Abstract
Monocytes are major mediators of inflammation, and apoptosis provides a mechanism for regulating the inflammatory response by eliminating activated macrophages. Furthermore, as a consequence of apoptosis, plasminogen binding is markedly increased on monocytoid cells. Therefore, we investigated the ability of plasminogen to modulate monocyte apoptosis. Apoptosis of monocytoid cells (human monocytes and U937 cells) was induced with either TNFα or cycloheximide. When apoptosis was induced in the presence of increasing concentrations of plasminogen, apoptosis was inhibited in a dose-dependent manner with full inhibition achieved at 2 μM plasminogen. Plasminogen treatment also markedly reduced internucleosomal DNA fragmentation and reduced levels of active caspase 3, caspase 8, and caspase 9 induced by TNFα or by cycloheximide. We examined the requirement for plasmin proteolytic activity in the cytoprotective function of plasminogen. A plasminogen active site mutant, [D(646)E]-Plg, failed to recapitulate the cytoprotective effect of wild-type plasminogen. Furthermore, antibodies against PAR1 blocked the antiapoptotic effect of plasminogen. Our results suggest that plasminogen inhibits monocyte apoptosis. The cytoprotective effect of plasminogen requires plasmin proteolytic activity and requires PAR1. Because apoptosis of monocytes plays a key role in inflammation and atherosclerosis, these results provide insight into a novel role of plasminogen in these processes.
Introduction
Monocytes are pivotal regulators of inflammation.1,2 In response to inflammatory stimulation, monocytes become activated and migrate to sites of inflammation. Monocytes and macrophages undergo apoptosis during inflammation in vivo.3 Induction of apoptosis thus provides a mechanism for homeostatic regulation of inflammation by eliminating activated and differentiated monocytes/macrophages.
Modulation of the inflammatory response by plasminogen is supported by observations in vivo. Monocyte recruitment is decreased in Plg–/– mice, compared with wild-type mice, following thioglycollate injection to induce a peritoneal inflammatory reaction.4,5 Plg–/– mice also exhibit reduced transplantation arteriosclerosis.6 In addition, elevated levels of α2-antiplasmin are detected in synovial fluid of affected joints in patients with arthritis.7 Furthermore, in response to inflammatory cytokines the plasminogen gene is up-regulated,8-11 leading to increased circulating levels of plasminogen.8
An emerging area of research strongly supports a role for the plasminogen activation system in regulation of apoptosis. With adherent cells, plasmin promotes anoikis, depriving cells of a necessary survival signal resulting from the loss of cell-matrix interactions, leading to programmed cell death. For example, plasmin degrades the hippocampal extracellular matrix protein, laminin, in response to excitotoxin treatment, leading to neuronal cell detachment and cell death.12,13 Similarly, plasmin-dependent anoikis is observed with adherent smooth muscle cells,14,15 retinal cells,16 and fibroblast cell lines.17
Plasminogen binding capacity is markedly enhanced on late apoptotic/necrotic U937 monocytoid cells following treatment with cycloheximide,18,19 suggesting a potential role for cell-associated plasminogen in regulation of apoptosis. Monocytoid cells do not depend on adherence for a survival signal. Thus, any effects of plasminogen on monocytoid cell apoptosis should be independent of anoikis. Therefore, we have investigated the role of plasminogen in cytokine-dependent apoptosis in monocytoid cells, using an autocrine physiologic modulator of monocytoid apoptosis, TNFα.
Materials and methods
Cells and cell culture
U937 cells were cultured in RPMI-1640 (Gibco, Gaithersburg, MD) with 10% heat-inactivated fetal bovine serum (FBS; Gemini Bioproducts, Woodland, CA), 2 mM glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 10 mM HEPES, 1 mM sodium pyruvate, 4.5 mg/mL glucose, and 1.5 mg/mL bicarbonate, at 37°C in humidified air with 5% CO2.
Mononuclear cells were isolated from freshly donated human blood by using the standard Ficoll-Hypaque isolation procedure. Fresh human blood, diluted with sterile phosphate-buffered saline (PBS),1 was layered over Ficoll-Hypaque (Pharmacia, Alameda, CA) and centrifuged at 400g for 35 minutes. The interfacial layer containing monocytic cells was washed 3 times with sterile PBS containing 2 mM EDTA. Cells were resuspended in monocyte media [DMEM, 15% heat-inactivated fetal bovine serum, 2 mM glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 0.5 μM β-mercaptoethanol, 0.4 nM macrophage colony-stimulating factor (Calbiochem, La Jolla, CA) and nonessential amino acids] and allowed to adhere to Petri dishes (Corning, Santa Clara, CA) for 2 hours to select for monocytes. Cells were then rinsed 5 times with sterile PBS and cultured for 1 day in media containing 10% FBS. Monocytes were identified by fluorescence-activated cell sorting (FACS) analysis using forward scatter and side scatter and lack of reactivity with PE-labeled anti–human CDllb/Mac1 (BD Biosciences, Oxnard, CA).
In preparation for apoptosis assays, cells were washed once with 200 mM ϵ-aminocaproic acid (EACA) to remove any cell-associated plasminogen and then washed with serum-free RPMI-1640 media. The cells were then resuspended at a concentration of 1 × 106 cells/mL in RPMI-1640 media or monocyte media containing 1% plasminogen-depleted FBS (unless otherwise indicated) and plated on 96-well plates (Corning).
Proteins
Glu-plasminogen was purified from fresh human blood as described previously.20 Blood was collected into 3 mM EDTA, 100 U/mL Trasylol (Serologicals, Norcross, GA), and 100 μg/mL soybean trypsin inhibitor (Sigma, St Louis, MO). The plasma was subjected to affinity chromatography on lysine-Sepharose in phosphate-buffered saline with 1 mM benzamadine, 0.02% NaN3, and 3 mM EDTA, followed by molecular exclusion chromatography on Biogel A 1.5 M (Bio-Rad, Hercules, CA). The plasminogen concentration was determined spectrophotometrically at 280 nm by using an extinction coefficient of 16.8. Plasminogen was filtered on a 0.22-μm filter (Costar, Santa Clara, CA).
Elastase degradation products of plasminogen, [kringle 1-3 (K1-3, Tyr79-Val337, orTyr79-Val353), kringle 4 (K4, Val354-Ala439), kringle 5-latent protease domain (K5-PD, Val442-Asn790)] were prepared according to the method of Sottrup-Jensen et al.21 The characteristics of such preparations from our laboratory have been described previously.22-24 Recombinant plasminogen kringle 5 (K5) was a gift from Dr Don Davidson, Abbott Laboratories (Abbott Park, IL).
[D(646)E]-Plg was generated by primer-directed mutagenesis of single-stranded p119/HPg25 as described26 and expressed in baculovirus-infected lepidopteran cells, followed by purification on lysine-Sepharose.27 The characteristics of this mutant have been published.28
Prothrombin, thrombin, and α2-antiplasmin were obtained from Enzyme Research Laboratories (South Bend, IN). Thrombin activity was assessed in kinetic assays using the thrombin substrate, S-2238 (Chromogenix, West Chester, OH). Bovine serum albumin was purchased from Calbiochem and RNase from Sigma.
Plasminogen-depleted FBS was prepared by incubating 20 mL heat-inactivated FBS with 10 mL lysine-Sepharose beads for 18 hours at 4°C. The unbound supernatant was incubated a second time with the lysine-Sepharose beads and then applied to a rabbit anti–human plasminogen polyclonal antibody affinity column. The unbound material was collected and filtered using a 0.22-μM filter (Corning). Depletion of plasminogen was greater than 99.9% as verified by Western blotting with an antiplasminogen monoclonal antibody (Mab 51).29
Mouse anti-PAR1 (ATAP2) and mouse primary antibody isotype control were purchased from Zymogen Laboratories (San Francisco, CA).
Fluorescence-activated cell sorting (FACS)
In preparation for FACS analysis, U937 cells were washed 3 times with Hanks Balanced Salt Solution (HBSS; pH 7.2), and subconfluent adherent monocytes were harvested by rinsing plates twice with HBSS and then detaching with Cell Dissociation Buffer Enzyme-Free Hanks-based (Gibco) for 15 minutes at 37°C. Cells were washed twice with HBSS. For FACS analysis, the cells were resuspended in 100 μL annexin V Binding Buffer (100 mM HEPES pH 7.4, 1.5 M NaCl, 50 mM KCl, 10 mM MgCl2, 18 mM CaCl2). Fluorescein isothiocynate (FITC)–labeled annexin V (2 μL; R&D Systems, Minneapolis MN) was added, and cells were incubated for 15 minutes at 22°C in the dark. Immediately prior to FACS analysis, 5 μg/mL propidium iodide (PI) was added for dual-color FACS analysis. Cell populations were gated according to the presence or absence of FITC and PI staining using a FACScan (Becton Dickinson, Oxnard CA). Cell viability status was defined as follows: viable, annexin V negative, PI negative; early apoptotic, annexin V positive, PI negative; late apoptotic/necrotic, annexin V positive, PI positive.
Apoptosis assays
To assess internucleosomal DNA fragmentation, DNA was harvested and purified using the Puregene DNA Purification Kit (Gentra Systems, Minneapolis, MN), and 1 μg DNA was electrophoresed on a 1.5% low-melt agarose gel containing ethidium bromide. Caspase 3 activity was measured using the Caspase 3 Activity Assay, a fluorometric immunosorbent enzyme assay (Roche, Palo Alto CA). Caspase 8 and 9 activities were measured, separately, using either the Carboxyfluorescein FLICAApoptosis Detection Kit Caspase Assay for caspase 8 or for caspase 9 (Immunochemistry Technologies, Bloomington, MN) followed by FACS analysis.
Reagents
Recombinant human tumor necrosis factor-α (TNFα) was from R&D Systems. Cycloheximide (CHX) was from Calbiochem. The thrombin substrate, S-2238, was from Chromogenix.
Statistics
All data are presented as mean ± SD or mean ± SE (at least 3 independent experiments). Statistical significance (P < .05) was determined using a 2-tailed Student t test.
Results
Plasminogen binding
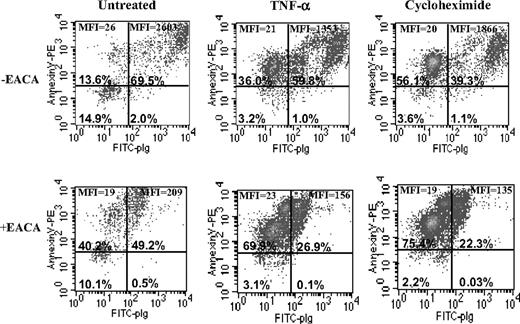
We studied the effect of a physiologic autocrine inducer of monocyte apoptosis, TNFα,30 on plasminogen binding to early apoptotic (annexin V positive, PI negative) monocytoid cells. The cells were treated with either TNFα or cycloheximide, and then 3-color FACS analysis was performed. In these experiments the cells were incubated simultaneously with PI, annexin V–phycoerythrin, and FITC-plasminogen, and fluorescence was monitored in 3 channels. Then, after gating cells for size (forward scatter) versus PI, 3 nonoverlapping subpopulations were identified: viable, early apoptotic, and late apoptotic/necrotic. We analyzed the early apoptotic populations only (after gating out only these cells) for FITC-plasminogen binding (Figure 1). The early apoptotic cell subpopulation contained cells with very high plasminogen-binding ability as shown in Figure 1, upper right quadrants. The binding of FITC-plasminogen was specific because the extent of binding was inhibited in the presence of EACA, a lysine analog that binds to the plasminogen kringles and interferes with the interaction of plasminogen with cells.31 Thus, plasminogen binding was markedly increased during the early stage of apoptosis.
Plasminogen inhibits apoptosis
We examined the effect of plasminogen on monocytoid cell apoptosis. U937 cells were incubated in either the presence or absence of TNFα and in the presence or absence of increasing concentrations of plasminogen. The viability status of the cells was determined by dual-color FACS analysis with annexin V–PE and PI. Treatment with TNFα induced apoptosis and decreased the percentage of viable cells (Figure 2A) while increasing the percentage of early apoptotic cells (Figure 2B) and slightly increasing the percentage of late apoptotic/necrotic cells within the population (Figure 2C). In the presence of plasminogen, monocytoid apoptosis induced with TNFα was neutralized (Figure 2). The effect was dose dependent, and, at its plasma and extracellular fluid concentration (2 μM32 ), plasminogen fully neutralized the effect of TNFα, by decreasing the percentage of both early apoptotic cells (Figure 2B) and late apoptotic cells (Figure 2C), and restoring the percentage of viable cells (Figure 2A) to that in the absence of TNFα. Plasminogen treatment, alone, did not affect cell viability (Figure 2A-C).
Plasminogen binding is stimulated on early apoptotic cells. U937 cells were either untreated or incubated with 30 ng/mL TNFα or 50 μg/mL cycloheximide for 18 hours at 37°C. To identify early apoptotic cells that specifically bound plasminogen, 3-color FACS analysis was performed with annexin V–phycoerythrin, PI, and FITC-plasminogen (0.2 μM) in the presence or absence of EACA (0.2 M). The cells were gated for size (forward scatter versus PI), and 3 nonoverlapping subpopulations were identified: viable, early apoptotic, and late apoptotic/necrotic. The early apoptotic cells were gated out and analyzed for FITC-plasminogen binding in the presence or absence of EACA, and only the early apoptotic cells are shown here. Because TNFα- and CHX-induced cells resulted in a larger population of early apoptotic cells, there are varying numbers of cells in each condition, but the total number of cells initially analyzed was 20 000. The percentage of early apoptotic cells in each quadrant is shown. MFI indicates mean fluorescence intensity.
Plasminogen binding is stimulated on early apoptotic cells. U937 cells were either untreated or incubated with 30 ng/mL TNFα or 50 μg/mL cycloheximide for 18 hours at 37°C. To identify early apoptotic cells that specifically bound plasminogen, 3-color FACS analysis was performed with annexin V–phycoerythrin, PI, and FITC-plasminogen (0.2 μM) in the presence or absence of EACA (0.2 M). The cells were gated for size (forward scatter versus PI), and 3 nonoverlapping subpopulations were identified: viable, early apoptotic, and late apoptotic/necrotic. The early apoptotic cells were gated out and analyzed for FITC-plasminogen binding in the presence or absence of EACA, and only the early apoptotic cells are shown here. Because TNFα- and CHX-induced cells resulted in a larger population of early apoptotic cells, there are varying numbers of cells in each condition, but the total number of cells initially analyzed was 20 000. The percentage of early apoptotic cells in each quadrant is shown. MFI indicates mean fluorescence intensity.
Because TNFα treatment results in cessation of protein synthesis, we examined the ability of plasminogen to neutralize induction of apoptosis by cycloheximide, in a second model of apoptosis. Cycloheximide treatment markedly decreased viability of U937 cells (Figure 2D) while increasing the percentage of early apoptotic cells (Figure 2E). Similar to the results shown in Figure 2A-C with TNFα-induced apoptosis, plasminogen neutralized the effect of cycloheximide-induced loss of cell viability, by restoring the percentage of viable cells to that in the absence of cycloheximide treatment (Figure 2D-E).
We examined the time dependence of the effect of plasminogen on apoptosis. U937 cells were either untreated or incubated with TNFα or cycloheximide. Plasminogen (2 μM) was added at different time points. The extent of plasminogen-dependent inhibition of apoptosis was the same whether plasminogen was added 3 hours prior to, simultaneously, or 3 hours following the addition of these inducers of apoptosis. When plasminogen was added 15 hours following treatment with the apoptosis inducers, 50% of the cytoprotective effect against TNFα treatment was retained and 25% of the cytoprotective effect against cycloheximide treatment was retained. However, when plasminogen was added 24 hours following induction of apoptosis, cytoprotection was not observed (data not shown).
Plasminogen inhibits U937 cell apoptosis in a dose-dependent manner. U937 cells were either untreated (♦) or incubated with 10 ng/mL TNFα (□; A-C) or 50 μg/mL cycloheximide (○; D-F), in either the absence or presence of increasing concentrations of plasminogen for 24 hours at 37°C. Cell viability status was determined by dual-color FACS analysis with annexin V–FITC and PI as described in “Materials and methods,” and the percentage of viable (annexin V negative, PI negative), early apoptotic (annexin V positive, PI negative), and late apoptotic/necrotic (annexin V positive, PI positive) cells within the entire cell population is shown. Data are shown as mean ± SE, n = 3, for a representative of 6 independent experiments.
Plasminogen inhibits U937 cell apoptosis in a dose-dependent manner. U937 cells were either untreated (♦) or incubated with 10 ng/mL TNFα (□; A-C) or 50 μg/mL cycloheximide (○; D-F), in either the absence or presence of increasing concentrations of plasminogen for 24 hours at 37°C. Cell viability status was determined by dual-color FACS analysis with annexin V–FITC and PI as described in “Materials and methods,” and the percentage of viable (annexin V negative, PI negative), early apoptotic (annexin V positive, PI negative), and late apoptotic/necrotic (annexin V positive, PI positive) cells within the entire cell population is shown. Data are shown as mean ± SE, n = 3, for a representative of 6 independent experiments.
We examined the effect of plasminogen on both TNFα-induced and cycloheximide-induced apoptosis in human peripheral blood monocytes. Viability status was determined in FACS analysis with annexin V–FITC and PI. Plasminogen fully neutralized the effect of TNFα on cell viability by restoring the percentage of viable cells within the population to that in the absence of an apoptosis inducer (Figure 3, compare B and E, lower left quadrants). When cycloheximide was used as the inducer of apoptosis, 93% of cell viability was restored in the presence of plasminogen (Figure 3, compare C and F, lower left quadrants). Furthermore, following cycloheximide treatment, 16% of the cells were late apoptotic/necrotic, and the percentage of late apoptotic/necrotic cells was decreased by 71% in the presence of plasminogen (Figure 3C and F, upper right quadrants). In the absence of an inducer of apoptosis, plasminogen treatment did not significantly affect cell viability (Figure 3A-B, lower left quadrants). Taken together, these data suggest that plasminogen at its physiologic concentration is cytoprotective for monocytes.
In controls to examine the specificity of the cytoprotective effect of plasminogen, prothrombin, thrombin, ovalbumin, and RNase (each at 2 μM) did not inhibit apoptosis induced by either TNFα or cycloheximide in either U937 cells or monocytes. In addition, we tested the effects of a full range of thrombin concentrations (0.02 nM to 2 μM, at 10-fold increments) on apoptosis induced by either TNFα or cycloheximide on both U937 cells and monocytes, and there was still no cytoprotective effect of thrombin within this broad concentration range. (We verified that thrombin was proteolytically active on the tripeptide substrate, S-2238).
Plasminogen treatment interferes with specific steps in apoptosis
We determined the effect of plasminogen treatment on internucleosomal DNA fragmentation. When cells were treated with cycloheximide, extensive laddering at intervals of approximately 200 nucleotides was observed, with less laddering following treatment with TNFα (Figure 4). When cells were incubated simultaneously with cycloheximide and plasminogen or TNFα and plasminogen, DNA fragmentation was not detected (Figure 4). Plasminogen treatment, alone, had no effect on DNA integrity. These data are consistent with inhibition of terminal steps of monocytoid apoptosis by plasminogen.
Plasminogen inhibits monocyte apoptosis in a dose-dependent manner. Human monocytes were cultured in the absence of an apoptotic inducer (A,D, untreated cells) or with the addition of 10 ng/mL TNFα (B,E) or 50 μg/mL cycloheximide (C,F) for 24 hours to induce apoptosis. In panels D, E, and F, 2 μM plasminogen was added simultaneously with the apoptotic inducers. The cells were analyzed by flow cytometry using annexin V–FITC and PI. The numbers in each quadrant indicate the percentage of the total cell population.
Plasminogen inhibits monocyte apoptosis in a dose-dependent manner. Human monocytes were cultured in the absence of an apoptotic inducer (A,D, untreated cells) or with the addition of 10 ng/mL TNFα (B,E) or 50 μg/mL cycloheximide (C,F) for 24 hours to induce apoptosis. In panels D, E, and F, 2 μM plasminogen was added simultaneously with the apoptotic inducers. The cells were analyzed by flow cytometry using annexin V–FITC and PI. The numbers in each quadrant indicate the percentage of the total cell population.
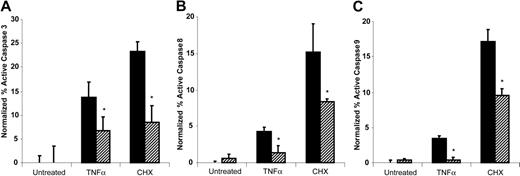
To investigate the mechanism of the antiapoptotic effect of plasminogen, we examined the effect of plasminogen treatment of the cells on activity of the effector caspase, caspase 3. U937 cells were either untreated or preincubated with 1 μM plasminogen for 30 minutes, and then either TNFα or cycloheximide was added. After an additional 24 hours, active caspase 3 was determined by using a fluorometric immunosorbent enzyme assay as described in “Materials and methods.” The cycloheximide-treated and TNFα-treated cells showed marked increases in active caspase 3 compared with the untreated control cells, consistent with induction of apoptosis (Figure 5A). Addition of plasminogen resulted in a 52% decrease in TNFα-induced active caspase 3 and a 64% decrease in cycloheximide-induced active caspase 3 (Figure 5A).
We examined whether decreased activation of caspase 3 was consistent with decreased activation of caspase 8 (activated via the receptor-mediated extrinsic pathway) and/or decreased activation of caspase 9 (activated via the mitochondrial-dependent intrinsic pathway), as determined in FACS analysis. Treatment with either TNFα or cycloheximide induced increases in both active caspase 8 and in active caspase 9 in U937 cells (Figure 5B-C, respectively). (The increase in active caspase 9 is consistent with activation of Bid by caspase 8, leading to activation of the mitochondrial-dependent intrinsic pathway and subsequent activation of procaspase 9.) Addition of plasminogen resulted in a 66% decrease in TNFα-induced active caspase 8 and a 45% decrease in cycloheximide-induced active caspase 8 with U937 cells (Figure 5B). Plasminogen treatment also decreased TNFα-induced active caspase 9 by 88% and cycloheximide-induced active caspase 9 by 56% (Figure 5C).
Structural requirements in plasminogen for inhibition of apoptosis
Because the lysine analog, EACA, inhibited the interaction of plasminogen with the cells (Figure 1), we examined the effect of EACA on the cytoprotective effect of plasminogen. A concentration of 100 mM EACA fully reversed the cytoprotective effect of plasminogen with both TNFα- and cycloheximide-treated cells (data not shown). These data suggested that unoccupied lysine binding sites within the kringle structures of plasminogen were required to inhibit apoptosis. Therefore, we tested kringle-containing domains of plasminogen for inhibition of apoptosis. Elastase degradation products composed of either kringle 1-3, kringle 4, or a recombinant plasminogen kringle 5 had no effect on monocyte apoptosis under conditions in which plasminogen fully neutralized the effect of both TNFα and cycloheximide. In contrast, a plasminogen fragment containing the latent active site and kringle 5 was able to inhibit apoptosis but was less effective than intact plasminogen (data not shown). Taken together, these data support a requirement for the protease domain of plasminogen as well as kringle domains, in addition to kringle 5, for the full cytoprotective effect of the plasminogen molecule.
Plasminogen activation takes place on its interaction with monocytoid cells, due to their synthesis and secretion of plasminogen activators.20,33 Therefore, we investigated the requirement for the proteolytic activity of plasmin in its cytoprotective effects. We compared the effects of a recombinant plasminogen variant, [D(646)E]-Plg, with wild-type plasminogen on apoptosis induced by either TNFα or cycloheximide. ([D(646)E]-Plg can be converted to the molecular form of plasmin, but it possesses greatly reduced plasmin activity because of the absence of the necessary Asp residue in the serine catalytic triad.20,28 ) As in the experiments in Figures 2, 3, wild-type plasminogen neutralized the effect of the apoptosis inducers by decreasing the percentage of early apoptotic cells with a consequent increase in viable cells. In contrast, [D(646)E]-Plg did not recapitulate the cytoprotective effect of plasminogen (Figure 6A-B). Thus, the full antiapoptotic function of plasminogen appears to require its ability to develop plasmin activity.
Role of PAR1 in the cytoprotective effect of plasminogen in monocytes
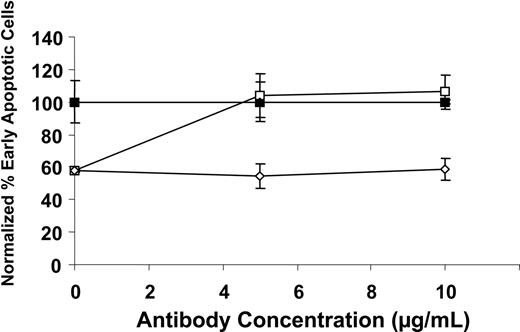
We examined the role of PAR1 in the cytoprotective effect of plasminogen on monocyte apoptosis. Human peripheral blood monocytes were incubated with TNFα in the presence or absence of plasminogen and in the presence of increasing concentrations of a specific anti-PAR1 cleavage site blocking antibody (ATAP2; that reacts with the tethered ligand domain34 ) or the isotype control, simultaneously for 24 hours. As shown in Figures 2, 3, plasminogen treatment decreased the percentage of early apoptotic cells within the population while the percentage of viable cells increased in parallel. In the presence of increasing concentrations of anti-PAR1, the effect of plasminogen was fully reversed (Figure 7). Taken together with our data showing a requirement for the proteolytic activity of plasmin in the cytoprotective functions of plasminogen, these data are consistent with a requirement for cleavage of PAR1 by plasmin for inhibition of apoptosis. The PAR1 agonist, TFLLRNPNDK (at 5 μM), did not inhibit monocyte apoptosis induced by either TNFα or cycloheximide. Thus, additional contacts of plasminogen with the cell surface appear to be required for its cytoprotective effect, consistent with the requirement for unoccupied kringle structures in the interaction.
Plasminogen retards DNA fragmentation. Plasminogen was preincubated with U937 cells for 30 minutes. Without washing the cells, TNFα and CHX were added, and the cells were incubated for an additional 24 hours. DNA was extracted as described in “Materials and methods” and electrophoresed on a 1.5% low-melt agarose gel. FACS analysis was performed simultaneously to verify the percentage of apoptosis within the total cell population.
Plasminogen retards DNA fragmentation. Plasminogen was preincubated with U937 cells for 30 minutes. Without washing the cells, TNFα and CHX were added, and the cells were incubated for an additional 24 hours. DNA was extracted as described in “Materials and methods” and electrophoresed on a 1.5% low-melt agarose gel. FACS analysis was performed simultaneously to verify the percentage of apoptosis within the total cell population.
Plasminogen decreases caspase activity. U937 cells were preincubated in either the absence (▪) or presence (▨) of 2 μM plasminogen for 30 minutes, and then 10 ng/mL TNFα, 50 μg/mL CHX, or buffer was added and further incubated for 24 hours. Active caspase 3 (A), active caspase 8 (B), and active caspase 9 (C) were determined as described in “Materials and methods.” Data are given as mean ± SE (n = 3). The raw data have been normalized to the untreated cells in the absence of plasminogen (*P < .05).
Plasminogen decreases caspase activity. U937 cells were preincubated in either the absence (▪) or presence (▨) of 2 μM plasminogen for 30 minutes, and then 10 ng/mL TNFα, 50 μg/mL CHX, or buffer was added and further incubated for 24 hours. Active caspase 3 (A), active caspase 8 (B), and active caspase 9 (C) were determined as described in “Materials and methods.” Data are given as mean ± SE (n = 3). The raw data have been normalized to the untreated cells in the absence of plasminogen (*P < .05).
Discussion
Monocyte apoptosis provides a feedback mechanism for down-regulating the inflammatory response. Dysregulation of this process can have pathophysiologic consequences (eg, persistence of an atherosclerotic plaque).35-38 Because plasminogen is required for the normal inflammatory response of macrophages,4,5,39 the goal of our study was to assess the participation of plasminogen in regulation of monocyte apoptosis. The main findings were that (1) early apoptotic monocytes, induced by TNFα treatment, had markedly enhanced ability to localize plasminogen on their surfaces, compared with viable cells; (2) plasminogen, at its physiologic concentration, inhibited monocyte apoptosis induced by TNFα as well as apoptosis induced by cycloheximide; (3) plasminogen treatment decreased levels of active caspase 3, caspase 8, and caspase 9 following induction of apoptosis; (4) the cytoprotective effect of plasminogen required its ability to develop proteolytic activity as well as the availability of unoccupied lysine binding sites within the plasminogen kringle domains; and (5) the cytoprotective effect of plasminogen required PAR1.
Effect of the plasminogen active site mutant, [D(646)E]-Plg, on apoptosis. U937 cells were either untreated (diamonds) or exposed to 10 ng/mL TNFα (squares) (A) or 50 μg/mL CHX (circles) (B) in the absence or presence of increasing concentrations of either the plasminogen active site mutant, [D(646)E]-Plg (closed symbols), or wild-type plasminogen (open symbols) for 24 hours at 37°C. The cells were analyzed by FACS with annexin V–FITC and PI. Data are shown as mean ± SE, n = 2, for a representative of 3 independent experiments.
Effect of the plasminogen active site mutant, [D(646)E]-Plg, on apoptosis. U937 cells were either untreated (diamonds) or exposed to 10 ng/mL TNFα (squares) (A) or 50 μg/mL CHX (circles) (B) in the absence or presence of increasing concentrations of either the plasminogen active site mutant, [D(646)E]-Plg (closed symbols), or wild-type plasminogen (open symbols) for 24 hours at 37°C. The cells were analyzed by FACS with annexin V–FITC and PI. Data are shown as mean ± SE, n = 2, for a representative of 3 independent experiments.
We found that the autocrine/paracrine cytokine, TNFα, induced apoptosis in monocytes, consistent with earlier reports.40,41 The early apoptotic cell population [characterized as cells exposing phosphatidyl serine on their surfaces (detected via annexin V binding) but not taking up PI] showed markedly enhanced specific plasminogen-binding ability compared with untreated cells. We obtained similar results following cycloheximide treatment of the cells. Previous reports have demonstrated markedly enhanced plasminogen binding to late apoptotic/necrotic U937 cells, following treatment with cycloheximide18,19 and to nonviable epithelial cells,42 necrotic breast cancer cells,43,44 and damaged amniotic epithelial cells.45,46 Our results demonstrate that plasminogen binding is markedly enhanced during early stages of apoptosis in response to treatment with a physiologic cytokine.
PAR1 mediates the cytoprotective effect of plasminogen. Human peripheral blood monocytes were exposed to 10 ng/mL TNFα in the presence (open symbols) or absence (closed symbols) of 2 μM plasminogen and either the presence of anti-PAR1 (ATAP2) (squares) or isotype control (diamonds) (each at 20 μg/mL) for 24 hours. The cells were analyzed by flow cytometry using annexin V–FITC and PI, and data were normalized to 100% for antibody in the absence of plasminogen. Symbols represent anti-PAR1 antibody without plasminogen (▪), isotype control without plasminogen (♦), anti-PAR1 antibody plus plasminogen (○), isotype control plus plasminogen (⋄). The closed diamonds are superimposed on the closed squares. Data are shown as mean ± SE, n = 2, for a representative of 3 independent experiments.
PAR1 mediates the cytoprotective effect of plasminogen. Human peripheral blood monocytes were exposed to 10 ng/mL TNFα in the presence (open symbols) or absence (closed symbols) of 2 μM plasminogen and either the presence of anti-PAR1 (ATAP2) (squares) or isotype control (diamonds) (each at 20 μg/mL) for 24 hours. The cells were analyzed by flow cytometry using annexin V–FITC and PI, and data were normalized to 100% for antibody in the absence of plasminogen. Symbols represent anti-PAR1 antibody without plasminogen (▪), isotype control without plasminogen (♦), anti-PAR1 antibody plus plasminogen (○), isotype control plus plasminogen (⋄). The closed diamonds are superimposed on the closed squares. Data are shown as mean ± SE, n = 2, for a representative of 3 independent experiments.
Treatment of monocytes and U937 cells with plasminogen neutralized the effect of both TNFα and cycloheximide by restoring the percentage of viable cells within the population. Both TNFα and cycloheximide increased caspase 3 and caspase 8 activities, consistent with activation of the extrinsic apoptotic pathway. In addition, caspase 9 activity was also increased, consistent with Bid activation via a caspase 8–dependent pathway. Plasminogen treatment decreased levels of each of these active caspases, suggesting a mechanism for its cytoprotective effect and consequently also inhibited DNA fragmentation induced by TNFα and cycloheximide. Our data also support the requirement for interaction of plasminogen with the cell surface in cytoprotection because EACA (that blocks the interactions of plasminogen kringles with cells31 ) also blocked the effect of plasminogen.
The effect of plasminogen required its ability to develop proteolytic activity because a plasminogen active site mutant, [D(646)E]-Plg, was not able to recapitulate the cytoprotective function of plasminogen. In addition, active plasmin did mimic the effect of plasminogen in a dose-dependent manner (data not shown), confirming the importance of the development of plasmin proteolytic activity on its role in apoptosis. Furthermore, a plasminogen fragment composed of the kringle 5 domain and the latent plasminogen active site was partially cytoprotective, whereas plasminogen kringle 5, alone, had no effect on apoptosis.
Our results suggest that the cytoprotective effect of plasminogen is dependent on cleavage of PAR1 by plasmin. This effect was not observed in the presence of either thrombin or prothrombin (data not shown). In purified systems, plasmin exhibits the ability to cleave recombinant PAR1 constructs at the thrombin activation site with an efficiency of approximately 650-fold less than thrombin.47 Previous studies have demonstrated that effective cleavage of PAR1 by FXa requires the formation of the TF-FVIIa-FXa complex48-50 and cleavage of PAR1 by activated protein C requires the EPCR as a coreceptor.51 Thus, it has been pointed out that under conditions of suboptimal scissile bond complementarity of PAR1 with the catalytic clefts of such enzymes, coreceptors are required to target these proteases to PAR1 to increase the efficiency of PAR cleavage.52 Likewise, when the local effective plasmin concentration is increased, via the interaction with plasminogen binding sites on monocytes, conditions for activation of PAR1 are likely to be achieved. When a lysine analog is present, plasmin is unable to cleave and activate PAR1, suggesting that the interaction of plasmin with the cell surface is necessary for PAR1 activation.47 This is likely to occur by an interaction of the plasminogen kringle structures with cell-surface carboxyl terminal lysines to increase the local concentration of the plasmin active site, for efficient cleavage of PAR1.23,31 Notably, recent reports demonstrate a role for PAR1 in other effects of plasmin on other cell types. Migration of α9-transfected Chinese hamster ovary cells on immobilized plasmin is blocked by PAR1 inhibitors.53 In addition, plasmin stimulates human lung fibroblasts to induce DNA synthesis and the effect is blocked by the activation blocking anti-PAR1 antibody (ATAP2).54
Activation of PAR1 results in inhibition of apoptosis in other cell types. Activated protein C (APC) inhibits staurosporine-induced apoptosis in endothelial cells in a PAR1-dependent mechanism55 and also prevents apoptosis in hypoxic human brain endothelium.56 However, cleavage of PAR1 is not sufficient to mimic the cytoprotective effect of APC.57 Similarly, in our study, although treatment with the anti-PAR1 inhibitory antibody, ATAP2, fully neutralized the inhibition of apoptosis by plasminogen, the PAR1 agonist, TFLLRNPNDK, alone did not inhibit monocyte apoptosis induced by either TNFα or cycloheximide, indicating that PAR1 activation is not sufficient for the cytoprotective effect of plasmin. Thus, contact with plasminogen binding sites on the apoptotic cells may be required, analogous to the requirement for contact of APC with the EPCR.58,59
An emerging area of research strongly supports a role for the plasminogen activation system in regulation of apoptosis. With adherent cells, plasmin promotes anoikis, depriving cells of a necessary survival signal by the loss of cell-matrix interactions, leading to programmed cell death.12,60-62 In contrast, with monocytoid cells in suspension (and thus not dependent on adherence for a survival signal), we found that plasmin(ogen) exhibited a novel cytoprotective function following induction of monocyte apoptosis.
Studies in Plg–/– mice support a role for plasminogen in inhibition of apoptosis under pathologic conditions. In posttransplantation arteriosclerosis, lesions are less severe in Plg–/– mice, compared with wild-type mice.63 Monocyte/macrophage apoptosis is a key feature of the atherosclerotic lesion, leading to lesional progression and necrosis.35,36,64 Thus, a mechanism by which lesion development is reduced in Plg–/– mice may be increased apoptosis (in the absence of the cytoprotective effect of plasminogen) leading to increased lesional necrosis, compared with wild-type mice.
Other in vivo studies are consistent with a role for plasminogen in cytoprotection in cell types other than monocytes. In studies with tumor models in Plg–/– mice, primary Lewis lung carcinoma tumors are smaller with reduced skin ulceration and delayed dissemination to lymph nodes.65 Furthermore, lung metastases are reduced in Plg–/– mice crossed with transgenic mice expressing Polyoma middle T antigen.66 Decreased apoptosis is also a phenotype of tumor cells. Although the effect of plasminogen may be restricted to monocytes, it is interesting to hypothesize that the absence of plasminogen may lead to increased apoptosis and consequent decreased tumor growth and metastasis in Plg–/– mice.
Our results are consistent with a previous report that plasminogen protects against apoptosis in neuronal cells.67 Patients with severe type I plasminogen deficiency (plasminogen activity and antigen both extremely low) exhibit the neurologic developmental defect of symmetric internal hydrocephalus with a Dandy-Walker malformation, hypoplasia of the cerebellum, and a hypoplastic corpus callosum.68 Because development of neurologic pathways requires apoptosis, the presence of this defect is consistent with defective apoptosis in these patients and mice. In additional support for a role for plasminogen in apoptosis during tissue remodeling, Plg–/– mice also exhibit defects in mammary gland involution.69
In summary, our results demonstrating a novel cytoprotective function for plasmin(ogen) in monocyte apoptosis suggest a new mechanism for regulation of monocyte/macrophage function in inflammation. Furthermore, this mechanism may be broadly used in other normal physiologic developmental functions and pathologic situations in other cell types.
Prepublished online as Blood First Edition Paper, February 14, 2006; DOI 10.1182/blood-2005-07-2872.
Supported by the National Heart, Lung, and Blood Institute (grants HL38272 and HL45934 to L.A.M.; grant HL13423 to F.J.C.), by a training grant from the National Institutes of Health (HL07195) (J.W.M.), and by a Kirchstein National Service Research Award (F32 AI062042-01) (J.W.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Don Davidson, Abbott Laboratories (Abbott Park, IL) for the generous gift of recombinant plasminogen kringle 5 (K5).




![Figure 6. Effect of the plasminogen active site mutant, [D(646)E]-Plg, on apoptosis. U937 cells were either untreated (diamonds) or exposed to 10 ng/mL TNFα (squares) (A) or 50 μg/mL CHX (circles) (B) in the absence or presence of increasing concentrations of either the plasminogen active site mutant, [D(646)E]-Plg (closed symbols), or wild-type plasminogen (open symbols) for 24 hours at 37°C. The cells were analyzed by FACS with annexin V–FITC and PI. Data are shown as mean ± SE, n = 2, for a representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/11/10.1182_blood-2005-07-2872/4/m_zh80110696210006.jpeg?Expires=1768969657&Signature=miD48V5iDG-9fFKxf7UpArn~KIgP0wyaMCXxkIM2lhL8J5IJ4n~3BAlsDoLP0k2-3NsjeanpVypW~gRAsfoxYAY~UvKfQqODJaYthhpbLSP9n2ZGXpUM7b6ydM4GKlrvi8f9d1WUpzFTmxKxI05wpuJllhhSOiNqFqvxBo~s8B4enVMLTjeDRY6sok-4nvF2Wxk6EhG3WuUOD-9mzgPhndzWm9phtYKWZ1MdtWzKIF2a8lozlq3Ls0tUfWdjm2MyfsJR8WzkId9YPA9HkiykNKUAbd3vqHood6NogCSFW5OwfbpHQVMbYBLixr5urFkN7D8Hd4znZChRzt~12SZS8A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal