Abstract
Patients with chronic lymphocytic leukemia (CLL) treated with adenovirus CD154 (Ad-CD154, CD40 ligand [CD40L]) gene therapy experienced rapid reductions in leukemia cell counts and lymph node size associated with the induced expression of Fas (CD95). However, CLL cells initially resist CD95-mediated apoptosis within the first 3 days after CD40 ligation in vitro. Thereafter, they become sensitive, which is associated with the CD40-induced expression of the proapoptotic protein B-cell leukemia 2 homology 3 (BH3) interacting domain death agonist (Bid). We hypothesized that the initial resistance to CD95-mediated apoptosis may be due to the high-level expression of X-linked inhibitor of apoptosis protein (XIAP) by CLL cells. Consistent with this, CLL cells from patients 1 day after treatment with autologous Ad-CD154-transduced CLL cells became sensitive to CD95-mediated apoptosis following treatment with a novel XIAP inhibitor, 1540-14. Similarly, 1540-14 specifically enhanced CD95-mediated apoptosis of CLL cells following CD40 ligation in vitro. Immunoblot analyses demonstrated that treatment with 1540-14 allowed CD40-stimulated CLL cells to experience high-level activation of caspases-8 and -3 and cleavage of poly(adenosine diphosphate [ADP]-ribose) polymerase following CD95 ligation. This study demonstrates that distal apoptosis regulators contribute to the initial resistance of CD40-activated CLL cells to CD95-mediated apoptosis and suggests that XIAP inhibitors might enhance the effectiveness of immune-based treatment strategies that target CD40, such as CD154 gene therapy. (Blood. 2005;106:1742-1748)
Introduction
Patients with chronic lymphocytic leukemia (CLL) who received intravenous infusions of autologous leukemia cells transfected with an adenovirus vector encoding the CD40 ligand (Ad-CD154) experienced acute reductions in leukemia cell counts and lymph node size.1 This rapid cytoreduction was unexpected and suggested the possible contribution of innate immune-effector mechanisms to the early clearance of CLL cells following CD154 gene therapy.
Following intravenous infusion of Ad-CD154-transduced CLL cells, we observed that bystander, nontransduced CLL cells were induced to express Fas (CD95) and DR5,1,2 a receptor for the tumor necrosis factor (TNF)-receptor apoptosis-inducing ligand (TRAIL). Furthermore, activated CD4 T cells of patients treated with CD154 gene therapy were noted to express the ligands for such death receptors, namely Fas ligand (CD178) and TRAIL.2 In vitro studies demonstrated that cells that expressed both CD178 and TRAIL could kill CLL cells within 1 day after CD40 ligation in a CD95-dependent fashion through coligation of both CD95 and DR5.2 Moreover, CLL cells became increasingly sensitive to apoptosis induced by cells bearing CD178 and/or TRAIL over 3 to 5 days following CD40 activation.2,3
CLL cells also can be induced to express high levels of CD95 and DR5 following coculture with CD154-bearing cells in vitro. Although initially resistant to CD95- or DR5-mediated apoptosis 1 day after such coculture, CD40-activated CLL cells become increasingly sensitive to apoptosis triggered by ligation of such extrinsic death receptors over the ensuing 3 to 5 days, an phenomenon termed “latent sensitivity to Fas-mediated apoptosis”.2,3
The initial resistance of CLL cells to CD95-mediated apoptosis following CD40 ligation may be secondary to CLL cell expression of inhibitors of apoptosis proteins (IAPs), such as the X-linked IAP (XIAP). IAPs negatively regulate apoptosis by inhibiting caspase activity directly.4 Moreover, IAPs can block the execution phase of apoptosis through direct inhibition of the effector caspase-3 and/or caspase-7.5 In addition, IAPs can prevent initiation of the intrinsic caspase activation cascade by directly inhibiting the apical caspase-9. Finally, high-level expression of XIAP, such as that found in CLL,6-8 can inhibit CD95-mediated apoptosis of cells that express CD95.9
Conversely, the latent sensitivity of CLL cells to CD95-mediated apoptosis following CD40 ligation may be due to release of intrinsic inhibitors to the IAPs that are sequestered within the mitochondria. We found that CLL cells cocultured with CD154-bearing cells are induced to express a proapoptotic protein called the B-cell leukemia 2 homology 3 (BH3)-interacting domain death agonist (Bid).2,10 Expression of Bid is observed within 24 hours following CD40 activation and increases over time, reaching maximum levels within 3 to 5 days.2 In different cell lines, it has been shown that Bid is degraded following ligation of extrinsic death receptors, such as CD95 or DR5, thereby generating a small truncated Bid (tBid) that rapidly trafficks to the mitochondria where it can trigger the release of the second mitochondria-derived activator of caspases (Smac), a potent natural IAP inhibitor that also is referred to as the direct IAP-binding protein with low isoelectric point (pI) (DIABLO).11-14 Conceivably, inhibition of IAPs by Smac/DIABLO could allow the CLL cells to become sensitive to apoptosis triggered by ligation of the extrinsic death receptors that are induced on CLL cells following CD40 ligation. As such, we hypothesized that exogenous inhibitors of IAPs also may enhance CD95-mediated apoptosis of CD40-activated CLL cells.
Studies using mixture-based combinatorial libraries identified polyphenylureas that selectively target the baculoviral IAP repeat (BIR2) domain of XIAP and that do not compete for the Smac/DIABLO binding site in BIR2.15,16 These compounds dissociate effector caspase-3 from XIAP and restore caspase-3 activity. Active phenylurea-based compounds induced apoptosis in a variety of different tumor cells, including CLL cells, in a dose-dependent manner, which was associated with activation of cellular caspases.15,16 On the other hand, normal cells, including blood mononuclear cells, were significantly less sensitive than tumor cells to these compounds.15
Structural activity studies have defined analogs of the original polyphenylureas that have improved druglike characteristics (eg, improved solubility, increased stability, and lower molecular weight) while maintaining comparable activity in inhibiting XIAP. We examined whether an active (1540-14) or an inactive (1540-20) structural analog of such compounds could enhance the sensitivity of CD40-activated CLL cells to CD95-mediated apoptosis.
Patients, materials, and methods
Caspase derepression assay
The caspase derepression assay was performed as recently described.15 Briefly, GST-XIAP (46 nM) or GST-BIR2 (1.2 nM) was added to active caspase-3-His6 (0.36 nM) in 100 μL of 50-mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid pH 7.4), 10% sucrose, 1 mM EDTA (ethylenediaminetetraacetic acid), 0.1% CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonic acid), 100 mM NaCl, and 10 mM dithiothreitol (DTT) to achieve approximately 75% inhibition of protease activity. Recombinant GST-XIAP (50 nM) was added to active (ΛCard) caspase-9 E306A (2.2 nM). Activity of caspases-3 and -9 was measured by monitoring cleavage of the fluorogenic tetrapeptide substrates acetyl-Asp-Glu-Val-Asp-AFC and acetyl-Leu-Glu-His-Asp-AFC, respectively (BIOMOL, Plymouth, PA) at 100 M. Generation of fluorogenic AFC (7-amino-4-trifluoromethyl coumarin) product was measured with a spectrofluorometric plate reader in kinetic mode for 30 minutes at 37°C, using excitation and emission wavelengths of 405 nm and 510 nm, respectively.
Cells
Blood was obtained from patients with CLL upon written informed consent, as approved by the institutional review board of the University of California, San Diego. The mononuclear cells were isolated by density centrifugation over Ficoll-Paque (Amersham Biosciences, Piscataway, NJ) and viably frozen in fetal bovine serum (FBS; Omega Scientific, Tarzana, CA) containing 10% dimethylsulfoxide (DMSO; Sigma, St Louis, MO). Samples used in this study contained 97.2% ± 2.8% CD5+/CD19+ cells. A CD154-expressing HeLa cell line was generated by electroporation of an expression vector containing the full-length human CD154, followed by selection and cloning by limiting dilution in RPMI containing 1 mg/mL G418 (Mediatech, Herndon, VA).17 Finally, studies were performed on the cryopreserved blood mononuclear cells isolated from patients before and 1 day after infusion of Ad-CD154-transduced autologous CLL cells.1
CD40 activation
CLL cells were cocultured for 24 hours with HeLa or HeLa-CD154 cells in RPMI culture medium (Irvine Scientific, Santa Ana, CA) supplemented with 10% FBS (Gibco/Invitrogen, Carlsbad, CA), 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco, Grand Island, NY) in 5% CO2 at 37°C. After 24 hours, the CLL cells were gently separated from the adherent HeLa cells, replated for 1 hour in 12-well plates to allow contaminating HeLa cells to readhere to the plastic surface, washed, and then cultured alone at 5 × 106 cells/mL for 1 or 3 days prior to the apoptosis assay. Contaminating HeLa cells comprised less than 1% of the harvested cells, as assessed by light microscopy. All time points of CD40 activation refer to days after completion of the 24-hour coculture of the CLL cells with HeLa-CD154 cells. CD40 activation was monitored on CD19+ CLL cells by flow cytometry of cells stained with fluorochrome-conjugated monoclonal antibody (mAb, CD95; BD PharMingen, San Diego, CA). In each case, an isotype control mAb of irrelevant specificity was used to monitor for nonspecific cytophillic antibody binding. To compare expression levels of surface antigens, we calculated the mean fluorescence intensity ratio (MFIR) for each antigen. This is the ratio of mean fluorescence of the cells stained with the fluorochrome-conjugated, antigen-specific mAb, divided by the mean fluorescence intensity of the cells stained with a fluorochrome-conjugated, isotype-control mAb of irrelevant specificity.
Apoptosis assay
1540-14 and 1540-20 were dissolved as a 1-mg/mL stock solution in 10% DMSO and stored at -80°C. CD40-activated CLL target cells were brought in culture with different concentrations (4 μM, 5 μM, 10 μM, 15 μM) of the active compound 1540-14 or the inactive compound 1540-20, as indicated. For the induction of CD95-mediated apoptosis, anti-FAS mAb clone CH11 (MBL, Woburn, MA) in a final concentration of 1 μg/mL was added to the culture medium. Some samples were incubated with the pan-caspase inhibitor N-carbobenzoxy-Val-Ala-Asp fluoromethyl ketone (zVAD-fmk; Calbiochem, San Diego, CA). The zVAD-fmk was dissolved as a 50-mM stock solution in DMSO and stored at -20°C. After 24 hours, the cells were stained with 40 nM 3,3′ dihexyloxacarbocyanine iodide (DiOC6; Molecular Probes, Eugene, OR) and propidium iodide (PI; Molecular Probes) for 15 minutes at 37°C and analyzed using a fluorescence-activated cell sorter (FACS) Calibur (Becton Dickinson, San Jose, CA), as previously described.18 For some experiments, we also stained cells for annexin-V (BD, Palo Alto, CA). Data were obtained only from samples that had more than 60% viability after 24 hours of culture in medium alone. Data of individual samples are presented as the relative viability, which is the viability of the treated cell population relative to that of the medium control cell population. To compare the activity of the compounds in different patient samples, data are presented as the “% specific apoptosis,” which is calculated using the following formula: [(% viability of the cells in the control group - % viability of the cells test group)/% viability of the cells in the control group] × 100.
Caspase-3 assay
To measure caspase activity, the cells were cultured as described in “Apoptosis assay.” At various time points, the cells were harvested and lysed. Caspase-3 activity was detected by the addition of the cell-permeable DEVD-fmk substrate to 10 μg of the total protein extract. The assays were performed by measuring the release of fluorescent dye, using a fMax spectrofluorimeter plate reader (excitation at 485 nm and emission at 538 nm) (Molecular Dynamics, Sunnyvale, CA), as previously described.15 The relative Vmax (maximal velocity) is calculated by dividing the inducible velocity by the basal (medium) velocity.
Immunoblot analysis
CLL cells were cocultured with or without the compound 1540-14, CH11, and/or zVAD-fmk for 6 and 12 hours. For preparation of whole-cell lysates, the cells were collected by centrifugation at 250g for 10 minutes at 4°C, washed once in ice-cold phosphate-buffered saline (PBS), and lysed with sonification in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris [tris(hydroxymethyl)aminomethane]/HCl, pH 7.4, 1% nonidet P-40, 0.25% Na-Deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 150 mM NaCl, 5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride [PMSF], 10 μg/mL aprotinin, 10 μg/mL leupeptin, and 10 μg/mL pepstatin) for 15 minutes on ice. The lysates were cleared from insoluble debris by centrifugation at 21 000g for 10 minutes at 4°C, and the supernatants were stored at -80°C until examined. Protein lysate (15 μg) was loaded onto each lane of a 5% to 15% gradient SDS-polyacrylamide gel electrophoresis (PAGE) gel (BioRad, Hercules, CA) (for detection of Bid, 30 μg protein lysate was loaded onto each lane of a 5% to 20% gradient SDS-PAGE gel [ISC Bioexpress, Kaysville, UT]). After electrophoresis, the proteins were transferred onto a polyvinylidene fluoride microporous membrane (Millipore, Billerica, MA). Membranes were probed with anti-caspase-8 antibody (Alexis Biochemicals, San Diego, CA), goat anti-mouse immunoglobulin G (IgG)-horseradish peroxidase (HRP; Santa Cruz, Santa Cruz, CA), anti-caspase-3 antibody (Cell Signaling Technology, Beverly, MA), goat anti-rabbit IgG-HRP (Santa Cruz), anti-poly(adenosine diphosphate [ADP]-ribose) polymerase (anti-PARP; BD Pharmingen), goat anti-mouse IgG-HRP, anti-hILP/XIAP (BD Pharmingen), and goat anti-mouse IgG-HRP, or anti-Bid (Cell Signaling Technology) and goat anti-rabbit IgG-HRP. Stripped membranes were probed with anti-β-actin (Sigma) and then goat anti-mouse IgG-HRP to assess the amount of protein present in each sample lane. As a control, lysates of HeLa or HeLa-CD154 cells were loaded onto separate lanes of a gel and then examined for Bid by immunoblot analysis. We found that HeLa and HeLa-CD154 expressed the same amount of Bid (data not shown).
Statistical analysis
A one-way, repeated measure using analysis of variance (ANOVA) with Dunnett correction was performed to compare each drug treatment (0, 5, 10, or 15 micromolar with or without CH11) with CH11 alone. Paired t tests with Bonferroni correction were used to compare individual groups. P less than .05 was considered statistically significant.
Results
Small molecule antagonists of XIAP
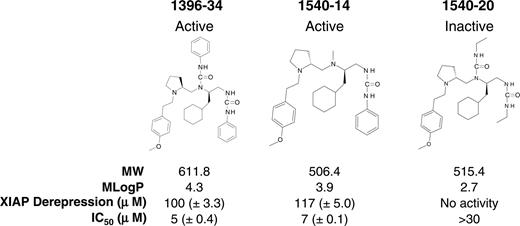
Using mixture-based combinatorial libraries, a series of polyphenylureas has been identified that selectively target the BIR2 domain of XIAP. These compounds stimulated increased caspase activity and induced apoptosis in a variety of tumor cells, including resting CLL cells.15 Structural activity experiments identified second-generation active analogs that had improved druglike characteristics, such as improved solubility as depicted by the Moriguchi logarithmic Partition (MlogP), lower molecular weight, and greater stability. The chemical structures and characteristics of one first-generation active compound (1396-3415 ), a second-generation active compound (1540-14), and an inactive structural analog of 1540-14 (1540-20) are shown in Figure 1. 1540-14 was as active as TPI 1396-34 in dissociating active caspase-3 from XIAP, as depicted by the mean effective concentration (EC50) measured with the caspase-3 derepression assay, and in inducing apoptosis of JURKAT cells, as depicted by the mean inhibitory concentration (IC50). However, the control compound, 1540-20, was inactive (Figure 1). We examined the effects that these second-generation molecules had on CLL cells before and after CD40 ligation.
CD40 stimulation induces CLL cells to increase expression of CD95 but not XIAP
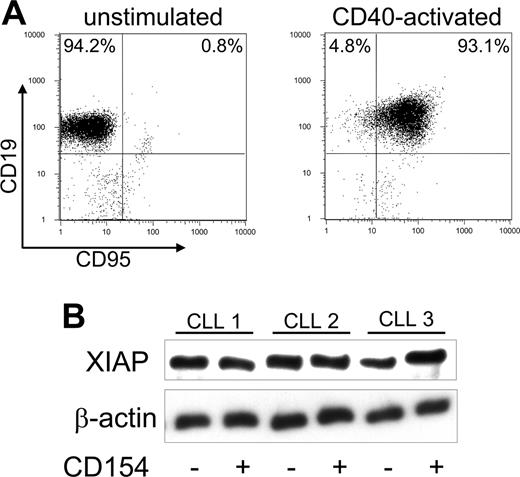
CLL cells were examined for expression of CD95 and XIAP before and 24 hours after CD40 ligation. Consistent with earlier reports,1-3 the expression level of CD95 was low to negligible on each of the samples prior to CD40 activation (Figure 2A). However, after 24 hours of coculture with CD154-expressing cells, more than 90% of CLL cells expressed CD95 (Figure 2A). On the other hand, XIAP was detectable in lysates prepared from resting CLL cells (Figure 2B), as noted in prior studies.6,8 The expression level of XIAP in CLL B cells did not detectably change following CD40 activation (Figure 2B).10,19
Chemical structure and characteristics of early generation active small molecule XIAP antagonistic compound (1396-34) and new generation active (1540-14) and inactive (1540-20) structural analog. The chemical structures of polyphenylurea compounds are provided. The 2 compounds that can inhibit XIAP (namely 1396-34 and 1540-14) are labeled as “Active,” while the third compound (1540-20) has no inhibitory activity and is labeled as “Inactive” at the top of the figure. Below each chemical structure is the name of the compound, the molecular weight (MW), the solubility (MLogP), the μM EC50 (± SE) observed in the XIAP derepression assay, and the μM IC50 (± SE) defined in the JURKAT cytotoxicity assay, as labeled at the bottom left-hand corner of the figure. For 1540-20, no activity in the XIAP derepression assay could be detected at the maximum concentration used (eg, 194 μM).
Chemical structure and characteristics of early generation active small molecule XIAP antagonistic compound (1396-34) and new generation active (1540-14) and inactive (1540-20) structural analog. The chemical structures of polyphenylurea compounds are provided. The 2 compounds that can inhibit XIAP (namely 1396-34 and 1540-14) are labeled as “Active,” while the third compound (1540-20) has no inhibitory activity and is labeled as “Inactive” at the top of the figure. Below each chemical structure is the name of the compound, the molecular weight (MW), the solubility (MLogP), the μM EC50 (± SE) observed in the XIAP derepression assay, and the μM IC50 (± SE) defined in the JURKAT cytotoxicity assay, as labeled at the bottom left-hand corner of the figure. For 1540-20, no activity in the XIAP derepression assay could be detected at the maximum concentration used (eg, 194 μM).
CLL cells of patients 1 day after infusion of Ad-CD154-transduced autologous CLL cells become sensitive to CD95-mediated apoptosis following treatment with 1540-14
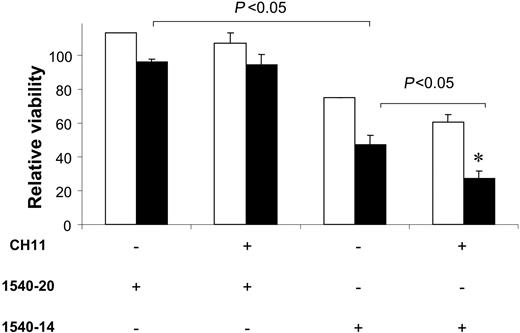
We examined the CLL cells isolated from patients before and 1 day after Ad-CD154 gene therapy for sensitivity to agonistic anti-CD95 antibody (CH11), with or without 1540-14. Despite having high-level expression of CD95 on the day after Ad-CD154 gene therapy, the CLL cells were not sensitive to CD95-mediated apoptosis (data not shown). However, such cells were sensitive to the cytotoxic effects of 1540-14, but not the control compound, at concentrations of 4 μM or higher, as noted from the decrease in viability of 1540-14-treated CLL cells relative to that of control-treated CLL cells (relative viability). However, the CLL cells isolated from patients prior to gene therapy were significantly less sensitive to 1540-14. The mean relative viability of the CLL cells from patients prior to gene therapy was 75% ± 0.3% (SD, n = 2) after treatment for 24 hours in vitro with 1540-14 at 4 μM. On the other hand, the relative viability of similarly treated CLL cells collected on the day after Ad-CD154 gene therapy was 47% ± 5% (SD, n = 2) (Figure 3). Adding CH11 to the 1540-14-treated CLL cells induced apoptosis in the CLL cells of patients after Ad-CD154 gene therapy (P < .05) (Figure 3). The mean relative viability of 1540-14-treated CLL cells after gene therapy was 24.4% ± 4.1% following treatment with CH11. These data indicate that 1540-14 can enhance the sensitivity of CD40-activated CLL cells to CD95-mediated apoptosis.
Expression of CD95 and XIAP before and after CD40 ligation on CLL B cells. (A) Surface expression of death receptors on CD19+ CLL cells was assessed by flow cytometry 24 hours after coculture with HeLa cells (control, left panel) or HeLa-CD154 cells (CD40 activated, right panel). The dot plots depict the fluorescence of CLL cells stained with a fluorescein isothiocyanate (FITC) fluorochrome-conjugated anti-CD95 mAb and a phycoerythrin (PE)-labeled fluorochrome-conjugated anti-CD19 mAb. The numbers in the upper quadrants represent the percentage of CD19+/CD95- cells (left) and the percentage of CD19+/CD95+ cells (right). (B) Immunoblot analyses were performed on the CLL cell samples of 3 different patients (CLL 1, CLL 2, and CLL 3) as indicated at the top of the figure. Prior to the preparation of the cell lysates, the CLL cells were cultured for 24 hours with HeLa cells (-) or HeLa-CD154 cells (+), as indicated by the symbols at the bottom of the figure. Cell lysate (15 μg) prepared from each sample was applied to each lane of a polyacrylamide gel for immunoblot analyses with anti-XIAP (top row) or anti-β-actin (bottom row), as indicated on the left.
Expression of CD95 and XIAP before and after CD40 ligation on CLL B cells. (A) Surface expression of death receptors on CD19+ CLL cells was assessed by flow cytometry 24 hours after coculture with HeLa cells (control, left panel) or HeLa-CD154 cells (CD40 activated, right panel). The dot plots depict the fluorescence of CLL cells stained with a fluorescein isothiocyanate (FITC) fluorochrome-conjugated anti-CD95 mAb and a phycoerythrin (PE)-labeled fluorochrome-conjugated anti-CD19 mAb. The numbers in the upper quadrants represent the percentage of CD19+/CD95- cells (left) and the percentage of CD19+/CD95+ cells (right). (B) Immunoblot analyses were performed on the CLL cell samples of 3 different patients (CLL 1, CLL 2, and CLL 3) as indicated at the top of the figure. Prior to the preparation of the cell lysates, the CLL cells were cultured for 24 hours with HeLa cells (-) or HeLa-CD154 cells (+), as indicated by the symbols at the bottom of the figure. Cell lysate (15 μg) prepared from each sample was applied to each lane of a polyacrylamide gel for immunoblot analyses with anti-XIAP (top row) or anti-β-actin (bottom row), as indicated on the left.
1540-14 sensitizes CD40-activated CLL B cells to CD95-mediated apoptosis
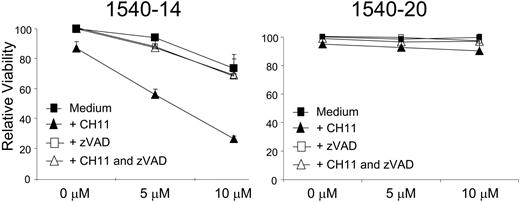
We examined the effects of 1540-14 on CLL cells that were activated in vitro through coculture with CD154-bearing cells. Again, the induction of apoptosis was deduced from the decrease in viability observed in the treated CLL cells relative to that of control-treated CLL cells. Consistent with earlier reports,3 CLL cells activated 1 day earlier via coculture with CD154-bearing cells were resistant to CD95-mediated apoptosis (Figure 4; 0 μM). However, when 1540-14 was added to the cultures, we observed that such CLL cells became sensitive to CH11-mediated apoptosis. Moreover, we found that CD95-ligation and the XIAP inhibitor acted synergistically to induce apoptosis of CD40-activated CLL cells (Figure 4; closed triangles). Addition of zVAD-fmk (at 50 μM) significantly inhibited the loss of CLL cell viability observed following treatment with the XIAP inhibitor or an agonistic anti-CD95 antibody (Figure 4, open squares and triangles). As a control, the same experiment was done using the nonactive structural analog 1540-20. As expected, no effect on CD95-mediated apoptosis was observed (Figure 4). The relative viability of CLL cells treated with CH11, zVAD-fmk, or medium alone is depicted in Figure 4 (0 μM).
Treatment with 1540-14 renders CLL cells of Ad-CD154-treated patients sensitive to CD95-mediated apoptosis. The bars represent the data obtained from CLL blood samples obtained before (□) or 24 hours after (▪) infusion of autologous Ad-CD154 CLL cells. After isolation, the CLL cells were cultured in one of the following conditions: (1) medium alone; (2) medium with CH11; (3) 1540-20 at 4 μM; (4) 1540-14 at 4 μM; (5) CH11 and 4 μM 1540-20; or (6) CH11 and 4 μM 1540-14. After 24 hours, the proportions of viable CLL cells were assessed by flow cytometry. Data are presented as the relative viability, which represents the proportion of viable CLL cells in the treatment wells relative to that of CLL cells cultured in medium alone. The viability of the CLL cells in the cultures containing only medium was always in excess of 80%. Addition of CH11 alone did not reduce the viability of pretherapy or posttherapy CLL cells relative to that of control cultures (data not shown). The error bars indicate the standard error about the mean values obtained from duplicate wells. Similar results were obtained in 2 independent experiments. The brackets with P < .05 indicate that there is a significant difference between (1) the relative viability of CLL cells obtained after Ad-CD154 gene therapy when treated with 1540-14 relative to that of such cells when treated with 1540-20, or (2) the relative viability of CLL cells obtained after Ad-CD154 gene therapy when treated with both CH11 and 1540-14 relative to that of such cells when treated with 1540-14 alone. An asterisk (*) indicates that the CLL cells obtained after Ad-CD154 gene therapy had a significantly lower relative viability than that of CLL cells obtained prior to therapy when treated with CH11 and 1540-14.
Treatment with 1540-14 renders CLL cells of Ad-CD154-treated patients sensitive to CD95-mediated apoptosis. The bars represent the data obtained from CLL blood samples obtained before (□) or 24 hours after (▪) infusion of autologous Ad-CD154 CLL cells. After isolation, the CLL cells were cultured in one of the following conditions: (1) medium alone; (2) medium with CH11; (3) 1540-20 at 4 μM; (4) 1540-14 at 4 μM; (5) CH11 and 4 μM 1540-20; or (6) CH11 and 4 μM 1540-14. After 24 hours, the proportions of viable CLL cells were assessed by flow cytometry. Data are presented as the relative viability, which represents the proportion of viable CLL cells in the treatment wells relative to that of CLL cells cultured in medium alone. The viability of the CLL cells in the cultures containing only medium was always in excess of 80%. Addition of CH11 alone did not reduce the viability of pretherapy or posttherapy CLL cells relative to that of control cultures (data not shown). The error bars indicate the standard error about the mean values obtained from duplicate wells. Similar results were obtained in 2 independent experiments. The brackets with P < .05 indicate that there is a significant difference between (1) the relative viability of CLL cells obtained after Ad-CD154 gene therapy when treated with 1540-14 relative to that of such cells when treated with 1540-20, or (2) the relative viability of CLL cells obtained after Ad-CD154 gene therapy when treated with both CH11 and 1540-14 relative to that of such cells when treated with 1540-14 alone. An asterisk (*) indicates that the CLL cells obtained after Ad-CD154 gene therapy had a significantly lower relative viability than that of CLL cells obtained prior to therapy when treated with CH11 and 1540-14.
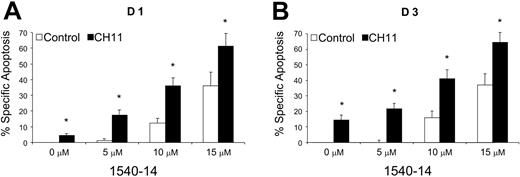
We tested the activity of 1540-14 on CLL cells of different patients (n = 16) who had been stimulated 1 day by coculture with CD154-bearing cells. As shown in Figure 5A, the CD40-activated CLL cells were not sensitive to CD95-mediated apoptosis. Moreover, when cultured alone with CH11, the mean percentage of specific apoptosis was 4.4% ± 1.4%. However, addition of 1540-14 to the cultures at 5 μM, 10 μM, or 15 μM significantly enhanced the levels of apoptosis achieved with CH11 to 17.3% ± 3.4%, 36.1% ± 5.0%, or 61.4% ± 7.9%, respectively. In contrast, without CH11, the levels of CLL cell apoptosis observed with 1540-14 at a concentration of 5 μM, 10 μM, or 15 μM were 1.1% ± 1.2%, 12.2% ± 3.1%, or 36.0% ± 5.0%, respectively (Figure 5A), revealing the synergistic activity between CH11 and the 1540-14 compound. In contrast, the control compound did not enhance the levels of apoptosis that could be achieved with CH11. Similar results were noted in assays measuring for binding of annexin-V to CLL cells in the various treatment groups (data not shown).
Effect of XIAP inhibitors on the sensitivity of CD40-activated CLL cells to CD95-mediated apoptosis. CLL cells were activated via coculture with CD154-bearing cells and then examined 24 hours later for sensitivity to CD95-mediated apoptosis (depicted as decrease in viability, measured by DiOC6 staining). These cells were treated with 1540-14 (left panel) or its inactive structural analog, 1540-20 (right panel), at various concentrations as indicated at the bottom of each panel. The viability of the treated CLL cells relative to that of CLL cells cultured in medium alone is indicated on the y-axis. In each experiment, the viability of CLL cells cultured in medium alone exceeded 80%. Depicted are the relative viability measurements of CLL cells cultured with either compound alone (▪), either compound with zVAD-fmk (□), either compound plus CH11 (▴), or either compound with both CH11 and zVAD-fmk (▵). Relative viability of treatment with CH11, zVAD-fmk, and medium alone is shown at 0 μM. The errors indicate the SE about the mean of triplicate wells.
Effect of XIAP inhibitors on the sensitivity of CD40-activated CLL cells to CD95-mediated apoptosis. CLL cells were activated via coculture with CD154-bearing cells and then examined 24 hours later for sensitivity to CD95-mediated apoptosis (depicted as decrease in viability, measured by DiOC6 staining). These cells were treated with 1540-14 (left panel) or its inactive structural analog, 1540-20 (right panel), at various concentrations as indicated at the bottom of each panel. The viability of the treated CLL cells relative to that of CLL cells cultured in medium alone is indicated on the y-axis. In each experiment, the viability of CLL cells cultured in medium alone exceeded 80%. Depicted are the relative viability measurements of CLL cells cultured with either compound alone (▪), either compound with zVAD-fmk (□), either compound plus CH11 (▴), or either compound with both CH11 and zVAD-fmk (▵). Relative viability of treatment with CH11, zVAD-fmk, and medium alone is shown at 0 μM. The errors indicate the SE about the mean of triplicate wells.
Treatment of CD40-activated CLL B cells with 1540-14 enhances sensitivity to CD95-mediated apoptosis
At 3 to 5 days after CD40 activation, CLL cells become increasingly sensitive to CD95-mediated apoptosis.2,3 For example, CLL cells that initially were resistant to CD95-mediated apoptosis could be induced to undergo apoptosis 3 days after activation by CD40 ligation upon incubation with CH11 (n = 13; mean percentage specific apoptosis, 14.6% ± 2.7%; Figure 5B). Addition of the 1540-14 at 5 μM did not further augment the killing of the CLL cells by CH11 using target cells that had been activated 3 days earlier by coculture with CD154-expressing cells (Figure 5B). However, 1540-14 did enhance the CH11-mediated killing of such CD40-activated CLL cells at higher concentrations (Figure 5B).
Synergistic effect of XIAP inhibitors on CD95-mediated apoptosis. (A) CLL cells of 16 patients were activated via CD40 ligation and examined 1 day later for sensitivity to CD95-mediated apoptosis. The activated CLL cells were treated with either medium alone, CH11, different concentrations of 1540-14, or CH11 and 1540-14 at various concentrations. After 24 hours, the relative percent number of cells undergoing apoptosis was measured by flow cytometry. The data are expressed as percent specific apoptosis as defined in “Patients, materials, and methods.” The differences in the level of apoptosis between cells treated with CH11 alone versus that of cells treated with CH11 and 1540-14 was statistically significant at all concentrations. An asterisk indicates that the level of apoptosis achieved with CH11 and 1540-14 was significantly greater than that achieved with 1540-14 alone at the indicated concentration. (B) CLL cells of 13 patients were activated via CD40 ligation and examined 3 days later for sensitivity to CH11. The differences in the level of apoptosis between treatment with CH11 alone and treatment with CH11 and 1540-14 were statistically significant only at 10 μM and 15 μM (P < .05). An asterisk indicates that the level of apoptosis achieved with CH11 and 1540-14 was significantly greater than that achieved with 1540-14 alone at the indicated concentration of 1540-14. Error bars indicate SD about the mean.
Synergistic effect of XIAP inhibitors on CD95-mediated apoptosis. (A) CLL cells of 16 patients were activated via CD40 ligation and examined 1 day later for sensitivity to CD95-mediated apoptosis. The activated CLL cells were treated with either medium alone, CH11, different concentrations of 1540-14, or CH11 and 1540-14 at various concentrations. After 24 hours, the relative percent number of cells undergoing apoptosis was measured by flow cytometry. The data are expressed as percent specific apoptosis as defined in “Patients, materials, and methods.” The differences in the level of apoptosis between cells treated with CH11 alone versus that of cells treated with CH11 and 1540-14 was statistically significant at all concentrations. An asterisk indicates that the level of apoptosis achieved with CH11 and 1540-14 was significantly greater than that achieved with 1540-14 alone at the indicated concentration. (B) CLL cells of 13 patients were activated via CD40 ligation and examined 3 days later for sensitivity to CH11. The differences in the level of apoptosis between treatment with CH11 alone and treatment with CH11 and 1540-14 were statistically significant only at 10 μM and 15 μM (P < .05). An asterisk indicates that the level of apoptosis achieved with CH11 and 1540-14 was significantly greater than that achieved with 1540-14 alone at the indicated concentration of 1540-14. Error bars indicate SD about the mean.
Expression of Bid following CD40 activation
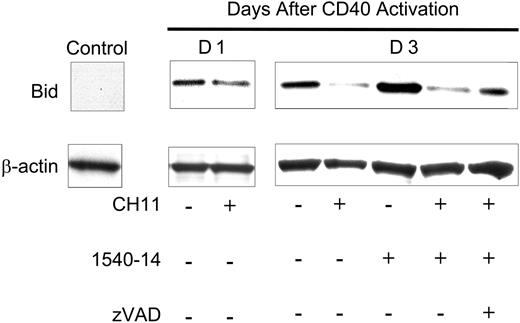
A key event in the latent sensitivity to Fas-mediated apoptosis could be the degradation of Bid into tBid, which links the death-receptor apoptotic pathway to the mitochondrial apoptotic pathway.11-14 CLL cells ordinarily lack expression of Bid. However, CD40 ligation induces CLL cells to express this proapoptotic factor.2,10 Low-level expression of Bid protein was detected in lysates prepared from CLL cells 1 day after CD40 activation (Figure 6). The levels of Bid increased in CLL cells at later time points after CD40 ligation both in vitro (Figure 6) and in vivo.2 CD95 ligation induced degradation of Bid, which in other studies has been associated with the formation of tBid.20,21 Consistent with this, the noted degradation of full-length Bid was inhibited by addition of zVAD-fmk to the cultures (Figure 6). Also, treatment of the CLL cells with 1540-14 alone did not result in Bid degradation (Figure 6).
Expression and cleavage of Bid in CD40-activated CLL cells. CLL cells were activated via coculture with HeLa cells (Control) or HeLa-CD154 cells and then isolated for further study. Twenty-four hours later, the CLL cells were treated for 12 hours with medium or CH11. Seventy-two hours later, the CLL cells were treated for 12 hours with medium, CH11, 1540-14, and/or zVAD-fmk, as indicated at the bottom of the figure. Protein lysates were prepared and normalized for total protein content. Cell lysate (30 μg) was applied to each lane of a polyacrylamide gel for immunoblot analyses with anti-Bid (top row) or anti-β-actin (bottom row), as indicated on the left side of the figure.
Expression and cleavage of Bid in CD40-activated CLL cells. CLL cells were activated via coculture with HeLa cells (Control) or HeLa-CD154 cells and then isolated for further study. Twenty-four hours later, the CLL cells were treated for 12 hours with medium or CH11. Seventy-two hours later, the CLL cells were treated for 12 hours with medium, CH11, 1540-14, and/or zVAD-fmk, as indicated at the bottom of the figure. Protein lysates were prepared and normalized for total protein content. Cell lysate (30 μg) was applied to each lane of a polyacrylamide gel for immunoblot analyses with anti-Bid (top row) or anti-β-actin (bottom row), as indicated on the left side of the figure.
Activation of caspases-3 and -8 and cleavage of PARP
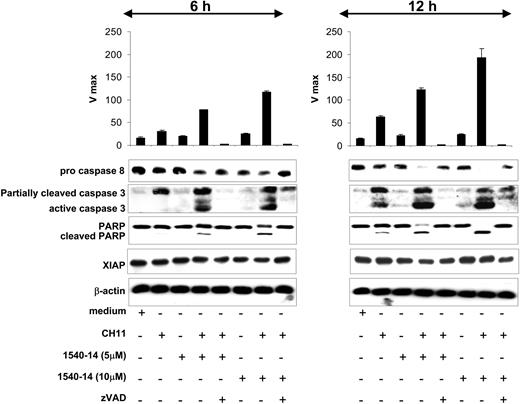
We examined the effect on CD95-mediated apoptosis by 1540-14 or the control compound on CLL cells 1 day after CD40 activation. Caspase-3 activity was measured at 2 time points (Figure 7). This demonstrated that both 1540-14 at 10 μM and ligation of CD95 induced only moderate activation of caspase-3. However, high-level activation of caspase-3 was achieved following CD95 ligation in the presence of 1540-14 both at 5 μM and 10 μM. This activation of caspase-3 could be inhibited by zVAD-fmk (Figure 7).
Activation of caspases-3 and -8 and cleavage of PARP in CD40-activated CLL cells. CLL cells were activated via CD40 ligation. Twenty-four hours later, these cells were treated without or with 1540-14 at 2 different concentrations and/or CH11, either alone or in combination, as indicated at the bottom of the figure. Incubation with the combination was done with or without the nonspecific caspase antagonist zVAD-fmk. After either 6 or 12 hours, the CLL cells were harvested and used to prepare lysates for immunoblot analysis. (Upper panel) Caspase-3 activity was detected by the addition of the DEVD substrate to 10 μg total protein lysates. The assay was performed measuring the release of fluorescent dye using a fMax spectrofluorometer plate reader (excitation at 485 nm and emission at 538 nm). Mean data are presented of 3 independent experiments ± SE. Y-axis represents the Vmax. (Lower panel) Protein lysates were prepared and normalized for total protein content. Cell lysate (15 μg) was applied to each lane of a polyacrylamide gel for immunoblot analyses with anti-caspase-8 (first row), anti-caspase-3 (second row), anti-PARP (third row), anti-XIAP (fourth row), or anti-β-actin (fifth row), as indicated on the left margin of the figure.
Activation of caspases-3 and -8 and cleavage of PARP in CD40-activated CLL cells. CLL cells were activated via CD40 ligation. Twenty-four hours later, these cells were treated without or with 1540-14 at 2 different concentrations and/or CH11, either alone or in combination, as indicated at the bottom of the figure. Incubation with the combination was done with or without the nonspecific caspase antagonist zVAD-fmk. After either 6 or 12 hours, the CLL cells were harvested and used to prepare lysates for immunoblot analysis. (Upper panel) Caspase-3 activity was detected by the addition of the DEVD substrate to 10 μg total protein lysates. The assay was performed measuring the release of fluorescent dye using a fMax spectrofluorometer plate reader (excitation at 485 nm and emission at 538 nm). Mean data are presented of 3 independent experiments ± SE. Y-axis represents the Vmax. (Lower panel) Protein lysates were prepared and normalized for total protein content. Cell lysate (15 μg) was applied to each lane of a polyacrylamide gel for immunoblot analyses with anti-caspase-8 (first row), anti-caspase-3 (second row), anti-PARP (third row), anti-XIAP (fourth row), or anti-β-actin (fifth row), as indicated on the left margin of the figure.
We also examined for cleavage of caspase-8, caspase-3, and PARP at 6 and 12 hours after treatment. Treatment of CD40-activated CLL cells for 6 hours with CH11 together with 1540-14 resulted in generation of active caspase-3, cleavage of PARP, and reduction of pro-caspase-8, consistent with its processing to caspase-8 (Figure 7). After 12 hours, the treated CLL cells had even greater activation of caspases-3 and -8 and more extensive PARP cleavage. However, treatment of CD40-activated CLL cells with CH11 generated only slight processing of pro-caspase-3 and failed to induce cleavage of PARP or processing of pro-caspase-8 (Figure 7). After 12-hour treatment with CH11, the CD40-activated CLL cells had only modest processing of pro-caspases-3 and -8 and moderate-to-negligible levels of cleaved PARP. The levels of XIAP remained unchanged after any of these conditions, indicating that treatment with 1540-14 did not induce degradation of XIAP. Treatment with the nonspecific caspase inhibitor zVAD-fmk prevented the observed cleavage of caspase-8, caspase-3, and PARP in all cases (Figure 7).
Discussion
Patients with CLL who received intravenous infusions of autologous leukemia cells transfected with an adenovirus vector encoding the CD40 ligand (Ad-CD154) experienced rapid reductions in leukemia cell counts and lymph node size.1 Subsequent studies found that the killing of CD40-activated CLL cells by autologous CD4 cytotoxic T cells was CD95 dependent.3 In the same study, it was found that CLL cells became increasingly sensitive to CD95-mediated apoptosis following CD40 ligation, despite initial resistance to apoptosis triggered by these extrinsic death-receptor pathways.3 Improved knowledge of the influence of CD40 ligation on the death-receptor pathway could lead to strategies that further enhance the effectiveness of CD40 ligand gene therapy.
Like many other tumors, CLL cells express high levels of the IAP family member XIAP.6-8 Both antisense and peptide inhibitor studies have demonstrated that XIAP plays an important role in tumor survival, allowing CLL cells to resist apoptosis induced by γ-radiation or anticancer drugs,22-24 and potentially also by ligation of extrinsic death receptors.25-27
We examined whether analogs of recently described phenylurea-based compounds could sensitize newly CD40-activated CLL cells to CD95-mediated apoptosis.15 These compounds dissociate XIAP from the effector caspase-3, thereby restoring caspase-3 activity. Of importance, these compounds do not compete with the natural XIAP inhibitor Smac in restoring caspase activity.15 We confirmed that CLL cells in the early time period after CD40 activation were resistant to CD95-mediated apoptosis (Figures 3, 4, and 5A). However, the CLL cells became increasingly sensitive to CD95-mediated apoptosis when treated with the XIAP inhibitor in a dose-dependent fashion. This effect was not observed when caspase activity was blocked by zVAD-fmk (Figure 4).
CLL cells isolated from patients 1 day after infusion of autologous CD154-transduced CLL cells were more sensitive to 1540-14 than CLL cells isolated from the same patients prior to gene therapy. The CLL cells of treated patients may have encountered cells bearing CD178 (FasL) and/or TRAIL in vivo, allowing them to become primed for apoptosis. Upon inhibition of XIAP in vitro, such cells may spontaneously undergo apoptosis without requiring additional CD95 ligation in vitro. Addition of an agonistic anti-CD95 antibody under these conditions further enhanced the killing of such leukemia cells.
In contrast to newly activated CLL cells, CLL cells that had been activated 3 days earlier via CD40 ligation were sensitive to CD95-mediated apoptosis (Figure 5B). Conceivably, increased expression of Bid at later time points following CD40 activation renders CLL cells sensitive to CD95-mediated apoptosis, partly by allowing for Smac-dependent inhibition of XIAP.11,12,14 In this study, we confirmed that Bid is not detectable in resting CLL cells. Following CD40 activation, expression of Bid increases over time and is present at high levels in CLL cells 3 days after CD40 activation (Figure 6). Ligation of CD95 or DR5 on such cells may then result in cleavage of both pro-caspase-8 and Bid, converting the latter into tBid, which in turn associates with Bax and induces mitochondria to release proapoptotic factors such as cytochrome c and Smac/DIABLO (reviewed in Barnhart et al28).29,30 Consistent with this notion, we found that ligation of CD95 on such CLL cells resulted in degradation of full-length Bid (Figure 6), which is required for the formation of tBid.20,21 It is therefore conceivable that degradative processing of Bid to tBid plays a role in sensitizing activated CLL cells to CD95-mediated apoptosis by affecting release of Smac, which in turn can inhibit IAPs.
A recent study using JURKAT T cells found that full-length XIAP not only prevented caspase-3 processing, but also partially inhibited the mitochondria from releasing Smac and cytochrome c.27 These studies support a model proposing that XIAP not only inhibits caspase-3, but also suppresses activation of mitochondria following death-receptor engagement.27 Consistent with this model, immunoblot analyses revealed that CD40-activated CLL cells experienced activation of the initiator caspase, caspase-8, in response to CD95 ligation, resulting in the initial cleavage of pro-caspase-3 to generate a partially processed fragment of caspase-3 (Figure 7). Consistent with this, it is possible to observe formation of the death-inducing signaling complex (DISC) following treatment of CD40-activated CLL cells with anti-CD95 even though such cells have increased levels of the surrogate caspase, FLICE (Fas-associating protein with death domain-like interleukin-1β-converting enzyme)-inhibitory protein (FLIP).3,31 Cotreatment with the XIAP inhibitor 1540-14 resulted in CD95-mediated autocleavage of the partially processed molecule to form an active caspase-3. This resulted not only in PARP cleavage, but also in enhanced processing of pro-caspase-8, creating an amplification loop, as described previously32,33 (Figure 7). As such, compounds that specifically inhibit BIR2 caspase-3 binding, such as the recently described polyphenylurea-based compounds and the active analog, 1540-14, used in this study, may function synergistically with signals that activate CD95 and/or DR5 to effect death-receptor-mediated pathways. This study also provides the first direct evidence that members of the IAP family, most likely XIAP, play a prominent role in the resistance of CLL cells to death-receptor-mediated apoptosis.
Prepublished online as Blood First Edition Paper, May 24, 2005; DOI 10.1182/blood-2005-02-0695.
Supported by grant PO1-CA81534 from the National Institute of Health to the Chronic Lymphocytic Leukemia Research Consortium and by a personal Dutch Cancer foundation and René Vogels foundation grant to A.P.K.
A.P.K. and F.D. contributed equally to this work.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Dr Laura Rassenti, Monica Cook, Lang Huynh, and Traci Toy for their technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal