listed. For the remaining cases the local institutional diagnosis is provided. A diagnosis of aggressive B-cell lymphoma was confirmed in 96% of the centrally reviewed cases.
AMC 010: pretreatment characteristics
Variable . | R-CHOP, no. (%) . | CHOP, no. (%) . | Total, no. (%) . |
|---|---|---|---|
| Age, y | 43.5 (26-69)* | 40 (26-73)* | 42 (26-73)* |
| Homosexual male | 65 (65) | 29 (57) | 94 (63) |
| Injection drug use | 11 (11) | 7 (14) | 18 (12) |
| Heterosexual contact | 19 (19) | 15 (29) | 34 (23) |
| Other risk group | 13 (13) | 8 (16) | 21 (14) |
| CD4 level, cells/mm3 | 130 (1-2457)† | 147 (2-986)† | 133 (1-2457)† |
| CD4 level less than 50 cells/mm3 | 22 (25) | 11 (22) | 33 (24) |
| CD4 level more than 200 cells/mm3 | 29 (33) | 16 (33) | 45 (33) |
| Prior opportunistic infection | 36 (36) | 15 (29) | 51 (34) |
| Stage III/IV | 79 (80) | 40 (78) | 119 (79) |
| Elevated LDH | 39 (39) | 24 (47) | 63 (42) |
| KPS, % | |||
| 100 | 26 (26) | 19 (37) | 45 (30) |
| 80/90 | 55 (56) | 17 (33) | 72 (48) |
| 60/70 | 17 (17) | 15 (29) | 32 (21) |
| 40/50 | 1 (1) | 0 (0) | 1 (1) |
| IPI score (age-adjusted) | |||
| 0 | 14 (14) | 6 (12) | 20 (13) |
| 1 | 42 (42) | 18 (35) | 60 (40) |
| 2 | 35 (35) | 20 (39) | 55 (37) |
| 3 | 8 (8) | 7 (14) | 15 (10) |
| Prior antiretrovirals | |||
| None | 26 (26) | 21 (41) | 47 (31) |
| Any 3 drugs | 66 (67) | 27 (53) | 93 (62) |
| Protease inhibitor | 58 (59) | 24 (48) | 82 (55) |
| Histology | |||
| Diffuse large B cell | 80 (81) | 40 (78) | 120 (80) |
| Burkitt | 8 (8) | 6 (12) | 14 (9) |
| HG NHL NOS | 4 (4) | 5 (10) | 9 (6) |
| Other | 7 (7) | 0 (0) | 7 (5) |
Variable . | R-CHOP, no. (%) . | CHOP, no. (%) . | Total, no. (%) . |
|---|---|---|---|
| Age, y | 43.5 (26-69)* | 40 (26-73)* | 42 (26-73)* |
| Homosexual male | 65 (65) | 29 (57) | 94 (63) |
| Injection drug use | 11 (11) | 7 (14) | 18 (12) |
| Heterosexual contact | 19 (19) | 15 (29) | 34 (23) |
| Other risk group | 13 (13) | 8 (16) | 21 (14) |
| CD4 level, cells/mm3 | 130 (1-2457)† | 147 (2-986)† | 133 (1-2457)† |
| CD4 level less than 50 cells/mm3 | 22 (25) | 11 (22) | 33 (24) |
| CD4 level more than 200 cells/mm3 | 29 (33) | 16 (33) | 45 (33) |
| Prior opportunistic infection | 36 (36) | 15 (29) | 51 (34) |
| Stage III/IV | 79 (80) | 40 (78) | 119 (79) |
| Elevated LDH | 39 (39) | 24 (47) | 63 (42) |
| KPS, % | |||
| 100 | 26 (26) | 19 (37) | 45 (30) |
| 80/90 | 55 (56) | 17 (33) | 72 (48) |
| 60/70 | 17 (17) | 15 (29) | 32 (21) |
| 40/50 | 1 (1) | 0 (0) | 1 (1) |
| IPI score (age-adjusted) | |||
| 0 | 14 (14) | 6 (12) | 20 (13) |
| 1 | 42 (42) | 18 (35) | 60 (40) |
| 2 | 35 (35) | 20 (39) | 55 (37) |
| 3 | 8 (8) | 7 (14) | 15 (10) |
| Prior antiretrovirals | |||
| None | 26 (26) | 21 (41) | 47 (31) |
| Any 3 drugs | 66 (67) | 27 (53) | 93 (62) |
| Protease inhibitor | 58 (59) | 24 (48) | 82 (55) |
| Histology | |||
| Diffuse large B cell | 80 (81) | 40 (78) | 120 (80) |
| Burkitt | 8 (8) | 6 (12) | 14 (9) |
| HG NHL NOS | 4 (4) | 5 (10) | 9 (6) |
| Other | 7 (7) | 0 (0) | 7 (5) |
Total patients treated with R-CHOP, 99; with CHOP, 51; with either, 150. HG NHL NOS indicates high-grade non-Hodgkin lymphoma not otherwise specified. Other includes polymorphic B-cell (2), mixed histology (1), primary effusion (1), and missing (1).
Mean (range).
Median (range).
Immune function is shown in Table 1. Viral load (VL) data were available from a minority of patients. In 46 individuals randomized to R-CHOP, the median VL was 13 173 copies/mL (range, 94-750 000 copies/mL) whereas 17 randomized to CHOP had median VL of 38 517 copies/mL (range, 130-750 000 copies/mL); P = .68. The vast majority had stage III or IV disease (79%) and 77% of all patients fell into the age-adjusted IPI score 1 or 2 categories. The majority of patients (80%) had diffuse large B-cell lymphoma; only 9% had Burkitt lymphoma (Table 1). Most patients were receiving antiretroviral treatment at the time of enrollment. Thirty-four percent had a history of a prior opportunistic infection.
Treatment
Treatment was discontinued prematurely in 47 (47%) patients receiving R-CHOP and 28 (55%) receiving CHOP alone (Table 2). Most withdrew due to an adverse event, disease progression, or death. Withdrawal due to disease progression was more frequent in patients who did not receive rituximab (31%) as compared with those who received R-CHOP (11%) (P = .003).
Early withdrawal from study
Reason . | R-CHOP, no. (%) . | CHOP, no. (%) . |
|---|---|---|
| Adverse event | 12 (12) | 4 (8) |
| Disease progression | 11 (11) | 16 (31) |
| Death | 5 (5) | 1 (2) |
| Patient withdrawal | 8 (8) | 2 (4) |
| Physician decision | 5 (5) | 2 (4) |
| Lost to follow-up | 4 (4) | 1 (2) |
| Noncompliance | 2 (2) | 2 (4) |
Reason . | R-CHOP, no. (%) . | CHOP, no. (%) . |
|---|---|---|
| Adverse event | 12 (12) | 4 (8) |
| Disease progression | 11 (11) | 16 (31) |
| Death | 5 (5) | 1 (2) |
| Patient withdrawal | 8 (8) | 2 (4) |
| Physician decision | 5 (5) | 2 (4) |
| Lost to follow-up | 4 (4) | 1 (2) |
| Noncompliance | 2 (2) | 2 (4) |
Total early withdrawal from R-CHOP, 47; from CHOP, 28.
The median relative dose intensity for cyclophosphamide was .94 and .96 for R-CHOP and CHOP, respectively. For doxorubicin it was .95 and .95 and for vincristine .64 and .75, respectively. A median of 4.0 chemotherapy cycles (range, 1-11 cycles) were administered to those randomized to R-CHOP whereas those in the CHOP group received a median of 5.0 cycles (range, 1-8 cycles); P = not significant. The median number of rituximab maintenance cycles administered was 3.0 (range, 1-4 cycles) for the 35 patients in the rituximab arm who received maintenance therapy.
Response and survival
Response data for all patients is shown in Table 3. This was an intent-to-treat analysis in which patients unevaluable for response were considered to be nonresponders. The overall complete response rates (CR+CRu) were 57.6% for R-CHOP and 47.0% for CHOP. This increase in complete response rate (representing a 22% improvement over CHOP alone) did not achieve statistical significance, P = .147. Progressive disease was more common in those receiving CHOP alone (21.6%) compared with those who received R-CHOP (8.1%). Of those individuals considered to be unevaluable, those who received rituximab were more likely to have had an early adverse event or death than those who did not (Table 3). Complete response rates (CR+CRu) were somewhat higher in those with IPI scores 0 or 1 (66% vs 54% for R-CHOP vs CHOP) compared with those having IPI scores of 2 or 3 (46.6% vs 40.7%).
Response summary
Response . | R-CHOP, no. (%) . | CHOP, no. (%) . |
|---|---|---|
| Complete response | 49 (49.5) | 21 (41.2) |
| CRu | 8 (8.1) | 3 (5.9) |
| Total CR | 57 (57.6) | 24 (47.0) |
| Partial response | 8 (8.1) | 4 (7.8) |
| Progression | 8 (8.1) | 11 (21.6) |
| Stable | 8 (8.1) | 4 (7.8) |
| Unevaluable | 18 (18.2) | 7 (13.7) |
| Adverse event | 8 (8) | 1 (2) |
| Death | 6 (6) | 1 (2) |
| Patient withdrawal | 3 (3) | 1 (2) |
| Loss/noncompliance | 0 (0) | 3 (6) |
| Physician decision | 1 (1) | 1 (2) |
Response . | R-CHOP, no. (%) . | CHOP, no. (%) . |
|---|---|---|
| Complete response | 49 (49.5) | 21 (41.2) |
| CRu | 8 (8.1) | 3 (5.9) |
| Total CR | 57 (57.6) | 24 (47.0) |
| Partial response | 8 (8.1) | 4 (7.8) |
| Progression | 8 (8.1) | 11 (21.6) |
| Stable | 8 (8.1) | 4 (7.8) |
| Unevaluable | 18 (18.2) | 7 (13.7) |
| Adverse event | 8 (8) | 1 (2) |
| Death | 6 (6) | 1 (2) |
| Patient withdrawal | 3 (3) | 1 (2) |
| Loss/noncompliance | 0 (0) | 3 (6) |
| Physician decision | 1 (1) | 1 (2) |
Total patients treated with R-CHOP, 99; with CHOP, 50.
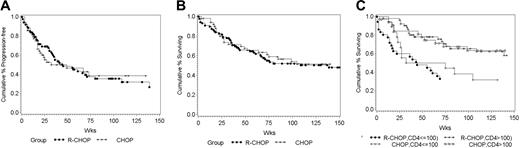
There were no statistically significant differences in time to progression (TTP), progression-free survival (PFS), or overall survival (OS) between the 2 treatment groups (Figure 1A-B). With a median follow-up of 137 weeks, the median TTP is 125 weeks for R-CHOP and 85 weeks for CHOP alone (P = .26). PFS and OS are 45 and 139 weeks, respectively, for R-CHOP and 38 and 110 weeks, respectively, for CHOP alone (P = .67, .76 respectively). Regardless of treatment arm, patients with baseline CD4 counts of less than 100/mm3 had a significantly shorter survival than did those with higher CD4 counts (P = .01; Figure 1C).
Kaplan-Meier plots. (A) Progression-free survival; R-CHOP (n=99), CHOP (n=51). (B) Overall survival; R-CHOP (n=99), CHOP (n=51). (C) Survival stratified by absolute CD4+ lymphocyte count; CD4 count > 100 R-CHOP (n=52), CD4 count > 100 CHOP (n=33), CD4 count ≥ 100 R-CHOP (n=36), CD4 ≥ 100 CHOP (n =18).
Kaplan-Meier plots. (A) Progression-free survival; R-CHOP (n=99), CHOP (n=51). (B) Overall survival; R-CHOP (n=99), CHOP (n=51). (C) Survival stratified by absolute CD4+ lymphocyte count; CD4 count > 100 R-CHOP (n=52), CD4 count > 100 CHOP (n=33), CD4 count ≥ 100 R-CHOP (n=36), CD4 ≥ 100 CHOP (n =18).
Logistic regression analysis of pretreatment characteristics and treatment group indicated that complete response rate was significantly associated with absolute CD4+ lymphocyte count (P < .001) and IPI score (P = .041). Proportional hazards analysis demonstrated PFS to be significantly related to CD4 count (P < .001) and IPI score (P = .022). There was a trend toward better outcome in those with non-Burkitt histology. Median TTP was 22 weeks and 157 weeks for Burkitt (n = 14) and non-Burkitt (n = 136) diagnoses, respectively (P = .072). Median PFS was 22 weeks and 55 weeks, respectively (P = .124). There were 5 meningeal relapses, 3 in the R-CHOP and 2 in the CHOP arm of the study.
Adverse events
Grades 3 and 4 toxicities are listed in Table 4. While there are trends toward an increased risk of neutropenia, febrile-neutropenia, and infection with R-CHOP, the risk of infection-related death was significantly higher in those randomized to R-CHOP compared with those receiving CHOP alone (Table 5). Fourteen percent of patients who received rituximab died of treatment-related infection compared with 2% (one patient) in the chemotherapy alone group (P = .035). If patients with CD4 counts less than 50/mm3 are excluded, the infection-related death rate is 5 of 77 (6.5%) for rituximab versus 1 of 40 (2.5%) for control patients (P = .66).
Grade 3/4 adverse events
Event . | R-CHOP, no. (%) . | CHOP, no. (%) . | P . |
|---|---|---|---|
| ANC < 500/mm3, patients | 61 (62) | 25 (48) | .11 |
| ANC < 500/mm3, cycles | 96 (21) | 39 (17) | .19 |
| Thrombocytopenia, grade 4 | 3 (3) | 4 (8) | .23 |
| Anemia, grade 4 | 8 (8) | 3 (6) | .75 |
| Febrile neutropenia | 31 (31) | 12 (24) | .35 |
| Akaline phosphatase, grade 3/4 | 10 (10) | 0 | .02 |
| AST, grade 3/4 | 11 (11) | 3 (6) | .38 |
| ALT | 1 (1) | 3 (6) | .114 |
| Infusion reaction | 2 (2) | 0 (0) | .55 |
Event . | R-CHOP, no. (%) . | CHOP, no. (%) . | P . |
|---|---|---|---|
| ANC < 500/mm3, patients | 61 (62) | 25 (48) | .11 |
| ANC < 500/mm3, cycles | 96 (21) | 39 (17) | .19 |
| Thrombocytopenia, grade 4 | 3 (3) | 4 (8) | .23 |
| Anemia, grade 4 | 8 (8) | 3 (6) | .75 |
| Febrile neutropenia | 31 (31) | 12 (24) | .35 |
| Akaline phosphatase, grade 3/4 | 10 (10) | 0 | .02 |
| AST, grade 3/4 | 11 (11) | 3 (6) | .38 |
| ALT | 1 (1) | 3 (6) | .114 |
| Infusion reaction | 2 (2) | 0 (0) | .55 |
AST indicates aspartate aminotransferase; ALT, alanine aminotransferase.
Cause of death
Cause . | R-CHOP, no. (%) . | CHOP, no. (%) . |
|---|---|---|
| Lymphoma | 14 (14) | 15 (29) |
| HIV progression | 4 (4) | 2 (4) |
| Infection, not treatment-related | 2 (2) | 0 (0) |
| Treatment-related infection | 13 (14) | 1 (2)* |
| Other | 7 (7) | 5 (10) |
| Not reported | 2 (2) | 0 (0) |
| Total | 42 (42) | 23 (45) |
Cause . | R-CHOP, no. (%) . | CHOP, no. (%) . |
|---|---|---|
| Lymphoma | 14 (14) | 15 (29) |
| HIV progression | 4 (4) | 2 (4) |
| Infection, not treatment-related | 2 (2) | 0 (0) |
| Treatment-related infection | 13 (14) | 1 (2)* |
| Other | 7 (7) | 5 (10) |
| Not reported | 2 (2) | 0 (0) |
| Total | 42 (42) | 23 (45) |
Total patients treated with R-CHOP, 99; with CHOP, 51.
P = .035.
Of the 16 infectious deaths (treatment-related and non–treatment-related), there were 8 cases of culture-positive sepsis (5 Gram-negative, 3 Gram-positive), 6 sepsis syndromes with negative blood cultures, one unspecified pneumonia, and one fungal pneumonia. Fifteen of these deaths occurred in patients receiving rituximab, 9 (60%) had baseline absolute CD4+ lymphocyte counts below 50/mm3, 8 (53%) deaths occurred in cycles 1 or 2, 5 (33%) deaths occurred during or within 6 months of maintenance therapy, and 9 (60%) patients had neutrophil counts under 1000/mm3 at the time of death. Only one had a central venous catheter in place at the time of fatal infection.
In the R-CHOP group, the rate of treatment-related infectious death was 8 of 22 (36%) in patients with baseline CD4+ counts less than 50/mm3 and 5 of 77 (6%) in patients with baseline CD4+ counts greater than or equal to 50/mm3, a difference that was highly significant (P = .001).
A total of 8 HIV-associated opportunistic infections occurred in 6 individuals randomized to rituximab during or within 6 months of therapy; including Candida albicans fungemia (1), pneumocystis pneumonia (3), esophageal candidiasis (1), cytomegalovirus (CMV; 2), and Mycobacterium avium (1). In addition to these 8 cases, one death due to fungal pneumonia occurred 1 year after completion of therapy. The median pretreatment CD4 count in these patients was 57.5/mm3 (range, 1-181/mm3). There were no opportunistic infections observed in the CHOP group (P = .096).
Immunoglobulin levels
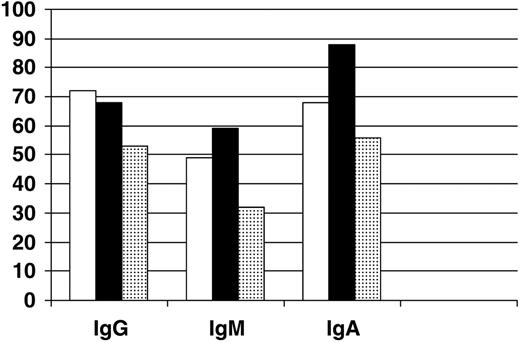
Follow-up Ig levels were not available for patients who died in the first 2 treatment cycles. Ig levels fell significantly from baseline to cycle 3 in both treatment groups (P = .01; Figure 2). IgG and IgA levels fell in both groups to about 70% of baseline values while IgM levels fell to about 50% of baseline. There were no statistically significant differences noted in Ig levels before or during therapy between the 2 treatment groups or those with late infectious deaths. Four patients treated with rituximab developed IgG levels less than 200 mg/dL during or after therapy. Two patients developed this in cycle 3 and both were still alive at last follow-up. One developed hypo IgG in cycle 7 and died 7 months later from sepsis. An additional patient developed low IgG after maintenance rituximab and died 1 year later from fungal pneumonia. Severe hypogammagolbulinemia was not observed in the CHOP alone group.
BCL-2 and outcome
Specimens were available from 69 patients for analysis. There were 36 positive (52%) and 33 negative tumors. No association was detected between positivity and clinical outcome (data not shown).
Immunoglobulin levels after completion of 2 cycles of chemotherapy. Levels are expressed as percent of baseline value for patients receiving R-CHOP (n=76; □); CHOP (n=31; ▪); and for those with infectious deaths that occurred after cycle 2 (n=7; ▦).
Immunoglobulin levels after completion of 2 cycles of chemotherapy. Levels are expressed as percent of baseline value for patients receiving R-CHOP (n=76; □); CHOP (n=31; ▪); and for those with infectious deaths that occurred after cycle 2 (n=7; ▦).
Discussion
In this randomized clinical trial of R-CHOP versus CHOP for HIV-NHL we were unable to demonstrate any statistically significant improvement in complete response rate, time to progression, event-free, or overall survival in the group treated with rituximab compared with the chemotherapy alone control group. Although the power to detect clinical benefit is limited by sample size, the data do indicate a trend toward better tumor control with the use of rituximab, in terms of complete remission rate (58% vs 47%), progressive disease (8% vs 22%), and withdrawal due to disease progression (11% R-CHOP vs 31% CHOP). However, there was a significantly increased risk of infectious death for HIV-NHL patients receiving rituximab.
Individuals in both study arms had survival times that exceeded expectations. Based on historical data,5,6 it was anticipated that patients treated with CHOP alone would have a median TTP of approximately 35 weeks. The observed median TTP of 85 weeks for this group is a likely benefit of the use of antiretroviral therapy during and following completion of chemotherapy rather than selection of patients for favorable characteristics. The median pretreatment absolute CD4 count of 130/mm3 is typical for patients with this disease enrolled on previous clinical trials.5,6,8
The addition of rituximab to CHOP chemotherapy improved tumor control in patients 60 years of age or older with diffuse large B-cell lymphoma treated in a randomized trial of individuals without HIV disease.16 With a median follow-up of 2 years, complete response rate, event-free, and overall survival times were significantly higher among patients who received rituximab.16 The 21% improvement in complete response rate is identical to that observed in our study, although our study was not powered to show significance with less than a 50% improvement. This study was therefore limited in its ability to identify response and survival differences similar to those observed in the immunocompetent population studied in the Groupe d'Etude des Lymphomes de l'Adulte (GELA) trial.16 This was largely a function of the limited size of the target patient population.
BCL-2 overexpression has been described as a prognostic factor in patients with aggressive B-cell lymphoma.17,18 The addition of rituximab to chemotherapy appears to specifically benefit individuals whose lymphomas overexpress bcl-2.19 Previous reports have indicated that bcl-2 positivity was rare in individuals with HIV-NHL.20 In our study, 52% of those with specimens available for evaluation had evidence of bcl-2 overexpression in their lymphomas; a frequency closer to that observed in the HIV-negative diffuse large B-cell lymphoma population.17-19 The addition of rituximab to CHOP in those patients whose tumors were bcl-2–positive did not statistically improve PFS or TTP (relative risk 0.93).
The trend toward increased response rate in our study has not translated into a survival benefit at least in part because of the incidence of infectious deaths in the R-CHOP arm. A significant decline in immunoglobulin levels was observed in both treatment arms of the study. Although patients who had later infectious deaths did show a trend toward lower immunoglobulin levels compared with other treated patients, this was not statistically significant, and follow-up Ig levels were never obtained from those patients with early infectious deaths. However, 2 of 4 individuals who developed severe hypogammaglobulinemia (IgG < 200 mg/dL) died from infectious causes. Although it is not possible to demonstrate a causal relationship between infectious death and hypogammaglobulinemia in this study, there may be individuals for whom this contributes to an increased risk of infection.
A recent prospective observational study demonstrated that low CD4+ lymphocyte count was associated with an increased risk of bacterial infection in HIV-infected neutropenic patients.21 Seven of the 12 individuals who developed infectious complications had CD4+ counts less than 50 cells/mm3. Borgetal22 recently reported that a low pretreatment CD4 count (< 450/mm3) was an independent risk factor for febrile-neutropenia and early death in patients receiving cytotoxic chemotherapy. Furthermore, the use of protease inhibitors may increase the risk of chemotherapy-induced neutropenia,23 thus potentially placing individuals with low CD4 counts who are receiving protease inhibitors at higher risk for life-threatening infection.
It is unclear what the contribution of rituximab was to the occurrence these infections. A recently reported analysis of 3 prospective trials of treatment for HIV-NHL in which rituximab was combined with infusional cyclophosphamide, doxorubicin, and etoposide (CDE) also suggested an increased risk of infectious deaths, which occurred in 8% of patients and included 3 septic deaths.24 Previous randomized controlled studies of chemotherapy with or without rituximab in HIV-negative individuals have not shown an increase in infectious deaths among those receiving rituximab.16 However, fludarabine is known to induce CD4 lymphopenia, to predispose to some of the same opportunistic infections that define AIDS, and when combined with rituximab the frequency of neutropenia is increased.25 Similarly, in the autologous transplantation setting rituximab has been associated with neutropenia.26 It is possible that there is an interaction between CD4 lymphopenia and CD20 lymphopenia in the risk of bacteremia and that part of this risk is associated with neutropenia. In the present study deaths were more common in those with CD4+ count less than 50 cells/mm3, and there was a trend toward more neutropenia in the rituximab arm (P = .11). It may be the confluence of impaired innate, neutrophil-mediated and adaptive T-cell and B-cell immunity that contributed to the unanticipated rate of infection-related death in this study.
These observations suggest that although the addition of rituximab to CHOP chemotherapy in patients with AIDS lymphoma may be associated with improved tumor responses, these benefits may be offset by the increase in infectious deaths. The high incidence of infectious deaths observed among patients receiving rituximab in this study raises concerns regarding its routine use with chemotherapy in individuals with immunodeficiency disease. Whether the dangers associated with rituximab therapy can be minimized by sequential administration of chemotherapy and rituximab or reduced by the use of prophylactic antibiotics in patients with low CD4+ counts will await the results of studies that are currently in progress. Efforts to further enhance the beneficial effects of rituximab while decreasing the infectious risks attendant with its use in this patient population are warranted.
Prepublished online as Blood First Edition Paper, May 24, 2005; DOI 10.1182/blood-2005-04-1437.
Supported in part by grants from the National Cancer Institute (U01CA083 118, U01CA070 079, U01CA70 058, U01CA070 047, U01CA070 054, U01C-A070 072, U01CA070 080, U01CA083 035, U01CA071 375, U01CA083 038, U01CA070 062, U01CA070 081, U01CA070 019) and IDEC Pharmaceuticals. L.D.K. designed the study, served as principal investigator, and wrote the manuscript; J.Y.L. designed the statistical section and analyzed data; R.F.A. designed the laboratory section of trial, performed EBV studies, and edited the manuscript; J.A.S. assisted with the design of the study and the writing of the manuscript; E.C. performed pathologic review and bcl-2 studies; A.C. performed pathologic review and bcl-2 studies; A.M.L. helped with the design of the study and the writing of the manuscript; D.T.S. codesigned the study with L.D.K., performed T-cell studies, and assisted in the writing of the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The following investigators participated in the study: Beth Israel Deaconess Medical Center, Boston, MA: B. Dezube; Baylor University, Houston, TX: M. Kroll; Boston Medical Center, Boston, MA: T. Cooley; Case Western Reserve University, Cleveland, OH: S. Remick; Columbia University, New York, NY: M. Keohan; Henry Ford Hospital, Detroit, MI: N. Janakiraman; Johns Hopkins University, Baltimore, MD: R. Ambinder; Massachusetts General Hospital, Boston, MA: D. Scadden; Memorial Sloan-Kettering Cancer Center, New York, NY: A. Noy and D. Straus; Montefiore Medical Center, New York, NY: J. Sparano; Northwestern University, Chicago, IL: J. Von Roenn; Ohio State University, Columbus, OH: M. Shah; Pennsylvania Oncology Hematology Associates, Philadelphia, PA: D. Henry; St Vincent's Hospital, Darlinghurst, Australia: M. Chipman; St Vincent's Hospital, New York, NY: J. Cho; UCSF, San Francisco, CA: L. Kaplan; UCLA, Los Angeles, CA: S. Miles and R. Mitsuyasu; UCSD Cancer Center, San Diego, CA: M. Saville and W. Wachsman; University of Miami, Miami, FL: W. Harrington Jr; University of Southern California, Norris Cancer Hospital, Los Angeles, CA: A. Levine and A. Tulpule; Virginia Mason Medical Center, Seattle, WA: D. Aboulafia; and Washington University, St Louis, MO: L. Ratner.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal