Abstract
5F11, a fully human monoclonal antibody directed against CD30, effectively induces killing of CD30-expressing lymphoma cell lines in vitro and in animal models. A recently conducted phase 1/2 study shows that 5F11 is well tolerated in heavily pretreated patients with relapsed and refractory CD30+ lymphoma and has some clinical activity. In the present study, we demonstrate that 5F11 activates nuclear factor κB (NF-κB) and the anti-apoptotic protein cellular FLICE (Fas-associating protein with death domain-like interleukin-1β-converting enzyme) inhibitory protein (c-flip) in Hodgkin lymphoma (HD)-derived cell lines, which might cause apoptosis resistance, thus limiting the clinical use of 5F11. To overcome this resistance, we combined 5F11 with the proteasome inhibitor bortezomib, which has been shown to suppress NF-κB activity. This combination revealed a synergistic cytotoxic effect in vitro and in a human HD xenograft model provided that 5F11 precedes bortezomib treatment. We conclude that initial 5F11-mediated NF-κB signaling sensitizes the tumor cells to bortezomib-induced cell death. These data suggest a therapeutic value of this combination for HD patients. (Blood. 2005;106:1839-1842)
Introduction
The CD30 receptor, a member of the tumor necrosis factor superfamily, is selectively overexpressed in the malignant cell population of Hodgkin lymphoma (HD) and anaplastic large-cell lymphoma (ALCL), rendering this antigen an excellent target for antibody-based immunotherapy. However CD30 signaling is pleiotropic since antibody binding may induce cell death in ALCL cells, whereas some CD30+ HD cells respond with growth stimulation.1-3 Recently 2 monoclonal antibodies, the human 5F114 and the humanized SGN-30,5 exhibited cytotoxicity against HD-derived cell lines as well as againstALCL cells, even though limitations in the sensitivity of HD-target cells were observed. Resistance against apoptosis upon CD30 stimulation might be due to the activation of the survival factor nuclear factor κB (NF-κB), a key antiapoptosis factor in HD.6-8 We found that CD30 stimulation via 5F11 activates NF-κB and its target cellular FLICE (Fas-associating protein with death domain-like interleukin-1β-converting enzyme) inhibitory protein (c-flip), which can be inhibited with bortezomib, a proteasome inhibitor known to suppress NF-κB activation. Combination of 5F11 and bortezomib results in cytotoxic synergy both in vitro and in vivo, and we conclude that down-regulation of NF-κB through bortezomib may be a therapeutic approach to improve CD30-based immunotherapy.
Study design
Cell lines, antibodies, reagents, and plasmids
The Hodgkin-derived cell lines (L540 and L428), the ALCL-line Karpas299, and the CD30- acute lymphoblastic leukemia-line REH were described previously.9 The anti-CD30 antibody 5F11 was kindly provided by Medarex (Bloomsbury, NJ). Other reagents used were goat anti-human Fc antibody (Dianova/Jackson, West Grove, PA), the proteasome inhibitor bortezomib (Velcade; Millennium, Cambridge, MA), rabbit anti-p65-immunoglobulin G (IgG) and fluorescein isothiocyanate (FITC)-labeled mouse anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA), anti-B-cell leukemia/lymphoma-2 (bcl-2) and anti-Bcl-2-associated X protein (bax) antibodies (Apoptosis Sampler Kits I and II; BD Bioscience, San Jose, CA), and the anti-c-flip antibody (F-6550; Sigma). NF-κB expression vectors and reporter constructs (NF-κB-luc, IkBαM) were purchased from Clontech (Palo Alto, CA) and BD Biosciences.
XTT assay
Luciferase reporter gene assay
Transfected L428 cells (electroporation efficacy, 5%-10%) were incubated with 5 μg/mL 5F11 plus 25 μg/mL GaH for 2 hours, or with 50 ng/mL bortezomib, 5F11/GaH and bortezomib, or plain medium for 24 hours at 37°C. Luciferase activity was measured as recommended by Promega (Madison, WI).
Electrophoretic mobility shift assay (EMSA)
The nuclear protein extracts (20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.9], 400 mM NaCl, 1 mM EDTA [ethylenediaminetetraacetic acid], and 1 mM EGTA [ethylene glycol tetraacetic acid]) derived from cells treated with different antibodies were incubated with 32P-labeled NF-κB binding site oligonucleotides (Santa Cruz Biotechnology) for 30 minutes at room temperature before electrophoresis on 6% native polyacrylamide gels.
Xenograft model of human HD
The xenograft model of human HD was described previously.4 Severe combined immunodeficient (SCID) mice with established tumors of about 100 mm3 were divided randomly into 4 groups; each group received 100 μg 5F11 intraperitoneally (in 200 μL phosphate-buffered saline [PBS]), 10 ng bortezomib via the tail vein 6 hours later, or each agent alone once weekly (for a total of 4 injections). Control mice received PBS only. The experiment was stopped and mice were killed when the median tumor diameter in the control group exceeded 2500 mm3 (day 50). Two independent experiments were performed.
Results and discussion
5F11-dependent CD30 signaling activates NF-κB and c-flip
The comparison of the subcellular distribution of NF-κB in the untreated Hodgkin cell line L540 (Figure 1A) and after incubation with 5F11/GaH (Figure 1B-C, 6- and 8-hour incubation) shows a nuclear accumulation of NF-κB after treatment, demonstrating its activation in response to CD30 signaling. This activation was inhibited when bortezomib was given 30 minutes after 5F11 stimulation, leading to the exclusion of NF-κB from the cell nucleus (Figure 1 D-E, shown after 6 and 8 hours). Similar results were obtained using L428 cells (data not shown). Accordingly, an NF-κB-responsive luciferase reporter gene was activated in L428 cells after stimulation with 5F11 (Figure 1F). This activation was suppressed in the presence of bortezomib. No luciferase activation was detected upon coexpression of IkBαM, the constitutive active mutant of the NF-κB inhibitor IkB, serving as a control. NF-κB DNA binding (Figure 1G) was detectable in untreated L540 and L428 cells. Exposure to cross-linked 5F11 enhanced DNA binding in both cell lines.
Since a number of factors have been reported to block the apoptotic cascade, we measured the expression level of prosurvival proteins related to NF-κB activation. Most changes were observed for the expression of the caspase inhibitor c-flip, which was up-regulated by NF-κB. C-flip, known to be processed by ubiquitination and proteasomal degradation,11,12 was strongly induced in L540 (Figure 1H) and L428 cells (data not shown) by 5F11 but was down-regulated after coincubation of the cells with bortezomib. For bcl-2 and bax, only minor changes were observed, and the expression levels of tumor necrosis factor receptor-associated death domain (TRADD) and Fas-associated death domain (FADD) remained unaltered (data not shown). Of interest, c-flip is the key regulator of death receptor resistance in Hodgkin/Reed-Sternberg cells, as down-regulation induces autonomous Fas-mediated cell death.13-15
These data provide evidence that resistance to CD30-mediated cell death in HD cells is due to activation of NF-κB.
Combination of 5F11 and bortezomib increases the cytotoxicity against HD- and ALCL-derived cell lines
We subsequently evaluated the cytotoxic potential of 5F11 to overcome apoptosis resistance when combined with bortezomib in XTT viability assays.
Regulation of NF-κB distribution and activity by 5F11 and bortezomib. Detection of the NF-κB subunit p65 with anti-p65 and a secondary FITC-labeled antibody (green) in untreated L540 cells (A), after stimulation with cross-linked 5F11 for 6 and 8 hours (B-C), and after stimulation with bortezomib (D-E). The cell nuclei are stained with 4′6-diamidino-2-phenylindole (DAPI). Imaging medium was Vectashield (Vector Laboratories, Burlingame, CA). Pictures were taken using the digital Nikon Eclipse E800 microscope with the LuciaG/F program (Nikon, Düsseldorf, Germany). (A) 10 ×/0.17 NA objective; (B-E) 40 ×/0.11-0.23 NA objective. (F) L428 cells were transfected with either reporter construct pCMV-luc (transfection control) or pNF-κB-luc (bars 2-4) or pNF-κB-luc together with the expression vector IkBαM (bars 5-7) and exposed to PBS (basal), 5F11, or bortezomib for 24 hours. The luciferase activity (relative light units [RLU]) of the cell lysates is indicated. Error bars indicate mean and SD of 2 independent experiments. (G) EMSA to detect DNA binding of NF-κB in nuclear extracts of L540 and L428 cells after 6-hour incubation with no antibody, cross-linked 5F11, and cross-linked Ber-H2 (BH2) antibody. Ber-H2 as a control anti-CD30 antibody is unable to mediate CD30 signaling10 and fails to activate NF-κB. (H) Western blotting of whole L540 cell extracts to detect c-flip, bcl-2, and bax in untreated cells after 16-hour incubation (n.t. indicates no treatment) and following treatment with either 5F11 or 5F11 + bortezomib after 6-, 8-, and 16-hour incubation.
Regulation of NF-κB distribution and activity by 5F11 and bortezomib. Detection of the NF-κB subunit p65 with anti-p65 and a secondary FITC-labeled antibody (green) in untreated L540 cells (A), after stimulation with cross-linked 5F11 for 6 and 8 hours (B-C), and after stimulation with bortezomib (D-E). The cell nuclei are stained with 4′6-diamidino-2-phenylindole (DAPI). Imaging medium was Vectashield (Vector Laboratories, Burlingame, CA). Pictures were taken using the digital Nikon Eclipse E800 microscope with the LuciaG/F program (Nikon, Düsseldorf, Germany). (A) 10 ×/0.17 NA objective; (B-E) 40 ×/0.11-0.23 NA objective. (F) L428 cells were transfected with either reporter construct pCMV-luc (transfection control) or pNF-κB-luc (bars 2-4) or pNF-κB-luc together with the expression vector IkBαM (bars 5-7) and exposed to PBS (basal), 5F11, or bortezomib for 24 hours. The luciferase activity (relative light units [RLU]) of the cell lysates is indicated. Error bars indicate mean and SD of 2 independent experiments. (G) EMSA to detect DNA binding of NF-κB in nuclear extracts of L540 and L428 cells after 6-hour incubation with no antibody, cross-linked 5F11, and cross-linked Ber-H2 (BH2) antibody. Ber-H2 as a control anti-CD30 antibody is unable to mediate CD30 signaling10 and fails to activate NF-κB. (H) Western blotting of whole L540 cell extracts to detect c-flip, bcl-2, and bax in untreated cells after 16-hour incubation (n.t. indicates no treatment) and following treatment with either 5F11 or 5F11 + bortezomib after 6-, 8-, and 16-hour incubation.
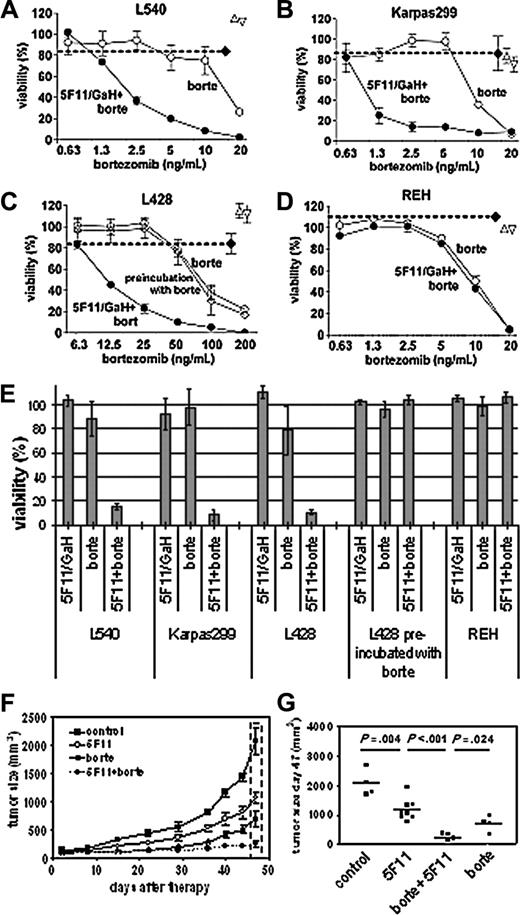
The cell lines tested were sensitive to bortezomib (median inhibitory concentration [IC50]: 15 ng/mL for L540 cells, 10 ng/mL for Karpas299, and 75 ng/mL for L428 cells). Figure 2A-D presents cell survival upon exposure to subtoxic concentrations of 5F11 and increasing concentrations of bortezomib. Combining these 2 reagents resulted in enhanced cell death for L540 and Karpas299 and interestingly also for L428 cells, which are resistant to 5F11 even at higher antibody concentrations.4 No effect was seen with the lymphoblastic leukemia line REH lacking CD30 expression. The preincubation with 5F11 is essential for this synergy, since preincubation with bortezomib for 30 minutes prior to antibody addition had no effect (shown for L428). A similar mechanism was recently reported for leukemic stem cells pretreated with NF-κB-activating anthracyclines before exposure to the proteasomes inhibitor MG132.16
In vivo activity of 5F11 and bortezomib in a human Hodgkin model
L540-derived tumor-bearing mice were treated either with 5F11, bortezomib, the combination of 5F11 and bortezomib, or PBS. No cross-linking goat antihuman antibody was given. Both 5F11 and bortezomib induced significant delayed tumor growth compared with the control (P = .004 and P = .002, respectively). Even more promising results were obtained in animals treated with the combination of 5F11 and bortezomib (significant compared with the control [P = .001], 5F11 [P = .001], and bortezomib [P = .024]), as the tumor growth was almost completely inhibited (Figure 2E-F). The growth inhibition was maintained for several weeks after treatment, whereas animals receiving either 5F11 or bortezomib alone showed tumor progression.
In conclusion, down-regulation of NF-κB through bortezomib after CD30-mediated NF-κB activation may become a therapeutic approach to improve immunotherapy using monoclonal antibodies against the CD30 antigen. These findings are supported by recently published data showing that bortezomib induces cell death irrespective of NF-κB inhibitor mutations frequently observed in HD-derived cells.17 Although bortezomib has significant single-agent activity in patients with multiple myeloma and mantle cell lymphoma,18 the efficacy in HD patients is less clear. Currently, there are only a few patients reported, although the responses seem less impressive.19,20 The German Hodgkin Study Group is currently evaluating a combination of bortezomib and dexamethasone in relapsed HD patients. Though it depends on the outcome of this phase 2 trial, these preclinical data presented provide a rationale for a clinical study combining bortezomib/dexamethasone with an anti-CD30 monoclonal antibody.
Cytotoxic synergy of 5F11 in combination with bortezomib. (A-D) XTT viability assays of various cell lines after 48-hour exposure to subtoxic concentrations of cross-linked 5F11 (5 μg/mL 5F11; 25 μg/mL GaH-IgG), increasing concentrations of bortezomib (○), and the combination of both (•). The cell viability after exposure to 5 μg/mL 5F11 (▵), 25 μg GaH (▿), and the same amounts of 5F11 plus GaH (♦) is indicated. The mAb 5F11 and GaH-IgG were distributed in 100-μL aliquots in 96-well plates with target cells (2 × 104; L540, Karpas299, L428, and REH), and the plates were incubated for 30 minutes before addition of bortezomib. A synergistic increase of cytotoxicity is observed for the CD30-expressing cell lines (A-C). The synergy is not seen upon preincubation with bortezomib before 5F11/GaH addition (C, ⋄) and for the CD30- cell line REH (D). Means and standard deviation of 3 independent experiments are given. (E) Depiction of the XTT data for subtoxic concentrations of cross-linked 5F11 (5F11/GaH: 5/25 μg/mL for each cell line), subtoxic concentrations of bortezomib (L540: 7.5 μg/mL; Karpas299: 2.5 μg/mL; L428: 50 μg/mL; REH: 5 μg/mL), and the combination of both. Error bars indicate mean and SD of 3 independent experiments. (F) Effect of 5F11 and bortezomib on the tumor growth of subcutaneous L540Cy Hodgkin tumors in SCID mice. One of 2 independent experiments is shown, and the standard error of the mean is given. (G) Statistical analysis of the tumor volumes measured on day 47 (see dashed box in panel F, and an independent series, each group with n = 4). The differences between 5F11, bortezomib, and the combination versus the control are significant (the P values are estimated with the paired t test using GraphPadPrism software; GraphPad Software, San Diego, CA).
Cytotoxic synergy of 5F11 in combination with bortezomib. (A-D) XTT viability assays of various cell lines after 48-hour exposure to subtoxic concentrations of cross-linked 5F11 (5 μg/mL 5F11; 25 μg/mL GaH-IgG), increasing concentrations of bortezomib (○), and the combination of both (•). The cell viability after exposure to 5 μg/mL 5F11 (▵), 25 μg GaH (▿), and the same amounts of 5F11 plus GaH (♦) is indicated. The mAb 5F11 and GaH-IgG were distributed in 100-μL aliquots in 96-well plates with target cells (2 × 104; L540, Karpas299, L428, and REH), and the plates were incubated for 30 minutes before addition of bortezomib. A synergistic increase of cytotoxicity is observed for the CD30-expressing cell lines (A-C). The synergy is not seen upon preincubation with bortezomib before 5F11/GaH addition (C, ⋄) and for the CD30- cell line REH (D). Means and standard deviation of 3 independent experiments are given. (E) Depiction of the XTT data for subtoxic concentrations of cross-linked 5F11 (5F11/GaH: 5/25 μg/mL for each cell line), subtoxic concentrations of bortezomib (L540: 7.5 μg/mL; Karpas299: 2.5 μg/mL; L428: 50 μg/mL; REH: 5 μg/mL), and the combination of both. Error bars indicate mean and SD of 3 independent experiments. (F) Effect of 5F11 and bortezomib on the tumor growth of subcutaneous L540Cy Hodgkin tumors in SCID mice. One of 2 independent experiments is shown, and the standard error of the mean is given. (G) Statistical analysis of the tumor volumes measured on day 47 (see dashed box in panel F, and an independent series, each group with n = 4). The differences between 5F11, bortezomib, and the combination versus the control are significant (the P values are estimated with the paired t test using GraphPadPrism software; GraphPad Software, San Diego, CA).
Prepublished online as Blood First Edition Paper, May 5, 2005; DOI 10.1182/blood-2005-01-0427.
Supported by a grant of the Deutsche Krebshilfe (70-3204EN8).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The excellent technical assistance of Katja Krönke with gel shift assays is gratefully acknowledged.

![Figure 1. Regulation of NF-κB distribution and activity by 5F11 and bortezomib. Detection of the NF-κB subunit p65 with anti-p65 and a secondary FITC-labeled antibody (green) in untreated L540 cells (A), after stimulation with cross-linked 5F11 for 6 and 8 hours (B-C), and after stimulation with bortezomib (D-E). The cell nuclei are stained with 4′6-diamidino-2-phenylindole (DAPI). Imaging medium was Vectashield (Vector Laboratories, Burlingame, CA). Pictures were taken using the digital Nikon Eclipse E800 microscope with the LuciaG/F program (Nikon, Düsseldorf, Germany). (A) 10 ×/0.17 NA objective; (B-E) 40 ×/0.11-0.23 NA objective. (F) L428 cells were transfected with either reporter construct pCMV-luc (transfection control) or pNF-κB-luc (bars 2-4) or pNF-κB-luc together with the expression vector IkBαM (bars 5-7) and exposed to PBS (basal), 5F11, or bortezomib for 24 hours. The luciferase activity (relative light units [RLU]) of the cell lysates is indicated. Error bars indicate mean and SD of 2 independent experiments. (G) EMSA to detect DNA binding of NF-κB in nuclear extracts of L540 and L428 cells after 6-hour incubation with no antibody, cross-linked 5F11, and cross-linked Ber-H2 (BH2) antibody. Ber-H2 as a control anti-CD30 antibody is unable to mediate CD30 signaling10 and fails to activate NF-κB. (H) Western blotting of whole L540 cell extracts to detect c-flip, bcl-2, and bax in untreated cells after 16-hour incubation (n.t. indicates no treatment) and following treatment with either 5F11 or 5F11 + bortezomib after 6-, 8-, and 16-hour incubation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/5/10.1182_blood-2005-01-0427/6/m_zh80170583230001.jpeg?Expires=1768454659&Signature=VjrFkn3ZNKiggwUBOtattR1QZGXRl89lz1cPn7OxPd0kJPh3zkv93ZVdD~BXSH8O~-QU-CN4NM9NCn3T3p1cJ7hu39NL4v2N91DoCOQD5RQl3wWx5CeX43RmpiBngwkpS~wxYLWC7xvFmF56EHifTsdoUawNcLpBeGJqpl5xAluMRUs~lpnZAxZIMRlkdNzOJMq3XLrOMClw3RClOl4-FKI-fTXaJ0HRaV4c9n4rnrbIvf2nd5lpFOXNIdyMYA-pWwA0Rn0q9abTXzvdMBnLf4rR7j5zpJL5fat5h-bL-SA~RxtwePLCRewX0qbK~QsGV-KSyczFOpEKgd0-BL41Ww__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal