Clinical success for gene therapy of hemophilia A will be judged by achievement of sustained, therapeutic levels of coagulation factor VIII (fVIII). Previous clinical trials have suffered from transient, subtherapeutic expression of human fVIII transgenes. Porcine fVIII contains sequence elements that enable more efficient biosynthesis than human fVIII due to enhanced posttranslational transit through the secretory pathway. In this study, we evaluated ex vivo retroviral gene transfer of a high-expression porcine fVIII transgene into bone marrow–derived stromal and hematopoietic stem/progenitor cells (MSCs and HSCs, respectively) and transplantation into genetically immunocompetent hemophilia A mice. Both MSCs and HSCs demonstrated high-level expression of porcine fVIII in vivo. However, following transplantation of gene-modified MSCs, fVIII activity levels rapidly returned to baseline due to the formation of anti–porcine fVIII–neutralizing antibodies. Alternatively, transplantation of HSCs into myeloablated and nonmyeloablated hemophilia A mice resulted in high-level fVIII expression despite low-level hematopoietic reconstitution by gene-modified cells. FVIII expression was sustained beyond 10 months, indicating that immunologic tolerance to porcine fVIII was achieved. Furthermore, transplantation of bone marrow from primary recipients into naive secondary recipients resulted in sustained, high-level fVIII expression demonstrating successful genetic modification and engraftment of HSCs.
Introduction
Congenital hemophilia A is an X-chromosome linked recessive bleeding disorder resulting from mutations within the F8 gene. Hemophilia A is treated effectively by administration of fVIII protein–containing products. Cloning of the F8 gene in 19841 facilitated the development of recombinant fVIII protein products, which became commercially available in the early 1990s. This was an important therapeutic improvement due to the safety advantage recombinant products have over plasma-derived products, which proved responsible for the infection of thousands of hemophilia patients with human immunodeficiency virus and/or hepatitis virus during the 1980s. The current limitations of treatment for hemophilia are (1) access to factor VIII replacement products, (2) the cost of goods for factor VIII replacement products, (3) the development of humoral anti–factor VIII immune responses, and (4) morbidity due to joint disease. Two basic strategies for improving hemophilia care are (1) to develop improved recombinant protein products (eg, manufactured more efficiently, have increased hemostatic efficacy, or are less immunogenic) and (2) to develop a cure (ie, to provide a lifelong source of endogenous factor VIII production that completely eliminates the need for protein replacement therapy). Over the past 2 decades, several academic and corporate laboratories have developed gene transfer–based strategies for treating hemophilia A, leading to the initiation of 3 human clinical trials.2-4 Due to transient low-level fVIII expression and lack of therapeutic efficacy, none of these trials proceeded beyond phase 1.
Significant advances have been made toward the development of fVIII transgenes that are expressed at higher levels.5-7 Recently, we demonstrated that B-domain–deleted (BDD) porcine fVIII is expressed at 10- to 14-fold greater levels than BDD human fVIII in vitro.8 In a subsequent study, we identified sequences within porcine fVIII that are sufficient for high-level expression and revealed that the interspecies expression differential results from enhanced secretion.5 However in these studies, we did not test whether these high-expression elements function in gene transfer–based settings in vivo.
The combination of ex vivo genetic modification and transplantation of bone marrow–derived cell types is a promising approach for the correction of many inherited diseases. Hematopoietic stem cells (HSCs), which have been used extensively in the clinic, are easily transplanted, undergo self-renewal, and repopulate the hematopoietic compartment following transplantation. Marrow-derived stromal cells (MSCs) are advantageous in that they are easily recovered from whole bone marrow, can be expanded in vitro, and can be genetically modified using standard gene-transfer techniques.9-11 MSCs contain a subpopulation of multipotent stem cells that possess the ability to differentiate into several mesenchymal tissues such as bone, adipose, cartilage, and myelosupportive stroma.10 The studies described here were designed to evaluate the potential of a high-expression porcine fVIII construct in gene therapy strategies incorporating ex vivo gene transfer into bone marrow–derived cell types.
Materials and methods
Materials
DNA and RNA extraction kits were purchased from Qiagen (Valencia, CA). Polymerase chain reaction (PCR) reagents were purchased from Applied Biosystems (Foster City, CA). All antibodies were purchased from BD Pharmingen (San Diego, CA). Purified coagulation factor proteins were obtained from Enzyme Research Laboratory (South Bend, IN), and the factor Xa chromogenic substrate was purchased from American Diagnostica (Stamford, CT). Cell culture media and supplements were purchased from Invitrogen (Carlsbad, CA). Magnetic separation columns were purchased from Miltenyi Biotec (Auburn, CA). Pooled normal human plasma (FACT) and human fVIII–deficient plasma was purchased from George King Biomedical (Overland Park, KS). Activated partial thromboplastin reagent was purchased from Organon Technika (Durham, NC).
Methods
Mice. Exon 16–disrupted hemophilia A mice12 backcrossed to a C57BL/6 genetic background were obtained from Dr Leon Hoyer (Holland Laboratories, American Red Cross) and a breeding colony was established. Transgenic mice expressing enhanced green fluorescence protein (EGFP) from the β-actin promoter on a C57BL/6 genetic background (strain designation: C57BL/6-Tg(Act-EGFP)C14-Y01-FM131 Osb) were a gift from Dr Masaru Okabe (Osaka University) and are maintained by Dr David Archer at Emory University.13 EGFP transgenic mice were used as congenic donors in the HSC transplantation experiments. Male and female mice aged 8 to 15 weeks were used in this study. Total body irradiation (TBI) was performed using a Gammacell 40 Exactor (Nordion, Ottawa, ON).
Production of MSCV-fVIII vectors and viral concentrates. BDD human fVIII and BDD porcine fVIII cDNAs were cloned into the murine stem cell virus (MSCV) expression plasmid pMSCV (BD Biosciences-Clontech, Mountain View, CA). Stable ecotropic-pseudotyped viral producer cell lines were established using the following method. First, a viral producer cell line (BD RetroPack PT67; BD Biosciences-Clontech) was transfected transiently with the MSCV–BDD human fVIII or MSCV–BDD porcine fVIII plasmids. Dualtropic (pseudotyped with 10A1 envelope) virus containing medium was collected daily and placed onto the ecotropic packaging cell line, BD EcoPack2 (BD Biosciences-Clontech). Following 3 rounds of transduction, conditioned medium was collected daily, filtered with a 0.45-μm filter, and stored at –80°C until use. Virus was concentrated overnight by centrifugation at 9000g, at 4°C, resuspended in 1/20th the original volume and stored at –80°C in 1-mL aliquots. The number of functional viral particles (fvp) per milliliter was determined by transduction of 105 NIH 3T3 mouse fibroblasts and analysis of the number of proviral genomes present per host cell genome equivalent using quantitative polymerase chain reaction (qPCR) of genomic DNA obtained 48 hours after transduction. The oligonucleotide sequences were as follows: human fVIII forward, 5′-GGCATGGAAGCTTATGTCAAAGTAGACAGC-3′ and reverse, 5′-AGTCCTCCTCTTCAGCAGCAATGT-3′; porcine fVIII forward, 5′-CGGAGGAAAGCTGATGAAGAGG-3′ and reverse, 5′-GCAGAGATGTAGTGCACCCAGG-3′. The PCR reaction conditions used were as described previously,8 except that the initial 30-minute incubation (reverse-transcription reaction) at 48°C was omitted. Calculation of the proviral copy number was achieved by interpolation from a standard curve consisting of MSCV-fVIII plasmid DNA serially diluted into untransduced NIH 3T3 genomic DNA. A conversion factor of 6 pg/diploid genome equivalent was used to calculate the copy number on a per cell basis. This protocol was used to establish stable ecotropic retroviral producer cell lines for MSCV–BDD human fVIII and MSCV–BDD porcine fVIII that routinely produced viral stocks containing 105 to 106 fvp/mL following concentration. Ecotropic-pseudotyped MSCV also was used to transduce PT67 packaging cells and generate stably modified cell lines producing recombinant (fVIII-expressing) 10A1-pseudotyped virus.
MSC isolation, transduction, and fVIII expression. MSCs were harvested and expanded in culture as described previously.14 Briefly, whole bone marrow was flushed from tibias and femurs of hemophilia A mice. Isolated cells were disaggregated and seeded onto tissue culture plates at a concentration of 5 × 106 cells/mL. MSCs were separated from hematopoietic cells by their selective adherence to tissue culture plastic and survival following serial passage. After passage 6, MSCs were subjected to viral transduction using MSCV–human fVIII or MSCV–porcine fVIII. Subsequently, the average number of proviral integration events was determined by qPCR as described in “Production of MSCV-fVIII vectors and viral concentrates” for viral titering. Steady-state fVIII mRNA levels and fVIII expression levels were determined as described previously.8
Isolation and transduction of murine stem cell antigen-1 (Sca-1)–positive cells. Positive immunomagnetic bead selection was used to isolate Sca-1+ and c-kit+ cells (populations containing HSCs and other progenitor cells) from bone marrow harvested from femurs and tibias of mice. Using this method, we routinely obtain more than 90% Sca-1+ cells after isolation, and these cells remain Sca-1+ throughout the stimulation and transduction period (data not shown). Following separation, the cells were stimulated for 2 days in serum-free media containing murine stem cell factor (100 ng/mL), murine interleukin-3 (20 ng/mL), human interleukin-11 (100 ng/mL), and human Flt-3 ligand (100 ng/mL). One million cells were transduced consecutively on days 3 and 4 with 106 fvp each day. Transduced cells were transplanted intravenously into recipient mice via tail-vein injection. Total donor cell (EGFP+) engraftment was analyzed by flow cytometry. Gene-marking analysis was performed using qPCR as described above for viral titering.
FVIII analyses. One-stage coagulation and chromogenic fVIII activity assays were performed as described previously.8,15 A standard curve was generated by reconstituting plasma derived from hemophilia A mice with recombinant porcine fVIII. Production, purification, and characterization of recombinant BDD porcine fVIII has been described.8 Total anti-fVIII IgG levels were determined by enzyme-linked immunosorbent assay (ELISA) as described previously.16,17 FVIII inhibitory activity was determined using a modified Bethesda assay18 in which experimental plasma samples were incubated with human fVIII–deficient plasma reconstituted with recombinant BDD porcine fVIII and assayed for residual fVIII activity.19
Results
In vitro fVIII expression from genetically modified MSCs
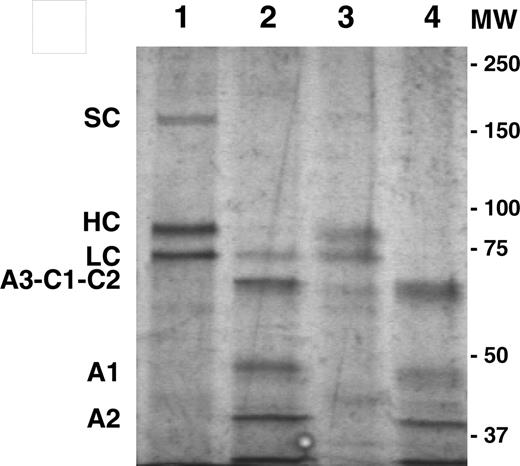
Previously, we demonstrated the superiority of BDD porcine fVIII and certain hybrid human/porcine fVIII transgenes for recombinant fVIII production from heterologous cell culture systems.5,8 In the current study, porcine fVIII expression from 2 promising target cell populations, MSCs and HSCs, was analyzed using an MSCV-based recombinant retroviral vector system and the murine model of hemophilia A. First, dualtropic (10A1) or ecotropic-pseudotyped MSCV-based retroviral particles were generated containing either the BDD human or porcine fVIII transgene. Next, MSCs were isolated from the bone marrow of syngeneic hemophilia A mice. Flow cytometry analysis of isolated MSCs demonstrated them to be CD106+, Sca-1+, CD45–, CD34low, Flk-1–, c-kit–, similar to the previously published cell surface phenotype for murine C57BL/6-derived MSCs (data not shown).20 MSCs were expanded in culture, transduced with MSCV–human fVIII or MSCV–porcine fVIII at equal multiplicity of infection (MOI), and analyzed for gene marking, viral RNA levels, and fVIII production (Table 1). Despite similar integration and RNA levels between the human and porcine fVIII transgene–modified cells, porcine fVIII production levels were 6-fold higher than that of human fVIII (P = .004, Mann-Whitney U test). Repeated transduction of MSCs with MSCV–porcine fVIII resulted in increased fVIII production. Following 3 rounds of transduction, genetically modified MSCs produced BDD porcine fVIII at a rate of 14 units/106 cells per 24 hours in serum-free medium and contained steady-state fVIII mRNA levels of 950 transcripts/cell. MSC-derived BDD porcine fVIII was purified and displayed mobility indistinguishable from BHK-M–derived BDD porcine fVIII upon sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; Figure 1).
Human and porcine fVIII expression from genetically modified MSCs
Transgene . | DNA copy no., fVIII transgenes per diploid genome equivalent . | FVIII transcripts per cell* . | FVIII activity, milliunits/24 h† . |
|---|---|---|---|
| Human fVIII | 0.15‡ | 140 | 14 |
| Porcine fVIII | 0.18§ | 116 | 84 |
Transgene . | DNA copy no., fVIII transgenes per diploid genome equivalent . | FVIII transcripts per cell* . | FVIII activity, milliunits/24 h† . |
|---|---|---|---|
| Human fVIII | 0.15‡ | 140 | 14 |
| Porcine fVIII | 0.18§ | 116 | 84 |
MSCs were transduced with MSCV-human fVIII or MSCV-porcine fVIII at equal MOI and analyzed for gene marking, viral RNA levels, and fVIII production by qPCR, qRT-PCR, and chromogenic fVIII activity assay.
n = 3.
n = 6.
n = 2.
n = 3.
In vivo fVIII expression from genetically modified MSCs
We next tested whether genetically modified MSCs continue to express BDD porcine fVIII in vivo. Five million MSCV–porcine fVIII–transduced or untransduced (control) MSCs were seeded onto microcarrier beads (Figure 2A) and transplanted into the peritoneal cavity of immunocompetent hemophilia A mice. Micro-carrier beads were used to provide an attachment substrate for the MSCs. Plasma collected from mice that received a transplant of untransduced MSCs did not contain detectable fVIII activity (data not shown), while mice that received a transplant of genetically modified MSCs demonstrated plasma fVIII activity levels of 0.91 ± 0.25 units/mL (all data are presented as mean ± sample standard deviation) 5 days after transplantation (Figure 2B). However, fVIII activity levels rapidly returned to baseline by day 7 after transplantation. Subsequent analysis of plasma samples from day 7 and beyond revealed the presence of anti–porcine fVIII–neutralizing antibodies. The anti-fVIII immune response intensified over the next 6 weeks in terms of total anti-fVIII IgG and anti-fVIII–neutralizing activity.
In vitro characterization of genetically modified MSCs. SDS-PAGE analysis of MSC-derived (lanes 1-2) and BHK-M–derived (lanes 3-4) BDD porcine fVIII. Samples in lanes 2 and 4 were pretreated with thrombin to demonstrate proteolytic activation. Single chain (SC), heavy chain (HC), light chain (LC), and A3-C1-C2, A1, and A2 polypeptides are labeled. Proteins were visualized by silver stain. The positions of the molecular weight (MW) standards (in kDa) are shown to the right of the gel image.
In vitro characterization of genetically modified MSCs. SDS-PAGE analysis of MSC-derived (lanes 1-2) and BHK-M–derived (lanes 3-4) BDD porcine fVIII. Samples in lanes 2 and 4 were pretreated with thrombin to demonstrate proteolytic activation. Single chain (SC), heavy chain (HC), light chain (LC), and A3-C1-C2, A1, and A2 polypeptides are labeled. Proteins were visualized by silver stain. The positions of the molecular weight (MW) standards (in kDa) are shown to the right of the gel image.
In vivo expression of BDD porcine fVIII from MSCs. (A) Micrograph of BDD porcine fVIII–expressing MSCs bound to dextran microcarrier beads. Image was visualized under an Olympus IX50 inverted microscope equipped with a 4 ×/0.13 objective lens (Olympus, Melville, NY). An Optronics Magnafire camera (Optronics, Goleta, CA) was used to capture images, and Magnafire 2.1 software to acquire and process them. (B) Plasma fVIII activity levels (•) following intraperitoneal transplantation of porcine fVIII–expressing MSCs bound to microcarrier beads into hemophilia A mice (n = 4). Anti–porcine fVIII ELISA (○) and Bethesda titers (▵) are shown (mean ± standard deviation).
In vivo expression of BDD porcine fVIII from MSCs. (A) Micrograph of BDD porcine fVIII–expressing MSCs bound to dextran microcarrier beads. Image was visualized under an Olympus IX50 inverted microscope equipped with a 4 ×/0.13 objective lens (Olympus, Melville, NY). An Optronics Magnafire camera (Optronics, Goleta, CA) was used to capture images, and Magnafire 2.1 software to acquire and process them. (B) Plasma fVIII activity levels (•) following intraperitoneal transplantation of porcine fVIII–expressing MSCs bound to microcarrier beads into hemophilia A mice (n = 4). Anti–porcine fVIII ELISA (○) and Bethesda titers (▵) are shown (mean ± standard deviation).
It was possible that the dextran microcarrier beads or intraperitoneal route of administration was critical to the development of anti-fVIII immune responses. To test this hypothesis, we introduced unbound MSCs into hemophilia A mice, either into the peritoneal cavity or intravenously, and monitored plasma fVIII activity (Figure 3A-B). Again, all mice developed high-titer, anti–porcine fVIII inhibitory antibodies. Therefore, neither the microcarrier beads nor the intraperitoneal route of administration was the sole determinant for the development of anti-fVIII immune responses.
In vivo fVIII expression from genetically modified hematopoietic cells
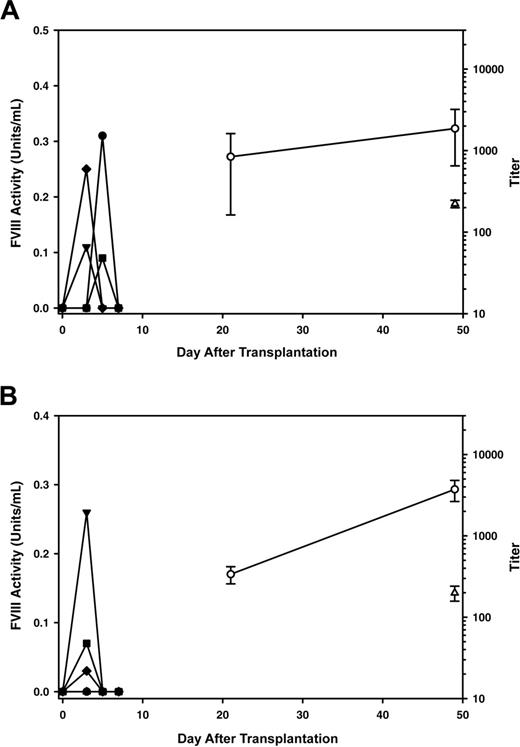
A similar ex vivo gene-transfer strategy was used to test whether sustained, high-level expression of BDD porcine fVIII could be achieved in vivo following transplantation of genetically modified HSCs. Sca-1+ cells isolated from EGFP-transgenic mice, a population of bone marrow cells that contains hematopoietic stem/progenitor cells,21 were transduced ex vivo with MSCV–porcine fVIII. Following transduction, the cells contained 0.8 proviral genomes per diploid genome equivalent. Recipient mice were preconditioned with a lethal dose of TBI prior to transplantation of 3 × 105 transduced cells. All mice that underwent transplantation demonstrated sustained, high-level plasma fVIII activity for more than 10 months after transplantation (Figure 4A). Flow cytometry analysis of peripheral blood mononuclear cells (PBMCs) harvested 8 weeks after transplantation demonstrated more than 95% total donor (EGFP+) cell engraftment and the presence of donor cells in each hematopoietic compartment (data not shown). However, engraftment of genetically modified cells was lower than expected based on pretransplantation marking levels, with posttransplantation levels ranging from 0.06 to 0.4 proviral genomes per diploid genome equivalent.
Comparison of intraperitoneal versus intravenous delivery of BDD porcine fVIII–expressing MSCs. Plasma fVIII activity levels (closed symbols) were measured at several time points following intraperitoneal (A, n = 4) or intravenous (B, n = 5) transplantation of unbound BDD porcine fVIII–expressing MSCs into hemophilia A mice. Anti–porcine fVIII ELISA (○) and Bethesda titers (▵) are shown (mean ± standard deviation). In panels A and B, one plasma sample was not available in sufficient quantity for Bethesda analysis (n = 3 and n = 4, respectively).
Comparison of intraperitoneal versus intravenous delivery of BDD porcine fVIII–expressing MSCs. Plasma fVIII activity levels (closed symbols) were measured at several time points following intraperitoneal (A, n = 4) or intravenous (B, n = 5) transplantation of unbound BDD porcine fVIII–expressing MSCs into hemophilia A mice. Anti–porcine fVIII ELISA (○) and Bethesda titers (▵) are shown (mean ± standard deviation). In panels A and B, one plasma sample was not available in sufficient quantity for Bethesda analysis (n = 3 and n = 4, respectively).
MSCs derived from C57BL/6 mice, including the ones that were used and described in the current study, have been shown to express Sca-1. Therefore, it was possible that the fVIII expression observed in the animals that received a transplant of transduced Sca-1+ cells originated from genetically modified MSCs cells and not hematopoietic cells. In order to verify that hematopoietic cells can express fVIII in vivo, we isolated a second population of cells expressing c-kit+. C-kit is not expressed on the surface of MSCs, but is expressed on HSCs and other hematopoietic cells. C-kit+ cells were transduced as described for the Sca-1+ cells and transplanted into recipient hemophilia A mice that had been subjected to 11 Gy TBI (Figure 4B). Sustained circulating fVIII levels of 1.5 ± 0.8 were observed at 13 weeks after transplantation.
In vivo expression of BDD porcine fVIII from genetically modified hematopoietic cells. Plasma fVIII activity levels (symbols) were determined following transplantation of BDD porcine fVIII transgene–modified hematopoietic progenitor cells into hemophilia A mice. Recipient mice were preconditioned with a lethal dose of TBI (11 Gy) and received a transplant of 3 × 105 transduced Sca-1+ cells (A), 3 × 105 transduced c-kit+ cells (B), or 2 × 106 whole bone marrow cells (C) derived from one of the animals that underwent transplantation in panel A. Each line represents an individual mouse.
In vivo expression of BDD porcine fVIII from genetically modified hematopoietic cells. Plasma fVIII activity levels (symbols) were determined following transplantation of BDD porcine fVIII transgene–modified hematopoietic progenitor cells into hemophilia A mice. Recipient mice were preconditioned with a lethal dose of TBI (11 Gy) and received a transplant of 3 × 105 transduced Sca-1+ cells (A), 3 × 105 transduced c-kit+ cells (B), or 2 × 106 whole bone marrow cells (C) derived from one of the animals that underwent transplantation in panel A. Each line represents an individual mouse.
Long-term restoration of circulating fVIII to the Sca-1+ animals that underwent transplantation suggested that genetically modified HSCs engrafted and proliferated, and the progeny expressed fVIII. To corroborate this conclusion, we performed secondary transplantation of bone marrow cells from one of the primary recipient animals into naive recipient hemophilia A mice (Figure 4C). All secondary recipient animals demonstrated sustained fVIII production at levels similar to the primary recipient animal (1 ± 1 unit/mL) for more than 15 weeks after transplantation.
The success of the myeloablative transplantation protocol prompted us to test whether similar results could be achieved using a reduced-intensity, sublethal conditioning regimen, which is decidedly more attractive for the treatment of hemophilia A. Similar to the previous experiment, Sca-1+ cells were transduced with MSCV–porcine fVIII at an MOI = 2, and 3 × 105 cells were transplanted into hemophilia A mice that received 5.5 Gy TBI before transplantation (Figure 5). To confirm that this dose of TBI was sublethal to hemophilia A mice, a cohort of hemophilia A mice (n = 3) was irradiated without subsequent bone marrow transplantation and all mice survived. Sustained fVIII levels of 2.1 ± 2 units/mL were observed out to 35 weeks after transplantation in all mice that received MSCV–porcine fVIII–transduced Sca-1+ cells. At 7 weeks after transplantation, the mice contained 70% to 80% donor PBMCs and an average proviral genome copy number of 0.05 ± 0.02 (range, 0.02-0.07) per diploid genome equivalent. Furthermore, no anti-fVIII antibodies were detected by ELISA (data not shown).
Discussion
To date, the treatment of hemophilia A in humans using gene transfer has been unsuccessful due to subtherapeutic fVIII expression levels.2-4 One strategy for increasing fVIII expression levels is to bioengineer the fVIII transgene itself. We and others have been successful in increasing fVIII expression levels by introducing genetic changes into the human fVIII transgene that enhance the secretory efficiency.5-7 Our strategy is founded on the observation that BDD porcine fVIII and certain hybrid human/porcine fVIII constructs containing the porcine high-expression elements are expressed at 10-fold or greater levels than BDD human fVIII from baby hamster kidney cells.8 The studies described here were designed to test the functionality of porcine high-expression sequence elements in vivo using 2 clinically viable gene therapy strategies.
In vivo expression of BDD porcine fVIII under reduced-intensity transplantation conditioning. Plasma fVIII activity levels (symbols) were determined following transplantation of BDD porcine fVIII transgene–modified Sca-1+ cells into hemophilia A mice. Recipient mice were preconditioned with a sublethal dose of TBI (5.5 Gy) and received a transplant of 3 × 105 transduced Sca-1+ cells. Each line represents an individual mouse (n = 5). One mouse died 2 weeks after transplantation due to isoflurane overexposure during blood collection.
In vivo expression of BDD porcine fVIII under reduced-intensity transplantation conditioning. Plasma fVIII activity levels (symbols) were determined following transplantation of BDD porcine fVIII transgene–modified Sca-1+ cells into hemophilia A mice. Recipient mice were preconditioned with a sublethal dose of TBI (5.5 Gy) and received a transplant of 3 × 105 transduced Sca-1+ cells. Each line represents an individual mouse (n = 5). One mouse died 2 weeks after transplantation due to isoflurane overexposure during blood collection.
The use of MSCs and HSCs for gene therapy of hemophilia A has been described previously.22-26 The first reported studies using transplantation of fVIII genetically modified bone marrow cells into fVIII-deficient mice did not result in the expression of therapeutic levels of fVIII, possibly due to inefficient secretion of human fVIII from bone marrow–derived cell types.22-24 In a study by Chuah et al, human and murine MSCs were transduced with a BDD human fVIII–containing retrovirus and transplanted into nonobese diabetic–severe combined immunodeficient mice.22 The estimated peak circulating human fVIII activity level achieved was 0.07 units/mL, which is less than 10% of that observed in the current study. Additionally, we directly compared human and porcine fVIII expression from gene-modified MSCs and observed that porcine fVIII was expressed at 6-fold greater levels than human fVIII following transduction at equivalent MOIs and similar steady-state fVIII mRNA levels (Table 1). Furthermore, following additional rounds of transduction, BDD porcine fVIII expression (on a per fVIII mRNA basis) from MSCs was comparable with that achieved previously from a baby hamster kidney–derived (BHK-M) cell line used in commercial manufacturing of recombinant fVIII.8 Therefore, we conclude that high-expression elements within porcine fVIII are functional in a viral gene transfer setting.
The brisk immune response to porcine fVIII following MSC transplantation apparently prevented the restoration of therapeutic fVIII levels. Currently, we do not know whether the transplanted MSCs (1) engrafted and continued to produce fVIII, (2) engrafted but stopped expressing fVIII, or 3) were cleared by a cytotoxic immune response. It is of note that several studies have reported that MSCs possess immunosuppressive properties resulting from both soluble and contact-dependent factors.27-29 Recently, Eliopoulos et al reported that they did not observe a humoral immune response to a specific transgene product, human erythropoietin, following transplantation of congenic MSCs into immunocompetent C57Bl/6 mice.30 In contrast, if major histocompatibility complex–mismatched allogeneic Balb/c MSCs expressing erythropoietin were transplanted, they observed both a cytotoxic immune response against the donor MSCs and a humoral immune response against human erythropoietin. Although we did not investigate the possibility of a cytotoxic immune response against the genetically modified MSCs, the difference in transgene product immunogenicity between our study and that of Eliopoulos et al30 could have resulted from several factors, including the different sites of MSC administration, the lack of use of an artificial collagen matrix in the present study, or intrinsic differences in the immunogenicity of fVIII compared with human erythropoietin. It is possible that approaches designed to suppress or avoid immunologic recognition (eg, administration of alkylating agents) could be used to facilitate the engraftment of genetically modified MSCs. Alternatively, transplantation of gene-modified MSCs could be performed following immune tolerance induction in humans or modeled in mice using neonatal tolerance induction.31
Initial studies of human fVIII expression from hematopoietic cells following ex vivo transduction and transplantation suggested that these cell populations do not express fVIII efficiently.23,24 Recently, Moayeri et al demonstrated long-term expression of a bioengineered human fVIII transgene, designed for increased secretory efficiency, from genetically modified bone marrow cells.25,26 The lack of anti-fVIII immune response observed by Moayeri et al, and confirmed in our study, using nonmyeloablative transplant-conditioning regimens, demonstrates the feasibility of performing low-risk hematopoietic stem cell gene therapy protocols in humans.
However, there are several factors that may hinder translation of hematopoietic stem cell gene therapy for hemophilia A to clinical application. These factors include (1) obtaining efficient genetic modification of HSCs, (2) risks associated with transplant conditioning, and (3) pathogenesis from insertional mutagenesis. In order to obtain a sufficient number of genetically modified bone marrow cells, Moayeri et al used a vector construct that expressed an EGFP reporter gene in addition to the fVIII transgene.25,26 The reporter gene facilitated the selection of transduced cells by fluorescence automated cell sorting. Therefore, the authors were able to transplant genetically modified cells exclusively, which may not be feasible in human clinical trials due to the larger number of cells required for transplantation. Additionally, the presence of a second neoantigen (EGFP) could decrease the engraftment of gene-modified cells due to cytotoxic targeting from the host immune system. In nonhuman primate studies and human clinical trials performed to-date, the range of percent gene-modified cells following engraftment is typically less than 1% to 15%.32-37 In the study by Moayeri et al, mice conditioned with 5.5 Gy TBI displayed 20% to 30% genetically modified cells after transplantation and contained circulating fVIII levels of 0.12 ± 0.08 units/mL.25 Using a high-expression porcine fVIII transgene and a sublethal 5.5 Gy TBI preconditioning regimen, we obtained engraftment levels of gene-modified cells at 0.05 proviral genomes per diploid genome equivalent. This low-level engraftment was sufficient to restore fVIII levels to more than 200% of the normal human level. If one assumes that the circulating fVIII activity levels are linearly proportional to the gene-marking level, then even a 40-fold reduction in gene marking would result in therapeutic fVIII levels (≥ 5%). We conclude that bioengineered fVIII transgenes containing high-expression elements from porcine fVIII will be a critical component to overcoming the fVIII expression barrier that has plagued previous gene therapy of hemophilia A clinical trials.
Prepublished online as Blood First Edition Paper, January 31, 2006; DOI 10.1182/blood-2005-12-4961.
Supported by grants from the Emory University Research Committee (C.B.D.) and the Woodruff Health Sciences Center Woodruff Fund (C.B.D.). Additional financial support was provided by the Gene Therapy Program Initiative at Children's Healthcare of Atlanta (H.T.S. and C.B.D.).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal