Abstract
The precise mechanisms by which imatinib mesylate (STI571) and interferon α (IFNα) exhibit antileukemic effects are not known. We examined the effects of IFNs or imatinib mesylate on signaling pathways regulating initiation of mRNA translation in BCR-ABL-expressing cells. Treatment of IFN-sensitive KT-1 cells with IFNα resulted in phosphorylation/activation of mammalian target of rapamycin (mTOR) and downstream activation of p70 S6 kinase. The IFN-activated p70 S6 kinase was found to regulate phosphorylation of S6 ribosomal protein, which regulates translation of mRNAs with oligopyrimidine tracts in the 5′-untranslated region. In addition, IFNα treatment resulted in an mTOR- and/or phosphatidyl-inositol 3′(PI 3′) kinase-dependent phosphorylation of 4E-BP1 repressor of mRNA translation on sites that are required for its deactivation and dissociation from the eukaryotic initiation factor-4E (eIF4E) complex. In contrast to the effects of IFNs, imatinib mesylate suppressed p70 S6 kinase activity, consistent with inhibition of BCR-ABL-mediated activation of the mTOR/p70 S6 kinase pathway. Moreover, the mTOR inhibitor rapamycin enhanced the suppressive effects of imatinib mesylate on primary leukemic granulocyte macrophage-colony-forming unit (CFU-GM) progenitors from patients with chronic myelogenous leukemia (CML). Taken altogether, our data demonstrate that IFNs and imatinib mesylate differentially regulate PI 3′ kinase/mTOR-dependent signaling cascades in BCR-ABL-transformed cells, consistent with distinct effects of these agents on pathways regulating mRNA translation. They also support the concept that combined use of imatinib mesylate with mTOR inhibitors may be an appropriate future therapeutic strategy for the treatment of CML. (Blood. 2005;106:2436-2443)
Introduction
The hallmark of chronic myelogenous leukemia (CML) is the presence of the abnormal BCR-ABL oncoprotein in the leukemic cells. BCR-ABL is the protein product of the bcr-abl oncogene, which results from the reciprocal translocation between chromosomes 9 and 22, and the abnormal fusion of the bcr and c-abl genes.1-3 Extensive studies over the years have established that the constitutively activated tyrosine kinase activity of BCR-ABL promotes leukemic transformation by activation of multiple downstream mitogenic cascades.4,5 These include pathways involving the Shc oncoprotein,6 Ras-GAP,7 the phosphatidylinositol polyphosphate 5′-phosphatase Src homology 2-containing inositol phosphatase (SHIP),8 the c-Cbl proto-oncogene product (CBL),9,10 Hef1,11 CrkL,12 Vav,13,14 signal transducer and activator of transcription 5 (STAT5),15,16 and the phosphatidyl-inositol 3′(PI 3′) kinase pathway.17,18 Recent evidence has also implicated the mammalian target of rapamycin (mTOR) as a downstream effector of BCR-ABL-mediated signals.19
Imatinib mesylate (STI571) is an ABL tyrosine kinase inhibitor14 that induces remission in CML by selectively targeting the kinase activity of the BCR-ABL tyrosine kinase and blocking the activation of BCR-ABL-dependent mitogenic pathways.20-22 It is now well established that imatinib mesylate is highly effective in inducing durable remissions in patients with CML in the chronic phase of the disease, and has shown activity against the accelerated or blast phases.23-26 Although the precise mechanisms by which imatinib mesylate induces responses in patients with CML are not known, it is presumed that its antineoplastic effects are mediated to a large extent by inhibition of BCR-ABL-generated mitogenic signals. There is also evidence suggesting that imatinib mesylate acts by reversing the suppressive effects of BCR-ABL on the activation of growth inhibitory pathways, notably the p38 Map kinase pathway.27
Prior to the introduction of imatinib mesylate in the treatment of CML, interferon α (IFNα) alone or in combination with chemotherapy, was the treatment of choice for patients in the chronic phase of the disease who were not eligible for bone marrow transplantation.24,28-30 Despite its displacement by imatinib mesylate as a first line agent for the treatment of CML, IFNα may still have an important future role in the management of this disease. Currently there are ongoing clinical trials to evaluate the therapeutic efficacy of the combination of imatinib mesylate and IFNα, as compared with imatinib alone. In addition, IFNα may prove to be useful in the treatment of patients with CML who develop resistance to the effects of imatinib mesylate.
Multiple signaling pathways are engaged during binding of IFNα to the type I IFN receptor. Initially, the type I IFN receptor-associated Tyk-2 and Jak-1 kinases are activated and regulate downstream engagement of the IFN-activated Stat-pathway (reviewed in Stark et al,31 Platanias and Fish,32 and Parmar and Platanias33 ), the insulin receptor substrate/PI 3′ kinase pathway,34-37 the Crk-pathway,38,39 and the p38 Map kinase signaling cascade.40-43 There has been accumulating evidence implicating the p38 Map kinase signaling pathway in the generation of the effects of IFNα in normal and malignant cells.40-43 In fact, activation of this cascade appears to be essential for the generation of the effects of IFNα on CML cells.42 In recent studies, we have also demonstrated that the activation of the PI 3′ kinase by the type I IFN (α, β) or the type II IFN (γ) receptors results in downstream engagement of mTOR and the p70 S6 kinase,44,45 but the precise role of these kinases in the generation of IFN responses in leukemic cells remains to be defined.
In the present study, we examined the effects of IFNα and imatinib mesylate on the activation of mTOR and the p70 S6 kinase in BCR-ABL-expressing cells. Our data demonstrate that treatment of sensitive cells with IFNα results in activation of p70 S6 kinase and downstream phosphorylation of the S6 ribosomal protein in CML cells. Such activation is blocked by pharmacologic inhibitors of the PI 3′ kinase and is rapamycin sensitive, indicating that the IFN-activated forms of PI 3′ kinase and mTOR regulate such activation. IFNα also induces phosphorylation and deactivation of the transcriptional repressor 4E-BP1, to allow its dissociation from the eukaryotic initiation factor-4E (eIF4E) complex. On the other hand, treatment of BCR-ABL-expressing cell lines with imatinib mesylate results in dephosphorylation/deactivation of the p70 S6 kinase and its downstream effector S6 ribosomal protein, indicating that imatinib mesylate exhibits opposing regulatory effects on the activation of this signaling cascade, as compared with IFNα. Pharmacologic inhibition of the mTOR/p70 S6 kinase pathway using rapamycin inhibits the growth of primary leukemic granulocyte macrophage-colony-forming unit (CFU-GM) progenitors from patients with CML and enhances the suppressive effects of both imatinib mesylate and IFNα, indicating that the function of this cascade is important for survival of BCR-ABL-transformed cells.
Materials and methods
Cell lines and reagents
The CML-derived KT-1 and K562 cell lines were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum and antibiotics. Human recombinant IFNα2 was provided by Hoffmann-La Roche (Nutley, NJ). Human recombinant consensus IFNα was provided by Inter Mune (Brisbane, CA). Human recombinant IFNβ was provided by Biogen (Cambridge, MA). Imatinib mesylate was provided by Novartis (Basel, Switzerland). Antibodies against the phosphorylated forms of p70 S6 kinase, mTOR, and 4E-BP1 were obtained from Cell Signaling Technology (Beverly, MA). Antibodies against the phosphorylated form of ribosomal S6 kinase at Ser235/236 and Ser240/244 were obtained from Cell Signaling Technology. Antibodies against p70S6 kinase, mTOR, and 4E-BP1 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The FKBP12 rapamycin-associated protein (FRAP)/mTOR inhibitor rapamycin, and the PI 3′ kinase inhibitors LY294 002 and wortmannin, were obtained from Calbiochem (La Jolla, CA).
Cell lysis and immunoblotting
Cells were stimulated with 1 × 104 U/mL of the indicated IFNs or with the indicated concentrations of imatinib mesylate for the indicated times, and then lysed in phosphorylation lysis buffer as previously described.46,47 In the experiments in which the effects of imatinib mesylate were studied, treatment of the cells with dimethyl sulfoxide (DMSO; diluent) was used as control. Immunoprecipitations and immunoblotting, using an enhanced chemiluminescence (ECL) method, were performed as previously described.46,47 In the experiments in which pharmacologic inhibitors of FRAP/mTOR or the PI 3′ kinase were used, the cells were pretreated for 30 minutes with the indicated concentrations of the inhibitors and subsequently treated for the indicated times with IFNs, prior to lysis in phosphorylation lysis buffer.
p70 S6 kinase assays
Assays to detect the activation of p70 S6 kinase were performed as previously described.44,45 Briefly, cells were lysed in phosphorylation lysis buffer and lysates were immunoprecipitated with an antibody against p70 S6 kinase or control nonimmune rabbit immunoglobulin (RIgG). In vitro kinase assays were subsequently performed using a synthetic peptide substrate (AKRRRLSSLRA), and p70 S6 kinase activity was measured using an S6 kinase assay kit (Upstate Biotechnology, Lake Placid, NY), according to the manufacturer's instructions.44,45 Values were calculated by subtracting nonspecific activity, present in RIgG immunoprecipitates, from kinase activity detected in the immunoprecipitates with the anti-p70 S6K antibody.
Isolation of peripheral-blood granulocytes
Peripheral blood was obtained from patients with CML after obtaining informed consent according to the guidelines established by the institutional review board of Northwestern University Medical School. Polymorphonuclear leukocytes were separated from peripheral venous blood using the Mono-Poly resolving medium (ICN Biomedicals, Aurora, OH), as previously described.42 The cells were washed with culture medium and were subsequently resuspended in culture medium, prior to interferon treatment.
Hematopoietic progenitor-cell assays
The effects of imatinib mesylate or IFNα on the growth of hematopoietic progenitors from patients with CML were determined in methylcellulose assays as described previously.48,49 Bone marrow aspirate specimens were obtained under local anesthesia from patients with CML after obtaining informed consent. Bone marrow mononuclear cells were separated by Ficoll-Hypaque sedimentation, and cells were cultured in a methylcellulose mixture containing hematopoietic growth factors32 in the presence or absence of IFNα (1000 U/mL), imatinib mesylate (1 μM), and rapamycin (10 nM-20 nM). In the patients from whom bone marrow aspirates were not available, peripheral-blood mononuclear cells were separated and used for methylcellulose assays. CFU-GMs from leukemic bone marrow samples were scored on day 14 of culture.
Results
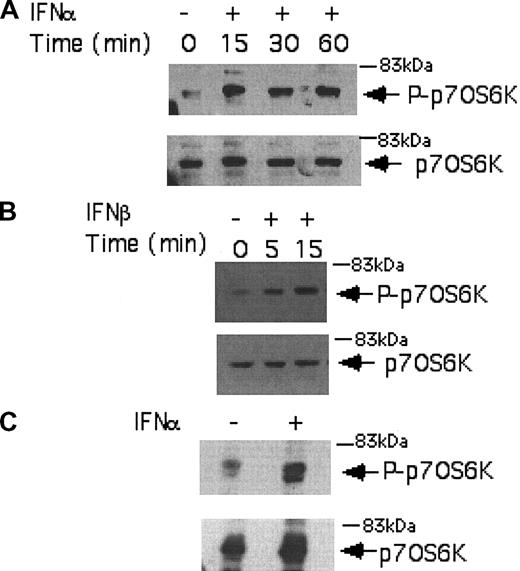
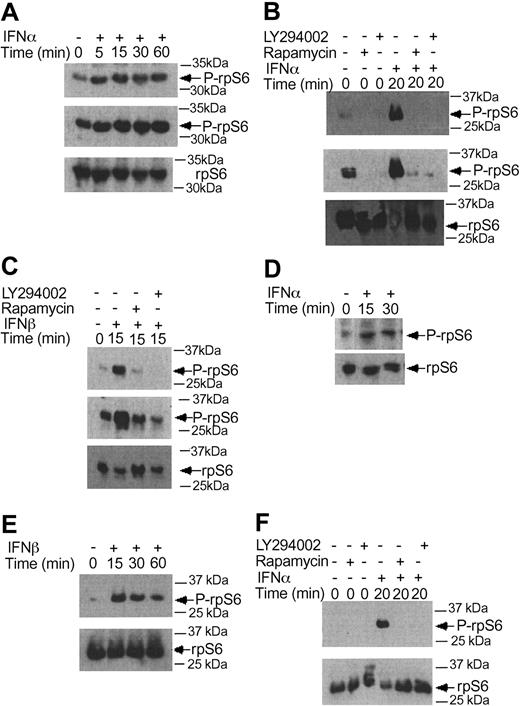
We initially performed studies using the IFN-sensitive, CML-derived KT-1 cell line, to examine the effects of IFNα on the phosphorylation/activation of the p70 S6 kinase. KT-1 cells were incubated for different time points in the presence or absence of IFNα, and total cell lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with an antibody against the phosphorylated form of the p70 S6 kinase on threonine 421 and serine 424. IFNα induced strong phosphorylation of the p70 S6 kinase in KT-1 cells (Figure 1A), suggesting that this kinase is a component of an IFN-activated signaling cascade and participates in the generation of IFNα signals in BCR-ABL-expressing cells. Similarly, treatment of KT-1 cells with IFNβ also resulted in strong phosphorylation of the p70 S6 kinase (Figure 1B), indicating that p70 S6 kinase is a common element in the signaling pathways of different type I IFNs in CML cells. Type I IFN-dependent phosphorylation of the p70 S6 kinase was also inducible when primary CML granulocytes were treated with IFNα (Figure 1C), suggesting that the activation of this kinase occurs under more physiologically relevant conditions, and may therefore be involved in the generation of the effects of IFNα in CML.
Type I IFN-dependent phosphorylation of the p70 S6 kinase in BCR-ABL-expressing cells. (A) KT-1 cells were treated with IFNα for different time points, as indicated. Equal amounts of total cell lysates were analyzed by SDS-PAGE and immunoblotted with an antibody against the phosphorylated/activated form of the p70 S6 kinase on threonine 421 and serine 424 (top panel). The same blot was then stripped and reprobed with an anti-p70 S6 kinase antibody, to control for protein loading (bottom panel). (B) Similar experiment to the one shown in panel A, except that IFNβ was used instead of IFNα. (C) Granulocytes isolated from the peripheral blood of a patient with CML were incubated with IFNα for 15 minutes. Top panel shows immunoblotting of total cell lysates with an anti-phospho-Thr421/Ser-424 p70 S6 kinase antibody. Bottom panel shows reprobing of the same blot with an anti-p70 S6K antibody, to control for protein loading.
Type I IFN-dependent phosphorylation of the p70 S6 kinase in BCR-ABL-expressing cells. (A) KT-1 cells were treated with IFNα for different time points, as indicated. Equal amounts of total cell lysates were analyzed by SDS-PAGE and immunoblotted with an antibody against the phosphorylated/activated form of the p70 S6 kinase on threonine 421 and serine 424 (top panel). The same blot was then stripped and reprobed with an anti-p70 S6 kinase antibody, to control for protein loading (bottom panel). (B) Similar experiment to the one shown in panel A, except that IFNβ was used instead of IFNα. (C) Granulocytes isolated from the peripheral blood of a patient with CML were incubated with IFNα for 15 minutes. Top panel shows immunoblotting of total cell lysates with an anti-phospho-Thr421/Ser-424 p70 S6 kinase antibody. Bottom panel shows reprobing of the same blot with an anti-p70 S6K antibody, to control for protein loading.
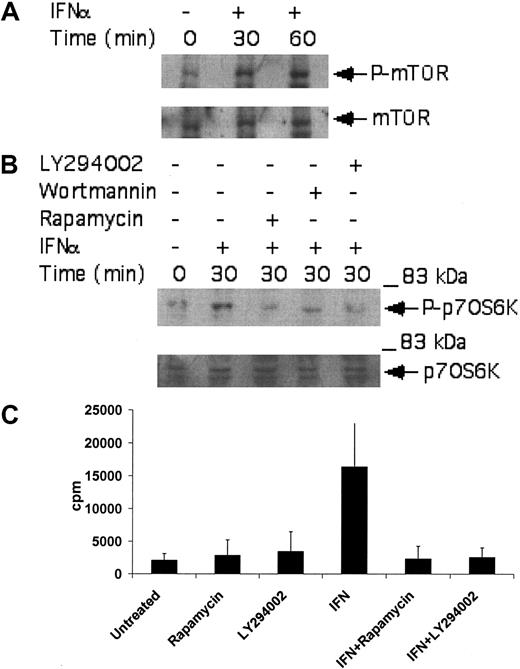
A known upstream regulator of the p70 S6 kinase is mTOR.50-53 As this kinase has been recently shown to be induced by the activated type I IFN receptor complex,44,54 we examined whether it is activated by IFNα in CML-derived cell lines, to regulate downstream engagement of the p70 S6 kinase. KT-1 cells were treated with IFNα, and after cell lysis total lysates were immunoblotted with an antibody that recognizes the phosphorylated/activated form of mTOR. IFNα treatment resulted in strong phosphorylation of mTOR over baseline (Figure 2A), suggesting that mTOR participates in the generation of IFNα signals. To determine whether such activation of mTOR plays a functional role in BCR-ABL-expressing cells, the effects of the mTOR inhibitor rapamycin on the phosphorylation/activation of p70 S6 kinase were examined. The potential regulatory effects of the IFN-activated PI 3′ kinase pathway34-38 on p70 S6 kinase activation were also analyzed in parallel. As shown in Figure 2B, the type I IFN-dependent phosphorylation of p70 S6 kinase in KT-1 cells was abolished by pretreatment of cells either with rapamycin or with the PI 3′ kinase inhibitors LY294 002 or wortmannin (Figure 2B). Treatment of KT-1 cells with IFNα also resulted in strong induction of p70 S6 kinase activity, as determined by in vitro kinase assay experiments in anti-S6 kinase immunoprecipitates from IFNα-treated cells (Figure 2C). Moreover, inhibition of the PI 3′ kinase by LY294 002, or inhibition of mTOR by rapamycin, abrogated the induction of p70 S6 kinase activity in anti-p70 S6K immunoprecipitates from IFNα-treated KT-1 cell lysates (Figure 2C). Together, these data established that the p70 S6 kinase is phosphorylated and activated in BCR-ABL-expressing cells, in an mTOR-dependent manner.
Activation of p70 S6 kinase by IFNα in BCR-ABL-expressing cells is PI 3′ kinase- and mTOR-dependent. (A) KT-1 cells were treated with IFNα for the indicated times. Top panel shows an immunoblot with an antibody against phosphorylated mTOR on serine 2448. Bottom panel shows reprobing of the same blot with an anti-mTOR antibody. (B) KT-1 cells were pretreated for 30 minutes with either LY294 002 (50 μM) or rapamycin (20 nM) prior to treatment with IFNα for 30 minutes as indicated. Top panel shows an anti-phospho-p70 S6 kinase immunoblot. Bottom panel shows reprobing of the same blot with an anti-p70 S6 kinase antibody. (C) KT-1 cells were pretreated for 30 minutes with the various pharmacologic inhibitors prior to treatment with IFNα for 30 minutes, as indicated. Cell lysates were immunoprecipitated with an anti-p70 S6 kinase antibody or control nonimmune rabbit immunoglobulin (RIgG). In vitro kinase assays to detect p70 S6 kinase activity were then carried out on the immunoprecipitates. The data represent means plus or minus the standard error (SE) of 3 independent experiments.
Activation of p70 S6 kinase by IFNα in BCR-ABL-expressing cells is PI 3′ kinase- and mTOR-dependent. (A) KT-1 cells were treated with IFNα for the indicated times. Top panel shows an immunoblot with an antibody against phosphorylated mTOR on serine 2448. Bottom panel shows reprobing of the same blot with an anti-mTOR antibody. (B) KT-1 cells were pretreated for 30 minutes with either LY294 002 (50 μM) or rapamycin (20 nM) prior to treatment with IFNα for 30 minutes as indicated. Top panel shows an anti-phospho-p70 S6 kinase immunoblot. Bottom panel shows reprobing of the same blot with an anti-p70 S6 kinase antibody. (C) KT-1 cells were pretreated for 30 minutes with the various pharmacologic inhibitors prior to treatment with IFNα for 30 minutes, as indicated. Cell lysates were immunoprecipitated with an anti-p70 S6 kinase antibody or control nonimmune rabbit immunoglobulin (RIgG). In vitro kinase assays to detect p70 S6 kinase activity were then carried out on the immunoprecipitates. The data represent means plus or minus the standard error (SE) of 3 independent experiments.
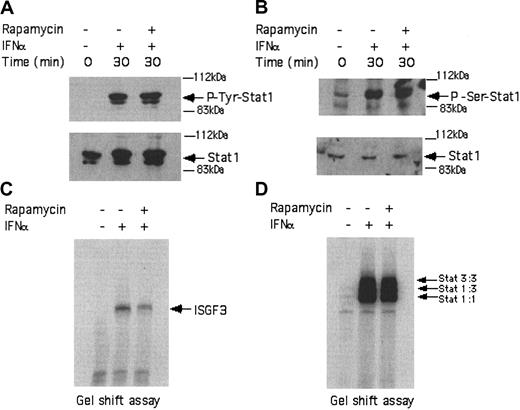
In subsequent studies, we examined whether activation of mTOR downstream of the PI 3′ kinase in BCR-ABL-expressing cells exhibits regulatory effects on the type I IFN-dependent Stat-pathway, which regulates transcriptional activation of IFN-sensitive genes. KT-1 cells were incubated in the presence or absence of IFNα for different times and total cell lysates were analyzed by SDS-PAGE and immunoblotted with antibodies against the phosphorylated forms of Stat1 on tyrosine 701 or serine 727. As expected, treatment of KT-1 cells with IFNα resulted in strong phosphorylation of Stat1 on both tyrosine 701 (Figure 3A) and serine 727 (Figure 3B). Concomitant treatment of the cells with rapamycin had no effect on the phosphorylation of Stat1 (Figure 3A-B), indicating that the mTOR/p70 S6 kinase pathway plays no role in the regulation of phosphorylation of this protein. Consistent with this, rapamycin did not affect the type I IFN-dependent formation of interferon-stimulated gene factor 3 (ISGF3) (Figure 3C) or sis-inducible element (SIE) (Figure 3D) DNA-binding complexes in KT-1 cells.
IFNα-dependent activation of Stat complexes in KT-1 cells is mTOR independent. (A) KT-1 cells were preincubated with rapamycin for 60 minutes and were subsequently treated with IFNα, as indicated. Top panel shows an immunoblot with an anti-phospho-Tyr701 Stat1 antibody. Bottom panel shows reprobing the same blot with an anti-Stat1 antibody. (B) Similar experiment as in panel A, except that an antibody against the phosphorylated form of Stat1 on serine 727 was used in the immunoblot shown in the top panel. (C) Actively growing KT-1 cells were preincubated for 30 minutes with rapamycin, as indicated. The cells were subsequently treated with IFNα, as indicated. Nuclear extracts were reacted with 40 000 cpm of a 32P-labeled interferon-stimulated response element (ISRE) probe and complexes were resolved by native gel electrophoresis and visualized by autoradiography. (D) Similar experiment to the one shown in panel C, except that a 32P-labeled SIE probe was used.
IFNα-dependent activation of Stat complexes in KT-1 cells is mTOR independent. (A) KT-1 cells were preincubated with rapamycin for 60 minutes and were subsequently treated with IFNα, as indicated. Top panel shows an immunoblot with an anti-phospho-Tyr701 Stat1 antibody. Bottom panel shows reprobing the same blot with an anti-Stat1 antibody. (B) Similar experiment as in panel A, except that an antibody against the phosphorylated form of Stat1 on serine 727 was used in the immunoblot shown in the top panel. (C) Actively growing KT-1 cells were preincubated for 30 minutes with rapamycin, as indicated. The cells were subsequently treated with IFNα, as indicated. Nuclear extracts were reacted with 40 000 cpm of a 32P-labeled interferon-stimulated response element (ISRE) probe and complexes were resolved by native gel electrophoresis and visualized by autoradiography. (D) Similar experiment to the one shown in panel C, except that a 32P-labeled SIE probe was used.
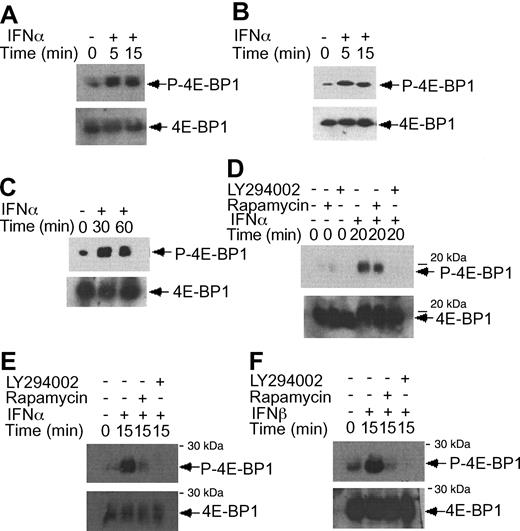
IFNα-dependent phosphorylation of 4E-BP1 repressor of mRNA translation in KT-1 cells. (A) KT-1 cells were treated with IFNα for different times, as indicated. Top panel shows an immunoblot with an antibody against phosphorylated 4E-BP1 on Thr 37/46. Bottom panel shows reprobing the same blot with an anti-4E-BP1 antibody. (B) Similar experiment to the one shown in panel B, except that immunoblotting was first performed using an antibody against the phosphorylated form of 4E-BP1 on Thr 70. (C) Similar experiment to the one shown in panel B, except that the cells were incubated for longer times with IFNα, as indicated. (D) KT-1 cells were preincubated with LY294 002 (50 μM) or rapamycin (20 nM) for 30 minutes and were subsequently treated with IFNα, as indicated. Top panel shows an immunoblot with an antibody against phosphorylated 4E-BP1 on Thr 37/46. Bottom panel shows reprobing of the same blot with an anti-4E-BP1 antibody. (E) KT-1 cells were pretreated with LY294 002 (50 μM) or rapamycin (20 nM) for 30 minutes and were subsequently incubated with IFNα, as indicated. Top panel shows an immunoblot with an antibody against phosphorylated 4E-BP1 on Thr 70. Bottom panel shows reprobing of the same blot with an anti-4E-BP1 antibody. (F) Similar experiment to the one shown in panel E, except that IFNβ was used instead of IFNα.
IFNα-dependent phosphorylation of 4E-BP1 repressor of mRNA translation in KT-1 cells. (A) KT-1 cells were treated with IFNα for different times, as indicated. Top panel shows an immunoblot with an antibody against phosphorylated 4E-BP1 on Thr 37/46. Bottom panel shows reprobing the same blot with an anti-4E-BP1 antibody. (B) Similar experiment to the one shown in panel B, except that immunoblotting was first performed using an antibody against the phosphorylated form of 4E-BP1 on Thr 70. (C) Similar experiment to the one shown in panel B, except that the cells were incubated for longer times with IFNα, as indicated. (D) KT-1 cells were preincubated with LY294 002 (50 μM) or rapamycin (20 nM) for 30 minutes and were subsequently treated with IFNα, as indicated. Top panel shows an immunoblot with an antibody against phosphorylated 4E-BP1 on Thr 37/46. Bottom panel shows reprobing of the same blot with an anti-4E-BP1 antibody. (E) KT-1 cells were pretreated with LY294 002 (50 μM) or rapamycin (20 nM) for 30 minutes and were subsequently incubated with IFNα, as indicated. Top panel shows an immunoblot with an antibody against phosphorylated 4E-BP1 on Thr 70. Bottom panel shows reprobing of the same blot with an anti-4E-BP1 antibody. (F) Similar experiment to the one shown in panel E, except that IFNβ was used instead of IFNα.
There is evidence that in addition to activation of the p70 S6 kinase mTOR mediates downstream phosphorylation of 4E-BP1, a transcriptional repressor that negatively regulates cap-dependent mRNA translation.43,44,55,56 It is also well established that phosphorylation of 4E-BP1 results in its inactivation and dissociation from the eIF4E complex, to allow initiation of translation.57-60 We examined whether type I IFNs induce phosphorylation of 4E-BP1 in KT-1 cells. IFNα treatment resulted in a strong phosphorylation of 4E-BP1 at threonines 37/46 (Figure 4A), and threonine 70 (Figure 4B-C), sites whose phosphorylation is essential for the inactivation of 4E-BP1.58-60 The IFNα-dependent phosphorylation of 4E-BP1 in KT-1 cells was also found to be PI 3′ kinase- and mTOR-dependent, as evidenced by the inhibition of phosphorylation of the protein when cells were concomitantly treated with either LY294 002 or rapamycin (Figure 4D-E). Interestingly, in these cells, the phosphorylation of 4E-BP1 on threonine 70 was both PI 3′ kinase- and mTOR-dependent, whereas the phosphorylation on threonines 37/46 was PI 3′ kinase-dependent, but rapamycin-insensitive. Similarly, treatment of KT-1 cells with another type I IFN, IFNβ, also resulted in induction of 4E-BP1 phosphorylation, which was also PI 3′ kinase-dependent (Figure 4F).
Beyond the phosphorylation of 4E-BP1 and its dissociation from eIF4E, another major mechanism by which mTOR ultimately regulates initiation of mRNA translation is the phosphorylation of the S6 ribosomal protein, an event of critical importance for the translation of mRNAs with oligopyrimidine tracts in the 5′-untranslated region.59-64 In experiments to determine the effects of type I IFNs on the phosphorylation of the S6 ribosomal protein, we found that both IFNα and IFNβ induced phosphorylation of the protein on serines 235/236 (Figure 5A-C), and that such phosphorylation was blocked by pretreatment of cells with either LY294 002 or rapamycin (Figure 5B-C). Similarly, type I IFN treatment induced phosphorylation of the S6 ribosomal protein on serine 240/244, and such phosphorylation was PI 3′ kinase- and mTOR-dependent (Figure 5D-F). Thus, activation of the PI 3′ kinase/mTOR signaling cascade by type I interferons in BCR-ABL-expressing cells appears to result in engagement of 2 distinct signals for the initiation of mRNA translation; one involving sequential activation/phosphorylation of the p70 S6 kinase and S6 ribosomal protein, and the other one involving phosphorylation and deactivation of the translational repressor 4E-BP1.
IFNα and IFNβ induce phosphorylation of the S6 ribosomal protein, in a PI 3′kinase- and mTOR-dependent manner. (A) KT-1 cells were treated with IFNα for different times, as indicated. Top panel shows an immunoblot with an antibody against the phosphorylated form of the S6 ribosomal protein on serines 235/236. Middle panel shows longer exposure of the same blot. Bottom panel shows reprobing of the same blot with an anti-S6 ribosomal protein antibody. (B) KT-1 cells were pre-incubated for 30 minutes with the indicated inhibitors and the cells were subsequently treated with IFNα. Top panel shows an immunoblot with an antibody against the phosphorylated form of the S6 ribosomal protein on serines 235/236. Middle panel shows longer exposure of the same blot. Bottom panel shows reprobing of the same blot with an anti-S6 ribosomal protein antibody. (C) Similar experiment to the one shown in panel B, except that IFNβ was used instead of IFNα. (D) KT-1 cells were treated with IFNα for different times, as indicated. Top panel shows an immunoblot with an antibody against the phosphorylated form of the S6 ribosomal protein on serines 240/244. Bottom panel shows reprobing of the same blot with an anti-S6 ribosomal protein antibody. (E) Similar experiment to the one shown in panel D, except that IFNβ was used instead of IFNα. (F) KT-1 cells were preincubated for 30 minutes in the presence or absence of LY294 002 or rapamycin, and were subsequently treated with IFNα, as indicated. Top panel shows an immunoblot with an antibody against the phosphorylated form of the S6 ribosomal protein on serines 240/244. Bottom panel shows reprobing of the same blot with an anti-S6 ribosomal protein antibody.
IFNα and IFNβ induce phosphorylation of the S6 ribosomal protein, in a PI 3′kinase- and mTOR-dependent manner. (A) KT-1 cells were treated with IFNα for different times, as indicated. Top panel shows an immunoblot with an antibody against the phosphorylated form of the S6 ribosomal protein on serines 235/236. Middle panel shows longer exposure of the same blot. Bottom panel shows reprobing of the same blot with an anti-S6 ribosomal protein antibody. (B) KT-1 cells were pre-incubated for 30 minutes with the indicated inhibitors and the cells were subsequently treated with IFNα. Top panel shows an immunoblot with an antibody against the phosphorylated form of the S6 ribosomal protein on serines 235/236. Middle panel shows longer exposure of the same blot. Bottom panel shows reprobing of the same blot with an anti-S6 ribosomal protein antibody. (C) Similar experiment to the one shown in panel B, except that IFNβ was used instead of IFNα. (D) KT-1 cells were treated with IFNα for different times, as indicated. Top panel shows an immunoblot with an antibody against the phosphorylated form of the S6 ribosomal protein on serines 240/244. Bottom panel shows reprobing of the same blot with an anti-S6 ribosomal protein antibody. (E) Similar experiment to the one shown in panel D, except that IFNβ was used instead of IFNα. (F) KT-1 cells were preincubated for 30 minutes in the presence or absence of LY294 002 or rapamycin, and were subsequently treated with IFNα, as indicated. Top panel shows an immunoblot with an antibody against the phosphorylated form of the S6 ribosomal protein on serines 240/244. Bottom panel shows reprobing of the same blot with an anti-S6 ribosomal protein antibody.
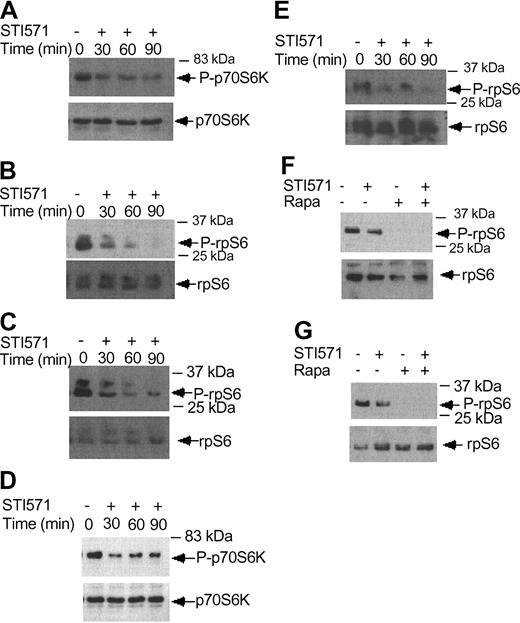
Imatinib mesylate inhibits phosphorylation/activation of p70 S6 kinase and the S6 ribosomal protein. (A) KT-1 cells were treated with imatinib mesylate (1 μM) for different time points, as indicated. Top panel shows immunoblotting of total cell lysates with an anti-phospho-Thr421/Ser-424 p70 S6 kinase antibody. Bottom panel shows reprobing of the same blot with an anti-p70 S6K antibody. (B) KT-1 cells were treated with imatinib mesylate for different times as indicated. Top panel shows an immunoblot of total cell lysates with an antibody against the phosphorylated form of the S6 ribosomal protein on serines 235/236. Bottom panel shows reprobing of the same blot with an anti-S6 ribosomal protein antibody. (C) KT-1 cells were treated with imatinib mesylate for different time points, as indicated. Top panel shows an immunoblot with an antibody against the phosphorylated form of the S6 ribosomal protein on serines 240/244. Bottom panel shows reprobing of the same blot with an anti-S6 ribosomal protein antibody. (D) K562 cells were treated with imatinib mesylate (1 μM) for different times, as indicated. Top panel shows immunoblotting of total cell lysates with an anti-phospho-Thr421/Ser-424 p70 S6 kinase antibody. Bottom panel shows reprobing of the same blot with an anti-p70 S6 kinase antibody. (E) K562 cells were treated with imatinib mesylate for different times as indicated. Top panel shows an immunoblot of total cell lysates with an antibody against the phosphorylated form of the S6 ribosomal protein on serines 235/236. Bottom panel shows reprobing of the same blot with an anti-S6 ribosomal protein antibody. (F) K562 cells were treated with imatinib mesylate and/or rapamycin for 120 minutes, as indicated. Top panel shows immunoblotting of total cell lysates with an antibody against the phosphorylated form of the S6 ribosomal protein on serines 235/236. Bottom panel shows reprobing of the same blot with an anti-S6 ribosomal protein antibody. (G) Similar experiment to the one shown in panel F, except that an antibody against the phosphorylated form of the S6 ribosomal protein on serines 240/244 was used for immunoblotting in the top panel.
Imatinib mesylate inhibits phosphorylation/activation of p70 S6 kinase and the S6 ribosomal protein. (A) KT-1 cells were treated with imatinib mesylate (1 μM) for different time points, as indicated. Top panel shows immunoblotting of total cell lysates with an anti-phospho-Thr421/Ser-424 p70 S6 kinase antibody. Bottom panel shows reprobing of the same blot with an anti-p70 S6K antibody. (B) KT-1 cells were treated with imatinib mesylate for different times as indicated. Top panel shows an immunoblot of total cell lysates with an antibody against the phosphorylated form of the S6 ribosomal protein on serines 235/236. Bottom panel shows reprobing of the same blot with an anti-S6 ribosomal protein antibody. (C) KT-1 cells were treated with imatinib mesylate for different time points, as indicated. Top panel shows an immunoblot with an antibody against the phosphorylated form of the S6 ribosomal protein on serines 240/244. Bottom panel shows reprobing of the same blot with an anti-S6 ribosomal protein antibody. (D) K562 cells were treated with imatinib mesylate (1 μM) for different times, as indicated. Top panel shows immunoblotting of total cell lysates with an anti-phospho-Thr421/Ser-424 p70 S6 kinase antibody. Bottom panel shows reprobing of the same blot with an anti-p70 S6 kinase antibody. (E) K562 cells were treated with imatinib mesylate for different times as indicated. Top panel shows an immunoblot of total cell lysates with an antibody against the phosphorylated form of the S6 ribosomal protein on serines 235/236. Bottom panel shows reprobing of the same blot with an anti-S6 ribosomal protein antibody. (F) K562 cells were treated with imatinib mesylate and/or rapamycin for 120 minutes, as indicated. Top panel shows immunoblotting of total cell lysates with an antibody against the phosphorylated form of the S6 ribosomal protein on serines 235/236. Bottom panel shows reprobing of the same blot with an anti-S6 ribosomal protein antibody. (G) Similar experiment to the one shown in panel F, except that an antibody against the phosphorylated form of the S6 ribosomal protein on serines 240/244 was used for immunoblotting in the top panel.
There is recent evidence that BCR-ABL positively modulates phosphorylation of the S6 ribosomal protein and 4E-BP1.19 We examined the effects of imatinib mesylate on the phosphorylation/activation of the p70 S6 kinase and the S6 ribosomal protein. KT-1 cells were treated for different times with imatinib mesylate and then cell lysates were resolved by SDS-PAGE and immunoblotted with an antibody against the phosphorylated form of p70 S6 kinase. Imatinib mesylate treatment resulted in a time-dependent dephosphorylation/inactivation of the p70 S6 kinase (Figure 6A). Consistent with this, inhibition of BCR-ABL kinase activity resulted in dephosphorylation of the S6 ribosomal protein, both on Ser235/236 (Figure 6B) and Ser 240/244 (Figure 6C). Such inhibitory effects of imatinib mesylate on the activation of the p70 S6 kinase and the phosphorylation of the S6 ribosomal protein were also seen when K562 cells were used in similar studies (Figure 6D-E). Treatment of cells with rapamycin alone or both rapamycin and imatinib mesylate also strongly inhibited the phosphorylation of S6 ribosomal protein on either Ser235/236 or Ser240/244 (Figure 6F-G). Thus, in contrast to IFNα, which induces activation of the p70 S6 kinase, inhibition of BCR-ABL kinase activity results in dephosphorylation of the p70 S6 kinase and its downstream effector S6 ribosomal protein.
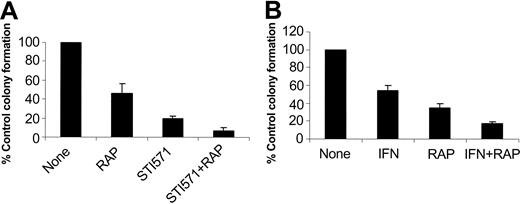
In subsequent studies we examined the effects of pharmacologic inhibition of mTOR on the generation of the suppressive effects of imatinib mesylate and IFNα on malignant hematopoietic progenitors. It has been previously established that imatinib mesylate selectively inhibits the growth of leukemic CFU-GM progenitors in methylcellulose assays, as compared with normal myeloid precursors.22 Bone marrow- or peripheral-blood-derived hematopoietic mononuclear cells from patients with chronic myelogenous leukemia were incubated with imatinib mesylate or IFNα, in the presence or absence of rapamycin, and the growth of leukemic CFU-GM precursors was assessed. As expected, treatment with either imatinib mesylate or IFNα suppressed leukemic CFU-GM colony formation in all different patient samples examined (Table 1). Addition of rapamycin to the cultures also inhibited leukemic CFU-GM growth in all patient samples (Table 1), indicating that activation of mTOR is essential for the growth of leukemic BCR-ABL-expressing hematopoietic progenitors. Importantly, addition of rapamycin to the cultures enhanced the suppressive effects of imatinib mesylate or IFNα on leukemic CFU-GM growth (see individual patient results in Table 1 and compilation of the data in Figure 7A). Paired t test analysis to compare the effects of imatinib mesylate alone with those of imatinib mesylate combined with rapamycin demonstrated that this effect was statistically significant (2-tailed P = .005). Similarly, rapamycin also exhibited statistically significant additive effects with IFNα (paired t test, 2-tailed P = .019), indicating that the mTOR/p70 S6 kinase pathway mediates a wide spectrum of signals that are important for cell growth and survival of BCR-ABL-transformed cells.
Effect of rapamycin on the inhibitory effects of imatinib mesylate and IFNα on leukemic CFU-GM progenitors
. | . | % control colony formation . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case . | Source . | Rapamycin . | IFNα . | IFNα + rapamycin . | imatinib mesylate . | imatinib mesylate + rapamycin . | ||||
| 1 | PB | 31 | — | — | 19 | 8 | ||||
| 2* | PB | 30 ± 3 | 39 ± 4 | 19 ± 4 | 22 ± 6 | 5 ± 2 | ||||
| 3 | PB | 48 | 53 | 23 | 23 | 14 | ||||
| 4* | BM | 26 ± 4.5 | 54 ± 8 | 15 ± 2 | — | — | ||||
| 5 | BM | 75 | — | — | 16 | 1.5 | ||||
| 6 | PB | 34 | 70 | 13 | — | — | ||||
. | . | % control colony formation . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case . | Source . | Rapamycin . | IFNα . | IFNα + rapamycin . | imatinib mesylate . | imatinib mesylate + rapamycin . | ||||
| 1 | PB | 31 | — | — | 19 | 8 | ||||
| 2* | PB | 30 ± 3 | 39 ± 4 | 19 ± 4 | 22 ± 6 | 5 ± 2 | ||||
| 3 | PB | 48 | 53 | 23 | 23 | 14 | ||||
| 4* | BM | 26 ± 4.5 | 54 ± 8 | 15 ± 2 | — | — | ||||
| 5 | BM | 75 | — | — | 16 | 1.5 | ||||
| 6 | PB | 34 | 70 | 13 | — | — | ||||
PB indicates peripheral blood;—, not done; and BM, bone marrow.
For cases 2 and 4, values shown are means ± SE of 2 independent experiments.
Effects of pharmacologic inhibition of the mTOR/p70 S6 kinase pathway in the generation of the inhibitory effects of imatinib mesylate and IFNα on primary leukemic progenitors. Peripheral blood or bone marrow mononuclear cells isolated from different patients with CML were plated in a methylcellulose culture assay system with imatinib mesylate (1 μM; A) or IFNα (1000 IU/mL; B), in the presence or absence of rapamycin, as indicated. Leukemic CFU-GM progenitor colonies were scored on day 14 of culture. (A) Comparison of the effects of imatinib mesylate alone versus imatinib mesylate plus rapamycin. Means plus SE of the values from the experiments using different patient samples (cases 1, 2, 3, and 5 from Table 1) are shown. (B) Comparison of the effects of IFNα alone versus IFNα plus rapamycin. Means plus SE of the values from the experiments using different patient samples (cases 2, 3, 4, and 6 from Table 1) are shown.
Effects of pharmacologic inhibition of the mTOR/p70 S6 kinase pathway in the generation of the inhibitory effects of imatinib mesylate and IFNα on primary leukemic progenitors. Peripheral blood or bone marrow mononuclear cells isolated from different patients with CML were plated in a methylcellulose culture assay system with imatinib mesylate (1 μM; A) or IFNα (1000 IU/mL; B), in the presence or absence of rapamycin, as indicated. Leukemic CFU-GM progenitor colonies were scored on day 14 of culture. (A) Comparison of the effects of imatinib mesylate alone versus imatinib mesylate plus rapamycin. Means plus SE of the values from the experiments using different patient samples (cases 1, 2, 3, and 5 from Table 1) are shown. (B) Comparison of the effects of IFNα alone versus IFNα plus rapamycin. Means plus SE of the values from the experiments using different patient samples (cases 2, 3, 4, and 6 from Table 1) are shown.
Discussion
The introduction of imatinib mesylate (STI571) has had a major impact on the management and natural history of CML.22-26,65,66 imatinib mesylate has replaced IFNα as the first line agent for the treatment of CML and induces dramatic, long-lasting remissions in patients in the chronic phase of the disease.65,66 Despite the impressive advances in the field, the precise cellular mechanisms by which imatinib mesylate, as well as IFNα, mediate induction of antileukemic responses in CML cells remain to be defined. Understanding such mechanisms would be important for the development of future clinical-translational efforts, as there is accumulating evidence that resistance to imatinib mesylate ultimately emerges in many cases. Meanwhile, clinical trials are currently underway to examine whether combinations of imatinib mesylate with IFNα are more effective in the treatment of the disease than imatinib mesylate alone.
Although IFNα and imatinib mesylate apparently employ distinct mechanisms of action, there is evidence that they may share some downstream effector pathways in chronic myelogenous leukemia cells. At least one growth inhibitory pathway, the p38 Map kinase pathway, has been recently shown to be activated in response to either IFNα42 or imatinib mesylate treatment27 of CML-derived cell lines. This signaling cascade plays a critical role in the generation of the biologic activities of IFNs in a variety of cell types, including normal hematopoietic progenitors43,49 and leukemic progenitors from patients with CML.42 Its activation in response to imatinib mesylate apparently reflects inhibition of BCR-ABL kinase activity,27 which was recently shown to block p38 activation when ectopically expressed in normal hematopoietic progenitors.67 As in the case of IFNα,42 pharmacologic inhibition of the p38 Map kinase pathway reverses the suppressive effects of imatinib mesylate on primary leukemic progenitors from patients with CML,27 indicating that activation of this signaling cascade is essential for the generation of antileukemic effects of both imatinib mesylate and IFNα.
In the present study we examined the effects of IFNα and imatinib mesylate on the activation of pathways that regulate initiation of mRNA translation in BCR-ABL-expressing cells. We specifically examined the effects of either IFNα or imatinib mesylate on the activation of pathways activated downstream of the PI 3′ kinase and/or mTOR in CML-derived cell lines. Our data demonstrate that IFNα induces phosphorylation/activation of mTOR in the IFN-sensitive KT-1 cell line, and downstream phosphorylation of p70 S6 kinase and induction of its kinase domain. In addition, our data provide the first evidence that IFNα induces phosphorylation of the S6 ribosomal protein on Ser 235/236 and Ser 240/244 downstream of the p70 S6 kinase, providing a mechanism by which this cytokine may regulate translation of mRNAs bearing a 5′ terminal oligoyrimidine tract (5′-TOP).57,68,69
Our data also demonstrate that IFNα induces phosphorylation and deactivation of the translational repressor 4E-BP1 in CML-derived cells. We have previously shown that such IFNα-inducible, rapamycin-sensitive, phosphorylation/deactivation of 4E-BP1 also occurs in non-BCR-ABL cell lines and normal hematopoietic cells.44 Such an event is apparently important for IFN-dependent initiation of cap-dependent mRNA translation, as it results in the dissociation of 4E-BP1 from the eIF4E complex.44 Thus, based on these findings, it is likely that activation of the mTOR/p70 S6 kinase pathway plays a positive role in IFNα signaling in BCR-ABL-expressing cells, likely by facilitating initiation of mRNA translation for genes encoding for protein products that regulate generation of IFN responses. As the mTOR/p70 S6 kinase is also known to mediate mitogenic signals, an alternative explanation is that activation of this pathway by IFNα is part of a negative-feedback regulatory or survival response in CML cells. However, this appears unlikely, as a recent report has suggested that rapamycin can reverse the induction of apoptosis by IFNα in U266 myeloma cells, suggesting that mTOR is capable of mediating IFN-dependent proapoptotic signals.54
Recent evidence has shown that the S6 ribosomal protein and 4E-BP1 are phosphorylated in Ba/F3 murine hematopoietic cells, in which BCR-ABL is ectopically expressed.19 It has also been shown that combination of the mTOR inhibitor rapamycin with imatinib mesylate results in enhanced killing of BCR-ABL-expressing cell lines (K562, BCR-ABL-transformed Ba/F3)19,70 or BCR-ABL-transformed myeloid and lymphoid cells in a murine model.70 The findings of the current report are in agreement with these studies, while they provide direct evidence that BCR-ABL induces phosphorylation/activation of the p70 S6 kinase downstream of mTOR. Importantly, our data establish that rapamycin inhibits the growth of human leukemic CFU-CM progenitors from patients with CML, and enhances the effects of imatinib in such primary CML samples. Interestingly, rapamycin also appears to exhibit additive effects with IFNα in suppressing the growth of leukemic CFU-GM progenitors. It is intriguing that pharmacologic inhibition of mTOR enhances the effects of either IFNα or imatinib mesylate, as these agents appear to exhibit opposite effects on the activation of the mTOR/p70 S6 kinase pathway. One potential explanation for such effects is that this pathway mediates generation of diverse and sometimes opposing signals. It is conceivable that activation of mTOR is required for mRNA translation of IFN-inducible genes that mediate antiproliferative responses, as well as for mRNA translation of products that mediate BCR-ABL-dependent mitogenic responses. Competition for the use of this pathway by the activated type I IFN receptor complex and BCR-ABL may account in part for the generation of IFN-dependent antiproliferative responses in CML cells.
It should be pointed out that beyond the well-established role of mTOR in promoting cell growth and proliferation,71 there is accumulating evidence that pathways downstream of mTOR are also capable of promoting apoptosis in response to certain stimuli.54,72,73 Independently of the precise mechanisms involved, our findings demonstrate that rapamycin alone, or in combination with imatinib mesylate and/or IFNα, suppresses the growth of primitive CML precursors and suggest that combined use of these agents may offer a therapeutic advantage in CML. This is of interest as there are ongoing clinical trials aimed toward the development of combination therapies of imatinib mesylate and IFNα for the treatment of CML,74 and rapamycin or other mTOR inhibitors may enhance the effects of both of these agents in vivo. Further studies in that direction are warranted, as they may lead to new combination strategies for the treatment of this leukemia.
Prepublished online as Blood First Edition Paper, March 24, 2005; DOI 10.1182/blood-2004-10-4003.
Supported by National Institutes of Health grants CA94079 and CA77816 (L.C.P.), a Merit review grant from the Department of Veterans Affairs (L.C.P.), and Canadian Institutes of Health Research (CIHR) grant MOP 15094 (E.N.F.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Alfred Rademaker (Northwestern University, Chicago, IL) for providing advice and help with the statistical analysis.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal