Abstract
Flk1, the gene encoding the vascular endothelial growth factor receptor 2 (VEGFR-2), is a well-known marker for vascular and hematopoietic progenitors and is indispensable for normal hematopoiesis and vasculogenesis. Here we show that Flk1 expression in the early mouse embryo marks a broad spectrum of mesodermal progenitors exiting the primitive streak as well as later mesodermal cell types including some cardiomyocytes, portions of the somites, and all extraembryonic mesoderm cells. These findings made use of an Flk1-lacZ knock-in allele in which the neomycin selection cassette was removed, which resulted in full replication of the endogenous expression of Flk1. Targeted deletion of a region in intron 1 that has been proposed to direct endothelial expression produced no alteration in either endothelial or broader mesodermal expression of the Flk1-lacZ allele. Examination of lacZ expression in homozygotes for the Flk1lacZ neo-out allele revealed that lacZ-expressing mesodermal cells persisted in nonvascular regions. Thus, Flk1 expression marks progenitors with broad mesodermal potential but is not absolutely required for the development of all mesodermal lineages in which it is expressed.
Introduction
The mouse Flk1 gene encodes the major signaling receptor, vascular endothelial growth factor receptor 2 (VEGFR-2), for vascular endothelial growth factor A (VEGF-A), and is essential for development of the vascular and hematopoietic systems in the early embryo.1-4 VEGF signaling through VEGFR-2 continues to play key roles in controlling blood-vessel development throughout embryogenesis and into the adult.5,6 In the early embryo and in differentiating embryonic stem (ES) cells, Flk1 expression seems to mark a common progenitor for both blood and endothelium, the so-called hemangioblast.7,8 Recently, it has become evident that the expression of Flk1 may mark progenitors with broader potential than just endothelial formation and hematopoiesis. Single Flk1+ cells derived from differentiating ES cells can produce cells expressing smooth muscle cell or cardiomyocyte markers in vitro.9,10 In addition, multipotential cell lines derived from either the embryonic dorsal aorta11 or from adult bone marrow stroma12 have been shown to express Flk1. However, these experiments do not address whether Flk1 expression also marks cells with broad potential in vivo, nor the functional importance of Flk1 for differentiation of lineages other than endothelium and blood lineages.
In situ expression analysis of endogenous Flk113 or of a Flk1-lacZ knock-in allele3 has shown that Flk1 is expressed in some nonendothelial embryonic cell types, such as the posterior lateral plate mesoderm and the allantois. In addition, sorted Flk1+ cells from E9.5 day embryos have been shown to produce cells expressing smooth muscle cell markers in vitro.14 Lineage tracing of Flk1-expressing cells using Cre recombinase-mediated cell marking indicated that Flk1-expressing cells could also contribute progeny to the cardiac and skeletal muscle cell lineages,15 a finding not predicted by the expression of the endogenous gene. Here we show that the expression of a Flk1-lacZ knock-in allele can also be detected in some cardiomyocytes and in parts of the somites, as well as all extraembryonic mesoderm cells arising from the primitive streak, provided that the neomycin selection cassette is removed from the genome. In addition, deletion of a region in intron 1 that has been proposed to direct endothelial expression produced no alteration in either endothelial or broader mesodermal expression of the Flk1-lacZ allele. Examination of lacZ expression in homozygotes for the Flk1lacZ neo-out allele revealed that all Flk1-expressing lineages, other than endothelial and hematopoietic, were still present in the mutants. Thus, Flk1 expression marks progenitors with broad mesodermal potential but is not required for the development of all lineages in which it is expressed.
Materials and methods
Generation of the Flk1-lacZ neo-out mice
The floxed PGK-neo cassette in our original Flk1 heterozygous mice was removed by crossing with a ubiquitously expressing Cre deleter strain (a gift from A. Nagy, Samuel Lunenfeld Research Institute, Toronto, Canada). Deletion was verified by Southern blot analysis.
Generation of the Flk1-GFP neo-out mice
EGFP cDNA (Clontech, Palo Alto, CA) was introduced into the first exon of the Flk1 gene as before3 and the targeting vector was electroporated into R1 ES cells.16 Correctly targeted events were identified by Southern blot analysis (data not shown). After successful germline transmission of the targeted allele, the floxed PGK-neo cassette was removed by crossing with a Cre deleter strain, and was verified by Southern blot analysis.
Construction of the Flk1-lacZ knock-in vector lacking the first intronic enhancer and establishment of targeted ES cell lines
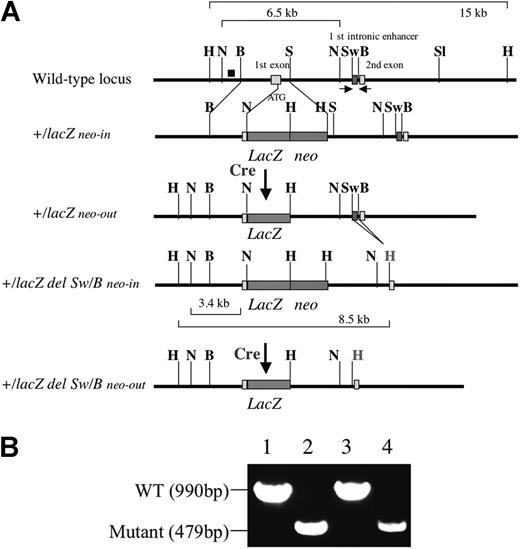
The Flk1-lacZ knock-in vector lacking the first intronic enhancer was constructed as follows. A 4-kb XhoI and SalI fragment harboring the first intronic enhancer was subcloned into the pBKS plasmid and 511 bp containing the putative first intronic enhancer sequence was deleted by SwaI and BamHI digestion and replaced by HindIII linker sequence. The gDNA was introduced between XhoI and NotI of the Flk1 gene in the previously reported Flk1-targeting vector.3 The vector backbone is pPNT-loxP, containing PGK-neo flanked by loxP sites and a PGK-tk cassette for negative selection. The targeting vector was electroporated into R1 ES cells. Correctly targeted ES cell lines were verified by Southern blot analysis using the probe shown in Figure 1. The deletion of the first intronic enhancer was verified by polymerase chain reaction (PCR) amplification using the following primers: forward, 5′-GTGGCCCAGGCTAGTCTCAAACTTGCGGTC-3′, and reverse, 5′-GGGATGGAGAAAATCGCCAGGCAAACCTAG-3′. The wild-type allele gives a 990-bp band, whereas the mutant allele gives a 479-bp band.
Generation of mice from the ES cell lines
Chimeric mice were generated by diploid aggregation with ICR outbred embryos as described previously.16 All animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the University of Tsukuba (Tsukuba, Japan) and the Samuel Lunenfeld Research Institute (Toronto, Canada).
Generation of Flk1+/lacZ neo-out mice and Flk1+/lacZ del Sw/B neo-out mice lacking the first intronic enhancer. (A) Schematic representation of mutant alleles of Flk1 gene used in this paper. The black box (top row) shows probe for Southern blot analysis. Red boxes show first intronic enhancer located between SwaI and BamHI sites. Arrows show a set of primers for PCR analysis. Characters in bold show restriction enzyme digestion sites. H indicates HindIII; N, NcoI; B, BamHI; S, SmaI; and Sw, SwaI. H in red shows HindIII site introduced by HindIII linker. (B) PCR analysis of Flk1 first intronic sequence. PCR was performed using a set of primers shown in panel A to verify the deletion event between SwaI and BamHI. Lane 1, 10 fg Flk1+/lacZ knock-in vector; lane 2, 10 fg Flk1+/lacZ del Sw/B knock-in vector; lane 3, DNA extracted from 8.5 dpc wild-type (WT) embryo; lane 4, DNA extracted from 8.5 dpc Flk1+/lacZ del Sw/B homozygous embryo.
Generation of Flk1+/lacZ neo-out mice and Flk1+/lacZ del Sw/B neo-out mice lacking the first intronic enhancer. (A) Schematic representation of mutant alleles of Flk1 gene used in this paper. The black box (top row) shows probe for Southern blot analysis. Red boxes show first intronic enhancer located between SwaI and BamHI sites. Arrows show a set of primers for PCR analysis. Characters in bold show restriction enzyme digestion sites. H indicates HindIII; N, NcoI; B, BamHI; S, SmaI; and Sw, SwaI. H in red shows HindIII site introduced by HindIII linker. (B) PCR analysis of Flk1 first intronic sequence. PCR was performed using a set of primers shown in panel A to verify the deletion event between SwaI and BamHI. Lane 1, 10 fg Flk1+/lacZ knock-in vector; lane 2, 10 fg Flk1+/lacZ del Sw/B knock-in vector; lane 3, DNA extracted from 8.5 dpc wild-type (WT) embryo; lane 4, DNA extracted from 8.5 dpc Flk1+/lacZ del Sw/B homozygous embryo.
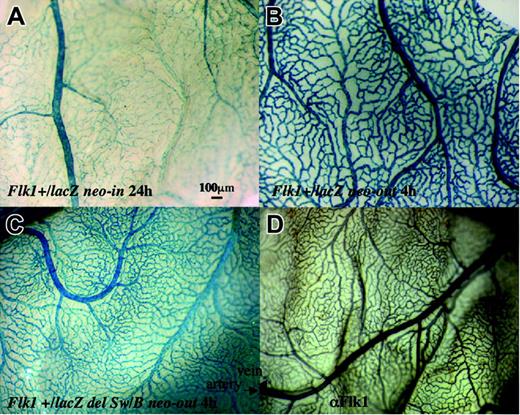
LacZ expression in the yolk sac at 13.5 dpc. (A) lacZ expression in Flk1+/lacZ neo-in embryo. Note small blood vessels between arteries and veins are not stained well after 24 hours of X-gal staining. (B) Expression of lacZ Flk1+lacZ neo-out embryo after 4 hours of X-gal staining. (C) Expression of lacZ in Flk1+/lacZ del Sw/B neo-out embryo. Note that most blood vessels are stained well after 4 hours of X-gal staining. (D) Immunostaining of a wild-type yolk sac using anti-Flk1 antibody. Note that Flk1 antibody stains most blood vessels.
LacZ expression in the yolk sac at 13.5 dpc. (A) lacZ expression in Flk1+/lacZ neo-in embryo. Note small blood vessels between arteries and veins are not stained well after 24 hours of X-gal staining. (B) Expression of lacZ Flk1+lacZ neo-out embryo after 4 hours of X-gal staining. (C) Expression of lacZ in Flk1+/lacZ del Sw/B neo-out embryo. Note that most blood vessels are stained well after 4 hours of X-gal staining. (D) Immunostaining of a wild-type yolk sac using anti-Flk1 antibody. Note that Flk1 antibody stains most blood vessels.
X-gal staining
Immunohistochemistry
The 8.5-dpc embryos were dissected and fixed with 4% PFA overnight. After washing with PBS, the embryos were soaked in 30% sucrose and mounted in OCT compound and 4-μm cryosections were prepared. After blocking reaction in PBS plus 2% skim milk plus 0.1% Tween 20 (PBSMT) for 1 hour, the sections were incubated with rat anti-Flk1 antibody (clone AVAS12; BD Biosciences, San Jose, CA), mouse anti-Pax3, mouse anti-sarcomeric myosin heavy chain (MHC) (clone MF20; Developmental Studies Hybridoma Bank, Iowa, IA), or goat anti-GATA4 (C-20; Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C overnight. The sections were washed twice in PBS plus 0.1% Tween 20 (PBT) for 5 minutes and incubated with Cy3-conjugated anti-rat IgG (Jackson Immunologicals, West Grove, PA), Cy3-conjugated anti-mouse IgG, or Alexa 488-conjugated anti-rat IgG at room temperature for 1 hour. After washing with PBT twice, nuclei were visualized by Hoechst 33342 staining. The images were captured by a Leica DC500 digital camera system (Leica, Wetzlar, Germany).
Results
Expression of lacZ in Flk1+/lacZ neo-out embryos
Introduction of the lacZ gene into the first exon of the Flk1 gene recapitulates the endogenous Flk1 expression in endothelial cells as well as some other mesodermal lineages including allantoic and splanchnic mesoderm in the mouse embryo.3 However, lacZ expression in minor blood vessels of the yolk sac at 13.5 dpc is weak compared with Flk1 antibody staining (Figure 2A,D), as previously reported by Kappel et al.18 They proposed that a cis-element in the small region of the 5′ untranslated region (UTR) that was removed in our lacZ knock-in mice might be critical for full recapitulation of Flk1 expression.18 However, it is also possible that the PGK-neo cassette in the targeted Flk1 allele interferes with lacZ transcription from the Flk1 promoter because transcriptional interference between 2 genes transcribed in parallel can occur very effectively.19
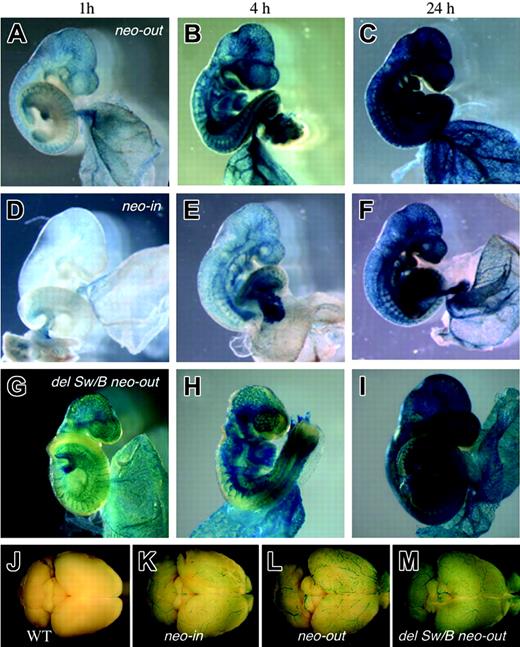
We removed the PGK-neo cassette of Flk1+/lacZ neo-in mice by crossing with a Cre-deleter strain to establish Flk1+/lacZ neo-out mice. The yolk sac of Flk1+/lacZ neo-out embryos at 13.5 dpc showed much stronger β-galactosidase activity than Flk1+/lacZ neo-in embryos (Figure 2B) with a β-galactosidase signal apparent in most, if not all, blood vessels. We also performed a time-course experiment by staining embryos for 1, 4, and 24 hours in X-gal and estimated that the blood vessels in both embryonic and extraembryonic regions were stained 4- to 10-fold more intensively in Flk1+/lacZ neo-out embryos than in Flk1+/lacZ neo-in embryos (Figure 3). The removal of the PGK-neo cassette also resulted in the up-regulation of lacZ expression in endothelial cells in the adult (Figure 3). We thus conclude that there is no evidence for any important regulatory element in the region of the 5′ UTR deleted in the Flk1-lacZ neo-out allele, but rather that the inclusion of the neo cassette in the knock-in allele interferes with full expression from the Flk1 locus.
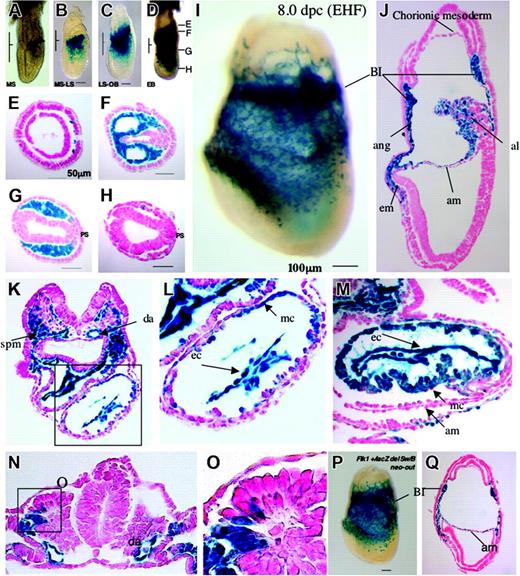
Because the expression of lacZ driven by the Flk1 promoter in the Flk1+/lacZ neo-out allele showed much stronger activity than the original knock-in allele, we reanalyzed the early developmental expression of the Flk1+/lacZ neo-out allele to determine the full range of expression of the gene. The earliest stage at which we could detect the lacZ signal was the mid-streak stage of gastrulation (Figure 4A). Expression of lacZ was seen in all mesoderm cells exiting from the primitive streak in the proximal-posterior region but not in cells arising from the distal-anterior region of the streak that will give rise to node and axial mesoderm (Figure 4D-H). The cells of the streak itself were negative for Flk1-lacZ expression but lacZ turned on as cells exited the streak (Figure 4G). By late streak/early head-fold stage Flk1-lacZ expression was observed in mesodermal cells in the developing cardiac crescent and almost all cells in the extraembryonic mesoderm lineage, including blood island cells, angioblasts, allantois, mesothelium, and amniotic and chorionic mesoderm (Figure 4I-J), whereas age-matched Flk1+/lacZ neo-in embryos showed lacZ expression in blood island cells, angioblasts, and allantois, but not mesothelium, amniotic and chorionic mesoderm, and heart crescent (data not shown). At 8.5 dpc, lacZ signal was seen in endothelial cells in dorsal aorta, splanchnic mesoderm (Figure 4K), and some myocardial cells (Figure 4L), as well as the dorsolateral part of somite that may give rise to dermis, myotome, and potential angioblast precursors (Figure 4N-O).
Flk1 expression in 9.5 dpc embryos carrying the various Flk1 mutant alleles after different reaction times. X-gal staining was performed for 1, 4, and 24 hours using Flk1+/lacZ neo-out mice (A-C), Flk1+/lacZ neo-in mice (D-F), and Flk1+/lacZ del Sw/B neo-out mice (G-I). Note marked increase of lacZ activity in Flk1+/lacZ neo-out embryo compared with that of Flk1+/lacZ neo-in embryo, and that there is no significant change in lacZ activity regardless of the absence of the first intronic enhancer. (J-M) Surface view of adult brains stained with X-gal for 16 hours isolated from wild-type (J), Flk1+/lacZ neo-in (K), Flk1+/lacZ neo-out (L), and Flk1+/lacZ Sw/B neo-out mice (M). Note that lacZ expression in Flk1+/lacZ neo-out mice is stronger than Flk1+/lacZ neo-in.
Flk1 expression in 9.5 dpc embryos carrying the various Flk1 mutant alleles after different reaction times. X-gal staining was performed for 1, 4, and 24 hours using Flk1+/lacZ neo-out mice (A-C), Flk1+/lacZ neo-in mice (D-F), and Flk1+/lacZ del Sw/B neo-out mice (G-I). Note marked increase of lacZ activity in Flk1+/lacZ neo-out embryo compared with that of Flk1+/lacZ neo-in embryo, and that there is no significant change in lacZ activity regardless of the absence of the first intronic enhancer. (J-M) Surface view of adult brains stained with X-gal for 16 hours isolated from wild-type (J), Flk1+/lacZ neo-in (K), Flk1+/lacZ neo-out (L), and Flk1+/lacZ Sw/B neo-out mice (M). Note that lacZ expression in Flk1+/lacZ neo-out mice is stronger than Flk1+/lacZ neo-in.
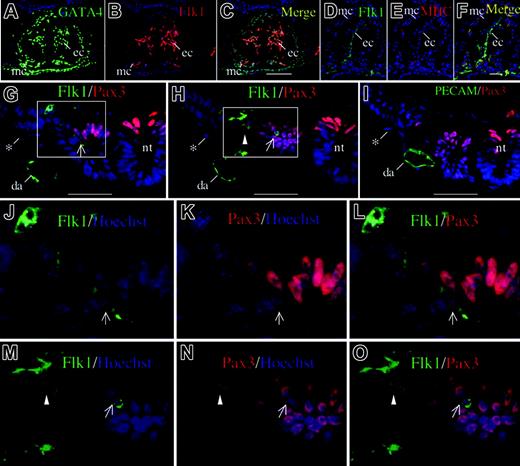
Thus, Flk1 expression, as revealed by the enhanced expression of the Flk1-lacZ neo-out allele, seems to be activated in all mesoderm cells arising from the posterior primitive streak, not just the endothelium and blood islands. However, expression of Flk1 lacZ in myocardium and in the somites cannot be easily explained by Flk1 expression in posterior streak progenitors, because these do not normally give rise to these structures.20 It is unlikely, then, that expression of the Flk1lacZ neo-out allele in myocardium reflects perdurance of β-galactosidase expression from any earlier progenitor. To confirm that the lacZ expression in myocardium reflects endogenous Flk1 protein expression, an immunohistochemical analysis was performed using anti-Flk1 antibody and cell lineage markers such as GATA4, Pax3, and sarcomeric MHC (Figure 5). Moderate Flk1 protein expression was confirmed in myocardial cells coexpressing MHC and GATA4, as well as the endocardial cells of the heart. Moderate Flk1 protein expression was also seen in the dorsolateral part of the somite colocalized with Pax3 (Figure 5) and cells migrating out of the somite (Figure 5). Flk1-Pax3 double-positive cells were observed inside the somite and migrating out of the somite along the whole body axis (data not shown).
Effect of deletion of first intronic enhancer on Flk1 gene expression
Kappel and coworkers identified an enhancer within the first intron as sufficient for Flk1 gene expression in angioblasts and endothelial cells in transgenic animals.16 They also showed that Tal1, GATA2, and Ets factors act cooperatively on the enhancer to direct endothelial expression.21 To determine whether this enhancer sequence is also required for Flk1 expression in other mesodermal lineages, we constructed a lacZ knock-in vector for the Flk1 locus that lacks the enhancer sequence from the SwaI to the BamHI site (Figure 1). The targeting vector was introduced into ES cells and targeting events were confirmed by Southern blot analysis (data not shown). The deletion of the enhancer sequence was confirmed by PCR analysis using the primers flanking the enhancer (Figure 1B). After germline transmission of the targeted allele, the PGK-neo cassette was removed by Cre excision as before. Removal of the neo cassette increased lacZ activity as observed in Flk1+/lacZ neo-in/out mice (data not shown). Comparison of the levels and location of X-gal staining between the Flk1+/lacZ del Sw/B neo-out and the Flk +/lacZ neo-out embryos revealed no significant differences in lacZ activity (Figure 3). LacZ signal was seen in most of the blood vessels in the yolk sac at 13.5 dpc after 4 hours of X-gal staining, just as observed in Flk1+/lacZ neo-out embryos (Figure 2C). At 8.0 dpc, lacZ was seen in a wide range of mesodermal lineages including blood island cells, allantoic, chorionic, and amniotic mesoderm, angioblasts, and heart crescent (Figure 4P-Q). LacZ expression was also observed in adult blood vessels (Figure 3). Thus deletion of the first intronic enhancer sequence did not change the pattern or level of the lacZ expression significantly, indicating that, although this enhancer may be sufficient for endothelial expression, it is not necessary for induction or maintenance of Flk1 expression in any of its well-characterized domains of expression.
Early developmental expression of the Flk1+/lacZ neo-out allele. Expression of lacZ in 6.5 to 8.5 dpc Flk1+/lacZ neo-out embryos (A-O) and Flk1+/lacZ del Sw/B neo-out embryos (P-Q). (A) Mid-streak stage. (B) Transition between mid-streak stage and late-streak stage. Note that Flk1+ cells are located in embryonic lateral mesoderm. (C) Transition between late-streak stage and no-bud (OB) stage. Note that Flk1+ cells are seen in extraembryonic mesoderm layer as well as embryonic mesoderm. (D) Early bud (EB) stage. Note that Flk1+ cells are seen in broad region in extraembryonic and intraembryonic mesoderm. Panels E-H show sections at the levels marked. (E) A proximal tip of Flk1+ cells located at extraembryonic mesoderm. (F) A section through the extraembryonic region. (G) A section at the embryonic region most proximal to extraembryonic region. Note that most of mesodermal cells except for primitive streak (PS) region are Flk1+. (H) A section at a distal embryonic region. (I) Lateral view of Flk1+/lacZ neo-out embryo at late head-fold (LHF) stage after 4 hours of X-gal staining. (J) A sagittal section of panel I. Note that lacZ signal is seen in most of the extraembryonic mesoderm including blood island cells, mesothelial, amniotic, chorionic and allantoic mesoderm, and also in some embryonic mesoderm. Bl indicates blood island; ang, angioblast; AM, amniotic mesoderm; al, allantois; em, embryonic mesoderm. (K) A transverse section at heart level after 4 hours of X-gal staining. Note that lacZ is seen in endothelial cells of dorsal aorta, splanchnic mesoderm, endocardium, and some cells of the myocardium. (L) Higher magnification of the heart in panel K. (M) A transverse section at heart level after 24 hours of X-gal staining. Note that many myocardial cells are Flk1+. (N) A transverse section at somite level. Activity of lacZ is seen in cells of the dorsal part of the somite. (O) Higher magnification of Flk1+ cells in panel N. (P) Lateral view of Flk1+/lacZ del Sw/B neo-out embryo at LHF stage after 4 hours of X-gal staining. Note that there is no significant difference between panels I and P. (Q) A sagittal section of panel P. Note that there is no significant expressional change between panels J and Q.
Early developmental expression of the Flk1+/lacZ neo-out allele. Expression of lacZ in 6.5 to 8.5 dpc Flk1+/lacZ neo-out embryos (A-O) and Flk1+/lacZ del Sw/B neo-out embryos (P-Q). (A) Mid-streak stage. (B) Transition between mid-streak stage and late-streak stage. Note that Flk1+ cells are located in embryonic lateral mesoderm. (C) Transition between late-streak stage and no-bud (OB) stage. Note that Flk1+ cells are seen in extraembryonic mesoderm layer as well as embryonic mesoderm. (D) Early bud (EB) stage. Note that Flk1+ cells are seen in broad region in extraembryonic and intraembryonic mesoderm. Panels E-H show sections at the levels marked. (E) A proximal tip of Flk1+ cells located at extraembryonic mesoderm. (F) A section through the extraembryonic region. (G) A section at the embryonic region most proximal to extraembryonic region. Note that most of mesodermal cells except for primitive streak (PS) region are Flk1+. (H) A section at a distal embryonic region. (I) Lateral view of Flk1+/lacZ neo-out embryo at late head-fold (LHF) stage after 4 hours of X-gal staining. (J) A sagittal section of panel I. Note that lacZ signal is seen in most of the extraembryonic mesoderm including blood island cells, mesothelial, amniotic, chorionic and allantoic mesoderm, and also in some embryonic mesoderm. Bl indicates blood island; ang, angioblast; AM, amniotic mesoderm; al, allantois; em, embryonic mesoderm. (K) A transverse section at heart level after 4 hours of X-gal staining. Note that lacZ is seen in endothelial cells of dorsal aorta, splanchnic mesoderm, endocardium, and some cells of the myocardium. (L) Higher magnification of the heart in panel K. (M) A transverse section at heart level after 24 hours of X-gal staining. Note that many myocardial cells are Flk1+. (N) A transverse section at somite level. Activity of lacZ is seen in cells of the dorsal part of the somite. (O) Higher magnification of Flk1+ cells in panel N. (P) Lateral view of Flk1+/lacZ del Sw/B neo-out embryo at LHF stage after 4 hours of X-gal staining. Note that there is no significant difference between panels I and P. (Q) A sagittal section of panel P. Note that there is no significant expressional change between panels J and Q.
Flk1 protein expression in heart and somite in 8.5 dpc wild-type embryo (7 somite pair stage). (A-C) Immunostaining of heart using anti-GATA4 antibody (A) and anti-Flk1 antibody (B) in a transverse section, and the merged image (C). Signals are seen in endothelial cells in endocardium (ec), and some myocardial cells (mc). Scale bar is 100 μm. (D-F) Immunostaining of heart using anti-Flk1 (D) and anti-sarcomeric MHC antibody MF20 (E) in a transverse section and the merged image (F). Scale bar is 50 μm. (G-I) Immunostaining of somite using anti-Flk1 (G-H) or anti-platelet endothelial cell adhesion molecule (anti-PECAM; I) in combination with anti-Pax3. *Nonspecific signal in visceral endoderm. Scale bar is 50 μm. (G) An Flk1+ cell coexpressing Pax3 is seen in the dorsolateral part of the somite. (H) Coexpression of Flk1 and Pax3 in a cell migrating out of somite (arrowhead). (I) Exclusive expression of PECAM and Pax3; da indicates dorsal aorta; nt, neural tube. (J-O) High-power magnification of Flk1-Pax3 double-positive cells. An arrowhead indicates an Flk1-Pax3 double-positive cell migrating out of the somite, and arrows indicate cells within the somite. Hoechst nuclear staining is shown in blue.
Flk1 protein expression in heart and somite in 8.5 dpc wild-type embryo (7 somite pair stage). (A-C) Immunostaining of heart using anti-GATA4 antibody (A) and anti-Flk1 antibody (B) in a transverse section, and the merged image (C). Signals are seen in endothelial cells in endocardium (ec), and some myocardial cells (mc). Scale bar is 100 μm. (D-F) Immunostaining of heart using anti-Flk1 (D) and anti-sarcomeric MHC antibody MF20 (E) in a transverse section and the merged image (F). Scale bar is 50 μm. (G-I) Immunostaining of somite using anti-Flk1 (G-H) or anti-platelet endothelial cell adhesion molecule (anti-PECAM; I) in combination with anti-Pax3. *Nonspecific signal in visceral endoderm. Scale bar is 50 μm. (G) An Flk1+ cell coexpressing Pax3 is seen in the dorsolateral part of the somite. (H) Coexpression of Flk1 and Pax3 in a cell migrating out of somite (arrowhead). (I) Exclusive expression of PECAM and Pax3; da indicates dorsal aorta; nt, neural tube. (J-O) High-power magnification of Flk1-Pax3 double-positive cells. An arrowhead indicates an Flk1-Pax3 double-positive cell migrating out of the somite, and arrows indicate cells within the somite. Hoechst nuclear staining is shown in blue.
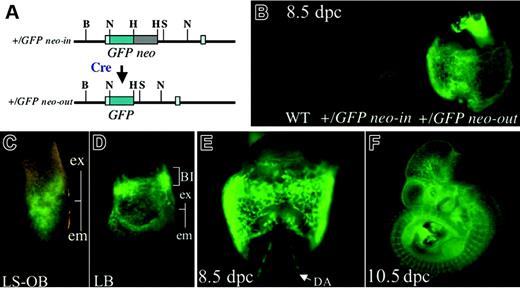
GFP expression in Flk1+/GFP embryo. (A) Schematic representation of GFP knock-in allele. (B) The effect of deletion of the PGK-neo cassette in Flk1+/GFP neo-in and Flk1+/GFP neo-out embryos at E8.5. (C) GFP expression during LS-OB stage. (D) GFP expression at the late bud (LB) stage. (E) GFP expression at 8.5 dpc. (F) GFP expression at 10.5 dpc; ex indicates extraembryonic region; em, embryonic region; BI, blood island; and DA, dorsal aorta.
GFP expression in Flk1+/GFP embryo. (A) Schematic representation of GFP knock-in allele. (B) The effect of deletion of the PGK-neo cassette in Flk1+/GFP neo-in and Flk1+/GFP neo-out embryos at E8.5. (C) GFP expression during LS-OB stage. (D) GFP expression at the late bud (LB) stage. (E) GFP expression at 8.5 dpc. (F) GFP expression at 10.5 dpc; ex indicates extraembryonic region; em, embryonic region; BI, blood island; and DA, dorsal aorta.
Generation of Flk1-GFP mice
Given the evidence that the knock-in allele of Flk1 can replicate the full endogenous expression of Flk1 once the selection cassette used for targeted clone selection was removed, we made another targeting construct replacing lacZ with the EGFP reporter. After germline transmission, without neo excision it was difficult to observe GFP expression (Figure 6B). However, after neo excision, the GFP knock-in allele produced clear fluorescence in all domains of Flk1 expression even from the primitive streak stage (Figure 6C-F). This allele should therefore prove very useful for separating and analyzing Flk1+ progenitors at different stages of development.
Contribution of Flk1-null cells to cardiac and skeletal muscle cells
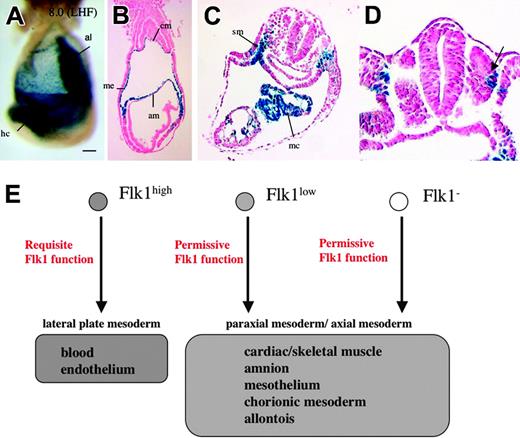
Flk1 is necessary for the development of hematopoietic and endothelial cells in the embryo.3,4 Because Flk1 appears to be expressed in a broader spectrum of mesodermal lineages, we asked whether or not Flk1 is also required for the development of these cells by investigating the contribution of Flk1-null cells (lacZ+ cells) to these lineages. At 8.0 dpc, strong or moderate lacZ expression was seen in heart crescent, amniotic, allantoic, and chorionic mesoderm, and mesothelium of homozygous Flk1lacZ neo-out embryos after 8 hours of X-gal staining, whereas blood island cells and angioblasts were absent (Figure 7A-B). At 8.5 dpc, lacZ signals persisted in splanchnic mesoderm, myocardium, and the dorsolateral part of the somites, although all blood vessels were absent (Figure 7C-D). No obvious morphologic abnormalities were seen in the nonendothelial Flk1-lacZ-expressing cell lineages in the null mutants. Thus, Flk1 seems to be indispensable for hematopoietic and endothelial development but not for other mesodermal lineages expressing the marker.
Discussion
By deleting the neomycin resistance cassette in the previously described Flk1-lacZ knock-in allele, we have found that Flk1 expression marks a broad spectrum of mesodermal progenitors including cardiac and skeletal muscle precursors in the early mouse embryo. Our findings are consistent with the recent cell fate tracing experiment by Motoike and coworkers, using Cre mice under the control of the Flk1 promoter, crossed with lacZ Cre-reporter mice.15 They claimed that Flk1+ cells contribute to parts of skeletal and cardiac muscle as early as E10.5. They did not see any contribution of Flk1-lineage marked cells to skeletal and cardiac muscle cells until E10.5, whereas the contribution of Flk1+ cells to cardiac and skeletal muscle are apparent at E8.5 in our study. This difference might reflect less than maximal Cre activity from their Flk1-Cre mice because these mice still carry the PGK-neo cassette in the Flk1-targeted allele.
As for the Flk1 expression in somites, it is of note that the dorsolateral part of the somite in chick embryo is shown to give rise to endothelium of the dorsal aorta22 and also lymphatic endothelium in the limb bud.23 Kardon and coworkers claimed that there is a common somitic precursor for endothelium and skeletal muscle in limb.24 However, it is not clear whether Flk1+ cells in the dorsolateral part of somite mark these endothelial progenitors in the mouse embryo because these marked cells persist in the Flk1 homozygous mutant embryos. In addition, at least some of the Flk1+ cells also express Pax3. Recent Cre lineage-tracing experiments using Pax3-Cre mice identified somitic derivatives and neural crest but not endothelial cells as Pax3 derivatives.25 Thus further investigation of the identity of the Flk1+ precursors in the somites is needed.
Expression of lacZ in Flk1-null embryos. (A) Lateral view of a Flk1-lacZ neo-out/EGFP embryo at LHF stage after 8 hours of X-gal staining. Scale bar is 100 μm; al indicates allantois; hc, heart crescent. (B) A transverse section of panel A. Note that lacZ signals are seen in amniotic (am), mesothelial (me), and chorionic mesoderm (cm) layer. (C) A transverse section of 8.5 dpc Flk1-lacZ neo-out/EGFP embryo at the level of heart. The lacZ+ cells are seen at splanchnic mesoderm (sm) and myocardium (mc). (D) Note that the dorsolateral part of somite is stained by X-gal. (E) A model for the differentiation of Flk1+ cells and the function of Flk1.
Expression of lacZ in Flk1-null embryos. (A) Lateral view of a Flk1-lacZ neo-out/EGFP embryo at LHF stage after 8 hours of X-gal staining. Scale bar is 100 μm; al indicates allantois; hc, heart crescent. (B) A transverse section of panel A. Note that lacZ signals are seen in amniotic (am), mesothelial (me), and chorionic mesoderm (cm) layer. (C) A transverse section of 8.5 dpc Flk1-lacZ neo-out/EGFP embryo at the level of heart. The lacZ+ cells are seen at splanchnic mesoderm (sm) and myocardium (mc). (D) Note that the dorsolateral part of somite is stained by X-gal. (E) A model for the differentiation of Flk1+ cells and the function of Flk1.
We also observed widespread expression of Flk1-lacZ in all the mesoderm lineages derived from the posterior of the primitive streak, namely, all extraembryonic mesoderm cells as well as embryonic splanchnic mesoderm. We previously reported that, in Flk1 null mutants, Flk1-lacZ-expressing cells were observed in the amniotic mesoderm and splanchnic mesoderm in apparently increased numbers over Flk1-lacZ heterozygotes.3 We suggested that, in the absence of active Flk1 signaling, Flk1-expressing progenitors that would normally make endothelium and blood islands were redirected into these other lineages. However, it now seems likely that this apparent redirection simply reflects the increased level of lacZ expression in the homozygous embryos, allowing detection of expression in these cells. In this current study, extensive Flk1-lacZ expression was observed in amniotic mesoderm, yolk sac mesothelium, and splanchnic mesoderm in heterozygous as well as homozygous mice. No obvious difference in patterns of expression was observed in these lineages between the 2 genotypes. Thus, it seems that Flk1 expression marks a broad range of mesoderm cells at gastrulation but is only required functionally in endothelium and hematopoietic cells.
Expression of Flk1, as revealed by the lacZ and GFP alleles generated here, seems to mark mesodermal progenitors arising from a specific region of the primitive streak, as well as particular subsets of mesodermal cells in the heart and somite, that may include muscle progenitors. Expression in the cells arising from the streak suggests that Flk1 is activated by patterning signals that determine the fate of cells exiting from different regions of the streak, such as fibroblast growth factors (FGFs), BMPs, and Wnts. Activation of Flk1 in a common progenitor that will give rise to multiple mesodermal cell types also is consistent with evidence that ES cells go through a stage during embryoid body formation, where many cells express Flk1, although only a subset of Flk1+ cells go on to form endothelium or blood cells.26,27 Expression of Flk1 in common mesodermal progenitors is also consistent with recent results showing that cells with hemangioblast properties can be isolated directly from the primitive streak of 7.5 day mouse embryos.8
Accumulating evidence indicates that Flk1 may mark other types of multipotent stem cell populations in the adult and during embryogenesis. Multipotential mesenchymal stem cells isolated from adult bone marrow express Flk1 protein, although the expression level is moderate.12 Flk1 is also a marker of hematopoietic stem cells in adult bone marrow.28 Multipotent mesodermal (mesoangioblast) cells derived from developing dorsal aorta express Flk1 and have the potential to differentiate into many types of mesodermal cells.11 Yamashita and coworkers claimed that there is a common Flk1+ progenitor that gives rise to both smooth muscle and endothelial cells in the embryo.9 We have proposed that the Flk1+ population represents a pool of progenitors that can make multiple mesodermal cell types, dependent on the coexpression of different transcription factors,29 and have shown that levels of one such transcription factor, Scl/Tal1, could alter the fate of the Flk1 progenitor.14 Gering and coworkers showed that overexpression of lmo2 and SCL, downstream genes from Flk1, in zebrafish causes cell-fate skewing from mesodermal cells toward endothelial cells in head, pronephros, and cardiac mesoderm in the absence of GATA-1.30
Exactly how only a subset of the Flk1+ cells respond to VEGF signaling and become hemangioblast progenitors in vivo, whereas other Flk1+ cells coming out of the streak do not require Flk1 for their survival is not yet clear. One possibility is that levels of Flk1 signaling activity may regulate the cell fate of Flk1+ cells. High Flk1 activity, perhaps in response to the VEGF-Aproduced in visceral endoderm, would result in migration of the cell to the extraembryonic region and promote differentiation toward hematopoietic and endothelial lineages, whereas low Flk1 activity would lead to retention of cells in the intraembryonic region where they would be exposed to other inductive signals along the anteroposterior (A-P) and dorsoventral (D-V) axis. Consistent with this idea, Fong and coworkers showed that Flt1-deficient embryos, which show deregulated VEGF signaling, develop increased blood progenitors and blood vessels at the expense of other mesodermal cell types.31 Thus, levels of VEGF signaling may play roles in influencing the fate of Flk1+ mesodermal progenitors in many different conditions. The Flk1-GFP neo-out allele we have generated will be very useful for analyzing the full potential of Flk1-expressing cells from different regions of the embryo and adult.
Little is known about what signals upstream of Flk1 activate its expression in the different regions of the embryo. Kappel and coworkers have identified an enhancer sequence located in intron 1 that appeared to be important for Flk1 expression in angioblasts and endothelial cells, but not early mesodermal cells.16 This might suggest that different upstream signals drive expression in endothelial versus other mesodermal progenitors. To test this, we generated mice lacking the enhancer by targeted deletion in the context of the endogenous gene. This enabled us to evaluate the impact of the deletion of the enhancer sequence on endogenous Flk1 expression as detected by the lacZ neo-out reporter. Our data clearly demonstrated that deletion of the enhancer sequences causes no loss of Flk1 expression in either endothelial cells or other mesodermal progenitors. Thus, the first intron enhancer is sufficient but not necessary for endothelial expression of Flk1. Major cis-acting elements required for the regulation of Flk1 expression in mesodermal lineages must reside elsewhere in the genome and need to be explored further to understand the regulation and function of Flk1 in different mesodermal progenitors.
Prepublished online as Blood First Edition Paper, September 15, 2005; DOI 10.1182/blood-2005-05-1970.
Supported by a Terry Fox Program project grant administered by the National Cancer Institute of Canada. M.E. was supported in part by Special Coordination Funds for Promoting Science and Technology and the Nakajima Foundation. J.R. is a Canadian Institutes of Health Research (CIHR) Distinguished Investigator.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Mr Ken Harpal and Ms Naomi Kaneko for histologic assistance. M.E. thanks Dr Yojiro Yamanaka for helpful comments on this project.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal