Abstract
We found that MEK1 inhibitor PD184352 strikingly increased apoptosis induced by arsenic trioxide (ATO) in 21 of 25 patients with primary acute myelogenous leukemia (AML). Isobologram analysis confirmed the synergistic (13 of 25 patients) or additive (8 of 25 patients) nature of this interaction. Moreover, we demonstrated that the p53-related gene p73 is a molecular target of the combined treatment in AML blasts. Indeed, ATO modulates the expression of the p73 gene by inducing the proapoptotic and antiproliferative TAp73 and the antiapoptotic and proproliferative ΔNp73 isoforms, thereby failing to elevate the TA/ΔNp73 ratio. Conversely, treatment with PD184352 reduces the level of ΔNp73 and blunts the arsenic-mediated up-regulation of ΔNp73, thus causing an increase in the TA/ΔNp73 ratio of dual-treated cells. High doses of ATO induced p53 accumulation in 11 of 21 patients. Combined treatment resulted in the induction of the proapoptotic p53/p73 target gene p53AIP1 (p53-regulated apoptosis-inducing protein 1) and greatly enhanced the apoptosis of treated cells.
Introduction
Arsenic trioxide (ATO) is the treatment of choice for patients with relapsed acute promyelocytic leukemia (APL), particularly those who have been exposed to all-trans retinoic acid (tRA) within the previous 12 months.1 Many trials involving ATO have been initiated to test its ability to treat hematologic malignancies such as refractory acute myelogenous leukemia (AML), myelodysplastic syndrome, non-Hodgkin lymphoma, chronic lymphocytic or chronic myelogenous leukemia, acute T cell leukemia, and multiple myeloma (for reviews, see Zhu et al2 and Ravandi3 ). Some of these trials have shown clinical effects, but nothing has come close to the success in APL.
Study design
Twenty-five patients with non–APL AML were investigated (Table 1). Leukemia cells were isolated and enriched as described.7
Synergistic, additive, and antagonistic effects of PD184352 with ATO on primary AML blasts: correlation with p73 isoform expression, relative TA/ΔN p73 ratio, and p53 protein levels in ATO- and PD + ATO–treated cells
. | . | . | . | . | . | . | . | TA-p73/ΔN-p73 ratio . | . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | PD + ATO . | PD + ATO . | p53 . | . | . | . | |||
| Patient no. . | FAB class . | Age, y . | Cytogenetic abnormalities . | PD + ATO Cl . | TA-p73α . | TA-p73β . | ΔN-p73 . | 1 μM vs ATO 1 μM . | 2 μM vs ATO 2 μM . | ATO, 1 μM . | PD + ATO, 1 μM . | ATO, 2 μM . | PD + ATO, 2 μM . | |||
| 1 | M0 | 50 | 45,XX,-7 | - | ND | ND | ND | ND | ND | ND | ND | ND | ND | |||
| 2 | M0 | 36 | 46,XY | ++ | 0.5 | 0.9 | 0.4 | 7.3 | 5.6 | - | - | ++ | +++ | |||
| 3 | M0 | 42 | 45,XY,-7 | ++ | 0.1 | 0.7 | 0.3 | 2.2 | 3.0 | ± | ± | ++ | + | |||
| 4 | M1 | 35 | 46,XX t(6;9)(p23;q34) | + | ND | ND | ND | ND | ND | ND | ND | ND | ND | |||
| 5 | M1 | 49 | 46,XX t(3;3)(q21;q26) | - | UL | UL | UL | NA | NA | - | - | - | - | |||
| 6 | M1 | 52 | 46,XX | ++ | 0.8 | 1.0 | 0.5 | 2.3 | 2.3 | - | - | - | - | |||
| 7 | M2 | 63 | 46,XY | ++ | ND | ND | ND | ND | ND | ND | ND | ND | ND | |||
| 8 | M2 | 61 | 46,XY t(8;21)(q22;q22) | ++ | 0.3 | 0.6 | 0.3 | 8.1 | 9.3 | - | - | - | - | |||
| 9 | M2 | 54 | 46,XY | ++ | 0.6 | 1.3 | 0.1 | 24.1 | 27.8 | - | - | + | + | |||
| 10 | M2 | 48 | 46,XY t(8;21)(q22;q22) | + | 0.7 | 0.5 | 0.8 | 3.1 | 3.6 | - | - | - | - | |||
| 11 | M2 | 65 | 47,XX,+8 | - | ND | ND | ND | ND | ND | ND | ND | ND | ND | |||
| 12 | M2 | 51 | 46,XY t(8;21)(q22;q22) | + | 0.1 | 0.7 | 1.6 | 2.7 | 2.5 | - | - | ++ | ++ | |||
| 13 | M2 | 28 | 46,XY t(8;21)(q22;q22) | + | 0.1 | 0.4 | 0.7 | 4.2 | 4.7 | - | - | - | - | |||
| 14 | M2 | 50 | 46,XX | + | 0.4 | 0.9 | 0.5 | 3.7 | 3.2 | - | - | - | - | |||
| 15 | M4 | 52 | 46,XY inv(16)(p13;q22) | ++ | 0.7 | 1.4 | 0.6 | 3.3 | 3.2 | - | ± | ++ | ++ | |||
| 16 | M4 | 44 | 46,XY | ++ | UL | 1.2 | UL | NA | NA | - | - | - | - | |||
| 17 | M4 | 77 | 46,XX inv(16)(p13;q22) | + | 1.0 | 1.2 | 0.9 | 3.3 | 2.9 | - | - | - | - | |||
| 18 | M4 | 60 | 46,XY | - | 0.5 | 0.6 | 1.3 | 0.7 | 1.1 | - | - | - | - | |||
| 19 | M4 | 47 | 47,XY,+8 inv(16)(p13;q22) | + | 0.5 | 0.8 | 0.1 | 23.7 | 29.0 | - | - | + | + | |||
| 20 | M4 | 73 | 46,XX t(16;16)(p13;q22) | ++ | 0.5 | 1.1 | 0.4 | 4.1 | 4.8 | ± | + | + | + | |||
| 21 | M4 | 49 | 47,XY,+8 inv(16)(p13;q22) | ++ | 0.6 | 0.5 | 0.1 | 5.5 | 9.8 | ± | ± | ++ | ++ | |||
| 22 | M4 | 43 | 46,XY | ++ | 0.2 | 0.4 | 0.4 | 5.1 | 5.9 | - | - | - | - | |||
| 23 | M5 | 32 | 46,XX | ++ | 0.8 | 1.5 | 0.9 | 5.9 | 5.3 | ± | ± | ++ | ++ | |||
| 24 | M5 | 65 | 46,XX t(8;16)(p11;p13) | + | 0.2 | 0.8 | 0.6 | 2.9 | 3.5 | - | - | + | + | |||
| 25 | M5b | 1 | 46,XX | ++ | 0.6 | 1.0 | 0.8 | 3.1 | 3.8 | - | ± | ++ | ++ | |||
. | . | . | . | . | . | . | . | TA-p73/ΔN-p73 ratio . | . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | PD + ATO . | PD + ATO . | p53 . | . | . | . | |||
| Patient no. . | FAB class . | Age, y . | Cytogenetic abnormalities . | PD + ATO Cl . | TA-p73α . | TA-p73β . | ΔN-p73 . | 1 μM vs ATO 1 μM . | 2 μM vs ATO 2 μM . | ATO, 1 μM . | PD + ATO, 1 μM . | ATO, 2 μM . | PD + ATO, 2 μM . | |||
| 1 | M0 | 50 | 45,XX,-7 | - | ND | ND | ND | ND | ND | ND | ND | ND | ND | |||
| 2 | M0 | 36 | 46,XY | ++ | 0.5 | 0.9 | 0.4 | 7.3 | 5.6 | - | - | ++ | +++ | |||
| 3 | M0 | 42 | 45,XY,-7 | ++ | 0.1 | 0.7 | 0.3 | 2.2 | 3.0 | ± | ± | ++ | + | |||
| 4 | M1 | 35 | 46,XX t(6;9)(p23;q34) | + | ND | ND | ND | ND | ND | ND | ND | ND | ND | |||
| 5 | M1 | 49 | 46,XX t(3;3)(q21;q26) | - | UL | UL | UL | NA | NA | - | - | - | - | |||
| 6 | M1 | 52 | 46,XX | ++ | 0.8 | 1.0 | 0.5 | 2.3 | 2.3 | - | - | - | - | |||
| 7 | M2 | 63 | 46,XY | ++ | ND | ND | ND | ND | ND | ND | ND | ND | ND | |||
| 8 | M2 | 61 | 46,XY t(8;21)(q22;q22) | ++ | 0.3 | 0.6 | 0.3 | 8.1 | 9.3 | - | - | - | - | |||
| 9 | M2 | 54 | 46,XY | ++ | 0.6 | 1.3 | 0.1 | 24.1 | 27.8 | - | - | + | + | |||
| 10 | M2 | 48 | 46,XY t(8;21)(q22;q22) | + | 0.7 | 0.5 | 0.8 | 3.1 | 3.6 | - | - | - | - | |||
| 11 | M2 | 65 | 47,XX,+8 | - | ND | ND | ND | ND | ND | ND | ND | ND | ND | |||
| 12 | M2 | 51 | 46,XY t(8;21)(q22;q22) | + | 0.1 | 0.7 | 1.6 | 2.7 | 2.5 | - | - | ++ | ++ | |||
| 13 | M2 | 28 | 46,XY t(8;21)(q22;q22) | + | 0.1 | 0.4 | 0.7 | 4.2 | 4.7 | - | - | - | - | |||
| 14 | M2 | 50 | 46,XX | + | 0.4 | 0.9 | 0.5 | 3.7 | 3.2 | - | - | - | - | |||
| 15 | M4 | 52 | 46,XY inv(16)(p13;q22) | ++ | 0.7 | 1.4 | 0.6 | 3.3 | 3.2 | - | ± | ++ | ++ | |||
| 16 | M4 | 44 | 46,XY | ++ | UL | 1.2 | UL | NA | NA | - | - | - | - | |||
| 17 | M4 | 77 | 46,XX inv(16)(p13;q22) | + | 1.0 | 1.2 | 0.9 | 3.3 | 2.9 | - | - | - | - | |||
| 18 | M4 | 60 | 46,XY | - | 0.5 | 0.6 | 1.3 | 0.7 | 1.1 | - | - | - | - | |||
| 19 | M4 | 47 | 47,XY,+8 inv(16)(p13;q22) | + | 0.5 | 0.8 | 0.1 | 23.7 | 29.0 | - | - | + | + | |||
| 20 | M4 | 73 | 46,XX t(16;16)(p13;q22) | ++ | 0.5 | 1.1 | 0.4 | 4.1 | 4.8 | ± | + | + | + | |||
| 21 | M4 | 49 | 47,XY,+8 inv(16)(p13;q22) | ++ | 0.6 | 0.5 | 0.1 | 5.5 | 9.8 | ± | ± | ++ | ++ | |||
| 22 | M4 | 43 | 46,XY | ++ | 0.2 | 0.4 | 0.4 | 5.1 | 5.9 | - | - | - | - | |||
| 23 | M5 | 32 | 46,XX | ++ | 0.8 | 1.5 | 0.9 | 5.9 | 5.3 | ± | ± | ++ | ++ | |||
| 24 | M5 | 65 | 46,XX t(8;16)(p11;p13) | + | 0.2 | 0.8 | 0.6 | 2.9 | 3.5 | - | - | + | + | |||
| 25 | M5b | 1 | 46,XX | ++ | 0.6 | 1.0 | 0.8 | 3.1 | 3.8 | - | ± | ++ | ++ | |||
Fresh bone marrow from 25 patients was taken at diagnosis and before therapy. After informed consent was obtained, marrow was extracted by aspiration from the posterior iliac crest. All patients studied had more than 90% blast cells. Patient ages, FAB classification, and cytogenetics are reported. To determine PD + ATO Cl, primary AML blasts from 25 patients were cultured (2.5 × 105/mL in RPMI 1640 medium containing 10% FBS, 2 mM L-glutamine, penicillin G [100 U/mL], streptomycin [100 mg/mL]) in the presence of escalating doses of PD (0.1-10 μM), ATO (0.125-10 μM), or combinations of the 2 agents at a 1:1 ratio (0.25/0.25, 0.5/0.5, 1/1, 1.5/1.5, and 2/2). After 48 hours of treatment, the cells were harvested for sub-G1 DNA content, annexin V, and mitochondrial transmembrane potential detection. Cl plots were then generated using the Chou-Talalay method and Calcusyn software. ++ indicates Cl ≤ 0.85 (indicating synergism); +, 0.85 < Cl < 1.10 (indicating additive effect); -, Cl ≥ 1.10 (indicating antagonistic effect). For TA-p73α, TA-p73β, and ΔN-p73, values indicate basal endogenous protein expression, detectable by Western blot analysis of whole cell lysates (100 μg), of p73 isoforms in AML blasts from 21 patients. Relative amounts of TA-p73α, TA-p73β, and ΔN-p73 isoforms in AML blasts were normalized to a positive control K562 cell line. TA-p73/ΔN-p73 ratio: TA-(p73α + p73β)/ΔN-p73 ratio expressed as fold increase in PD + ATO compared with ATO-treated cells. For p53 protein expression, - indicates fold increase ≤ 1.3 with respect to control; ±, fold increase between 1.4 and 1.9 with respect to control; +, fold increase between 2.0 and 4.0 with respect to control; ++, fold increase between 4.1 and 6.0 with respect to control; and +++, fold increase ≥ 6.1 with respect to control.
PD indicates PD184352; Cl, combination index; ND, not done; UL, undetectable levels; NA, not applicable.
ATO was purchased from Sigma (St Louis, MO). PD184352, a potent and highly selective MEK1/2 inhibitor,8 was kindly provided by Dr J. S. Sebolt-Leopold (Cancer Molecular Sciences, Pfizer Global Research & Development, Ann Arbor, MI).
Cell lysis, in vitro treatment, apoptosis assays, immunoblotting, transfection, and statistical analysis were carried out as previously described.4,7 Approval for the study was obtained from the institutional review board of the Department of Clinical Sciences, University of Parma. Informed consent for blood samples was provided according to the Declaration of Helsinki.
Results and discussion
Samples from 25 patients with primary non–APL AML of different French-American-British (FAB) classifications were used in this study (Table 1). We first analyzed the pharmacologic interactions between ATO and PD184352 using a fixed-ratio experimental design and found that combined treatment with PD plus ATO resulted in the synergistic (combination index [CI] values equal to 0.85 or less), additive (CI values greater than 0.85 and less than 1.1), and antagonistic (CI values equal to 1.1 or greater) induction of apoptosis in 13, 8, or 4 patients with primary AML blasts, respectively (Table 1).
We recently demonstrated the involvement of the proapoptotic p73-p53AIP1 pathway in NB4 and K562 cell lines treated with PD+ATO.4 p73 is a p53 paralogue9-11 able to transactivate the promoters of several p53-responsive genes involved in apoptosis and cell cycle regulation.9-11
Indeed, p73 is sufficient to trigger cell death independently of the status of p53.12-14 Conversely, p53 requires p63 and p73 for the induction of apoptosis in response to DNA-damaging drugs.15 However, p73 exists as multiple transactivation competent (TA) proapoptotic and antiproliferative p73 COOH-terminal splicing isoforms (α, β, γ, δ, ϵ, ζ), of which the 2 major forms are p73α and p73β.16 In addition, dominant-negative (ΔN) variants are expressed from a second promoter, lack the amino-terminal transactivation domain, act as trans-repressors of p53- and p73-dependent transcription, and have antiapoptotic and proproliferative potential.17-20
To evaluate whether the combined treatment modulates p73 isoforms in AML blasts, protein expression of TA-p73α, TA-p73β, and ΔN-p73 was evaluated before and after treatment with PD and/or ATO in 21 of 25 patients with AML, and the TA/ΔN-p73 ratio was calculated (Table 1; Figure 1A-B; representative data).
Basal expression of TA-p73α and TA-p73β was clearly evident in 19 of 21 and in 20 of 21 patients, respectively (Table 1; Figure 1A; representative data). In addition, ΔN-p73 expression was detectable in 19 of 21 patients. These latter observations are in good agreement with a recent report showing the expression of ΔN-p73 in more than 90% of non-APL AML patients analyzed.21
In the responsive patients, with the exception of patient 16 (who lacked TA-p73α and ΔN-p73 expression; Table 1), we found that MEK1 inhibitor reduced the levels of dominant-negative ΔNp73 proteins and promoted the accumulation of endogenous TA-p73α and/or TA-p73β, elevating the TA/ΔN ratio (Table 1; Figure 1A-B; representative data). ATO alone promoted the increase in TA and ΔN-p73 protein expression, failing to elevate or showing a lesser ability than PD or PD + ATO to elevate the TA/ΔN ratio (Table 1; Figure 1A-B; representative data). The expression of p73 isoforms in ATO-treated blasts was different from what we observed in NB4 and K562 cell lines, in which ATO treatment did not affect TA-p73 and partially reduced ΔN-p73 in both cell lines.4 Moreover, in patient 12 (responsive) who presented a strong basal expression of ΔN-p73, treatment with ATO did not modulate its expression. Conversely, PD strongly inhibited ΔN expression (Table 1; data not shown). The lack of p73 protein expression (patient 5) or the inability of PD to elevate the TA/ΔN-p73 ratio (patient 18) resulted in loss of efficacy by PD+ATO treatment (Figure 1A-B; Table 1).
MEK-1 inhibition sensitizes AML blasts to ATO-induced apoptosis. (A) Primary AML blasts were seeded at 2.5 × 105 in the presence of DMSO (vehicle) or PD184352 (1 μM) for 3 hours and then were incubated for 18 hours with the indicated concentration of ATO. Endogenous TA-p73α, TA-p73β, and ΔN-p73 proteins were revealed by immunoblotting analysis using a mouse monoclonal anti-p73 (clone 1288) or a mouse monoclonal anti-ΔNp73 (clone 38C674) antibody. Endogenous p53 was revealed by immunoblotting using a mouse monoclonal anti-p53 (DO-1) antibody. Anti–actin immunoblotting was performed as loading control. (B) TA-p73α, TA-p73β, ΔN-p73, and β-actin bands were subjected to densitometric scanning using the TINA 2 software, and the TA-(p73α+p73β)/ΔN-p73 ratio was calculated. (C) Expression of PARP cleavage, Bax, PUMA, and p53AIP1 was revealed after 48 hours of treatment. Cell lysates were analyzed by immunoblotting analysis using mouse monoclonal anti-PARP (F2), rabbit polyclonal anti-Bax, rabbit polyclonal anti-PUMA, rabbit polyclonal anti-p53AIP1 (CT), and goat polyclonal anti–human actin. Antiactin immunoblotting was performed as loading control. (D) Primary AML blasts were cultured as described. After 48 hours of treatment, the cells were harvested for ΔΨm detection by flow cytometry. Values are expressed as percentage of cells with low ΔΨm. (inset) CI plot for the combination of escalating doses of PD184352 and ATO at a 1:1 ratio, obtained as described in the Table 1 footnote. The dashed line indicates a CI value of 1. CI, 1. (E) Primary AML blasts were cultured as described and were stained for annexin V binding. CTR indicates control; PD, PD184352 (1 μM).
MEK-1 inhibition sensitizes AML blasts to ATO-induced apoptosis. (A) Primary AML blasts were seeded at 2.5 × 105 in the presence of DMSO (vehicle) or PD184352 (1 μM) for 3 hours and then were incubated for 18 hours with the indicated concentration of ATO. Endogenous TA-p73α, TA-p73β, and ΔN-p73 proteins were revealed by immunoblotting analysis using a mouse monoclonal anti-p73 (clone 1288) or a mouse monoclonal anti-ΔNp73 (clone 38C674) antibody. Endogenous p53 was revealed by immunoblotting using a mouse monoclonal anti-p53 (DO-1) antibody. Anti–actin immunoblotting was performed as loading control. (B) TA-p73α, TA-p73β, ΔN-p73, and β-actin bands were subjected to densitometric scanning using the TINA 2 software, and the TA-(p73α+p73β)/ΔN-p73 ratio was calculated. (C) Expression of PARP cleavage, Bax, PUMA, and p53AIP1 was revealed after 48 hours of treatment. Cell lysates were analyzed by immunoblotting analysis using mouse monoclonal anti-PARP (F2), rabbit polyclonal anti-Bax, rabbit polyclonal anti-PUMA, rabbit polyclonal anti-p53AIP1 (CT), and goat polyclonal anti–human actin. Antiactin immunoblotting was performed as loading control. (D) Primary AML blasts were cultured as described. After 48 hours of treatment, the cells were harvested for ΔΨm detection by flow cytometry. Values are expressed as percentage of cells with low ΔΨm. (inset) CI plot for the combination of escalating doses of PD184352 and ATO at a 1:1 ratio, obtained as described in the Table 1 footnote. The dashed line indicates a CI value of 1. CI, 1. (E) Primary AML blasts were cultured as described and were stained for annexin V binding. CTR indicates control; PD, PD184352 (1 μM).
Because p53 mutations, primarily 17p monosomy, have been detected in only approximately 5% of patients with AML,22-25 we also studied p53 protein expression in 21 of 25 patients. In 11 of 21 (52%) patients, p53 was strongly induced (more than 2-fold increase compared with control; Table 1) after treatment with ATO 2 μM or PD+ATO 2 μM, whereas in most (20 of 21) patients analyzed, monotreatment with neither PD nor ATO 1 μM (or their combination) was able to significantly promote p53 accumulation (Figure 1A; Table 1; representative data). Taken together, these data indicate that in AML blasts a high concentration of ATO (2 μM) promotes the accumulation of p73 and p53 protein levels, whereas at low concentration (1 μM), ATO induces only p73.
Next we evaluated whether the changes in TA/ΔN-p73 ratio observed in PD+ATO-compared with ATO-treated cells resulted in an increased expression of the proapoptotic p53/p73 target genes Bax, PUMA (p53 up-regulated modulator of apoptosis),26,27 and P53AIP1 (p53-regulated apoptosis-inducing protein 1). P53AIP1, a primary effector gene of wild-type p53 and TAp73-induced apoptosis,28,29 is located in mitochondria, and its overexpression induces massive apoptotic cell death through the dissipation of mitochondrial ΔΨ 28 m. Interestingly, after 48-hour treatment in 13 of 16 patients with AML, we found that Bax and PUMA proteins accumulated to a greater extent when cells were challenged with ATO treatment than when they were challenged with PD+ATO. p53AIP1 expression was greatly enhanced after PD+ATO treatment compared with ATO alone (2-fold or greater increase; Figure 1C; representative data), thereby confirming our previous findings that PD and ATO cooperate to induce and activate the p73-p53AIP1 pathway.4 In patient 23 (responsive), the combined treatment did not up-regulate P53AIP1, but the amount of Bax expression was 2.8-fold greater than was ATO treatment (data not shown). Conversely, no differences in p53AIP1, Bax, and PUMA expression between ATO and PD+ATO treatment were observed in nonresponsive patients (Figure 1C, patient 18). In responsive patients, combined treatment also led to increased poly–(ADP-ribose) polymerase (PARP) fragmentation that reflected the increased apoptosis (Figure 1C). In agreement with the biochemical findings indicating the activation of the mitochondrial apoptotic pathway, we could also demonstrate that combined treatment with PD184352 and ATO strikingly potentiates the loss of ΔΨm induced by ATO alone in the responsive AML blasts (Figure 1D; representative data). Indeed, isobologram analysis confirmed that the proapoptotic interaction between PD and ATO was synergistic (CI values equal to 0.85 or less; Figure 1D, inset). The loss of ΔΨm and a decrease in the DNA content to sub-G1 levels were paralleled by the exposure of phosphatidylserine on the outer leaflet of the plasma membrane (annexin V assay) (Figure 1E; representative data).
Treatment with 1 μM ATO, a dose at which p73 but not p53 is accumulated, either alone or in combination with PD, led to increased levels of PUMA, BAX, or p53AIP1, suggesting that p73 indeed plays an important role in the transcriptional activation of p53/p73 proapoptotic target genes and apoptosis induction in most patients with AML (Figure 1C; patients 3, 6, 22). At higher concentrations of ATO, the accumulation of p73 and p53 indicated that both pathways could contribute to ATO- or PD+ATO-induced apoptosis.
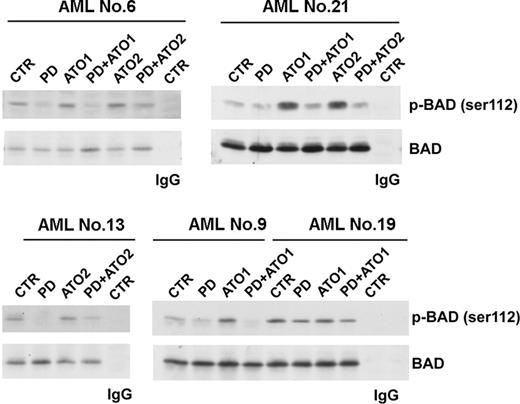
Recently, we demonstrated that MEK1 inhibition sensitizes parental and arsenic-resistant NB4 cell lines and APL primary blasts to ATO-induced apoptosis through the MEK1 inhibition-mediated dephosphorylation of Bad at Ser112, leading to an increased capacity to heterodimerize with Bcl-xL and Bcl-2, thereby blocking their antiapoptotic functions.7,30,31 Because Bcl-2 interacts with P53AIP1 in the mitochondrion and the overexpression of Bcl-2 blocks both the ΔΨm down-regulation and the p53AIP1 proapoptotic activity,28,32 we also investigated Ser112 phosphorylation and Bad protein levels after ATO treatment, with and without MEK1 inhibitor in primary AML blasts (13 patients). Pretreatment with MEK1 inhibitor strongly increased the expression of dephosphorylated Bad and blunted the ATO-mediated phosphorylation of Bad at Ser112 in all AML patients analyzed, thus suggesting that this pathway might contribute, together with the p73-P53AIP1 pathway, to the induction of apoptosis in dual-treated cells (Figure 2; representative data).
Effects of combined MEK inhibition and ATO treatment on Ser112 Bad phosphorylation and Bad protein levels in AML blasts. For the analysis of Bad phosphorylation, lysates were immunoprecipitated with rabbit polyclonal anti-Bad or with a control antibody, and the immunoprecipitated samples were subjected to 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blot analysis with the anti–phospho-Ser112-Bad antibody. Filters were then stripped and reprobed with the anti-Bad antibody.
Effects of combined MEK inhibition and ATO treatment on Ser112 Bad phosphorylation and Bad protein levels in AML blasts. For the analysis of Bad phosphorylation, lysates were immunoprecipitated with rabbit polyclonal anti-Bad or with a control antibody, and the immunoprecipitated samples were subjected to 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blot analysis with the anti–phospho-Ser112-Bad antibody. Filters were then stripped and reprobed with the anti-Bad antibody.
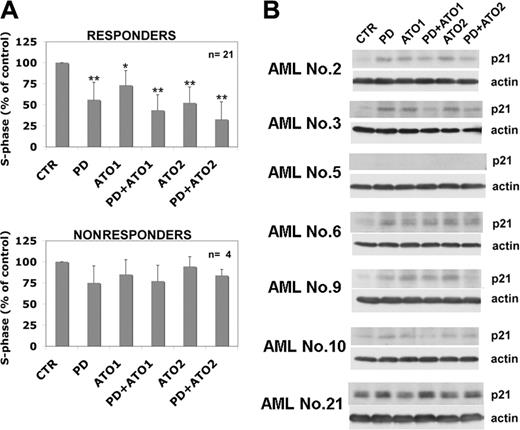
MEK blockade causes growth inhibition of leukemic cells and modulates p21Waf1/CIP1 expression in AML blasts. (A) Leukemic blasts from patients were cultured in the presence of PD 184352, ATO, or both for 48 hours and were stained for DNA content (*P < .05; **P < .001 vs control vehicle; Dunnett test). Values are mean ± SD. (B) AML blasts were cultured for 48 hours in the presence of PD 184352, ATO, or both and were subjected to Western blot analysis with mouse monoclonal anti-p21Waf1/Cip1 (DCS60).
MEK blockade causes growth inhibition of leukemic cells and modulates p21Waf1/CIP1 expression in AML blasts. (A) Leukemic blasts from patients were cultured in the presence of PD 184352, ATO, or both for 48 hours and were stained for DNA content (*P < .05; **P < .001 vs control vehicle; Dunnett test). Values are mean ± SD. (B) AML blasts were cultured for 48 hours in the presence of PD 184352, ATO, or both and were subjected to Western blot analysis with mouse monoclonal anti-p21Waf1/Cip1 (DCS60).
To better understand the effect of the ATO and MEK inhibitors on cell proliferation in AML blasts, we analyzed cell cycle (25 patients) and p21 modulation (14 patients). We found that treatment with the MEK1 inhibitor or ATO, or both, significantly decreased cell cycle progression (Figure 3A) and up-regulated p21Waf1/CIP1 (Figure 3B; representative data) in the responsive patients but not in nonresponsive patients.
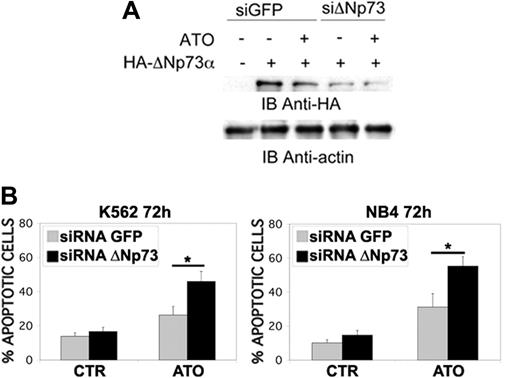
ΔN-p73 down-regulation potentiates ATO-induced apoptosis in K562 and NB4 cells. (A) Transfection of ΔN-p73 siRNA, but not the unrelated GFP siRNA, led to a decrease in ΔN-p73 in K562 cell counts without affecting the levels of the unrelated protein actin; K562 cells were transfected with an expression vector encoding for an HA-tagged version of ΔNp73 alone or in the presence of siRNAΔNp73 or siRNAGFP. Cells were lysed 24 hours after transfection, and ΔN-p73 expression was assessed by anti-HA immunoblot. Sequences of the siRNA ΔNp73 are siRNA ΔNp73 sense 5′-CGUCGGUGACCCCGCACGGUU-3′ and siRNA ΔNp73 antisense 5′-CCGUGCGGGGUCACCGACGUU-3′. (B) Percentages of sub-G1 apoptotic K562 and NB4 cells treated with ATO were significantly increased in cells transfected with ΔN-p73 siRNA relative to cells transfected with control siRNA (*P < .01; Dunnett test). K562 and NB4 cells were transfected with the indicated siRNAs and subsequently treated with ATO (2 μM for K562 and 1 μM for NB4) for 72 hours before apoptosis analysis. Values are mean ± SD of 4 independent experiments.
ΔN-p73 down-regulation potentiates ATO-induced apoptosis in K562 and NB4 cells. (A) Transfection of ΔN-p73 siRNA, but not the unrelated GFP siRNA, led to a decrease in ΔN-p73 in K562 cell counts without affecting the levels of the unrelated protein actin; K562 cells were transfected with an expression vector encoding for an HA-tagged version of ΔNp73 alone or in the presence of siRNAΔNp73 or siRNAGFP. Cells were lysed 24 hours after transfection, and ΔN-p73 expression was assessed by anti-HA immunoblot. Sequences of the siRNA ΔNp73 are siRNA ΔNp73 sense 5′-CGUCGGUGACCCCGCACGGUU-3′ and siRNA ΔNp73 antisense 5′-CCGUGCGGGGUCACCGACGUU-3′. (B) Percentages of sub-G1 apoptotic K562 and NB4 cells treated with ATO were significantly increased in cells transfected with ΔN-p73 siRNA relative to cells transfected with control siRNA (*P < .01; Dunnett test). K562 and NB4 cells were transfected with the indicated siRNAs and subsequently treated with ATO (2 μM for K562 and 1 μM for NB4) for 72 hours before apoptosis analysis. Values are mean ± SD of 4 independent experiments.
Finally, to confirm the biologic relevance of ΔN-p73 modulation in response to ATO treatment, we silenced the expression of endogenous p73 transcripts by using specific siRNA. The selective down-regulation of ΔNp73 sensitized K562 and NB4 leukemic cell lines to ATO-induced apoptosis (Figure 4i-ii), suggesting that it might have contributed to ATO resistance in leukemia cells. The importance of ΔN-p73 in regulating apoptosis and cell transformation is supported by the observation that ΔN-p73 indeed cooperates with oncogenic Ras in in vivo transformation assays33 and that Ras-MAPK pathway activation impairs ATO-induced apoptosis in leukemic cells.7
Taken together, our data indicate that the disruption of the MEK/MAPK pathway potentiates the antileukemic activity of ATO in AML blasts through the activation of p73 and Bad proapoptotic pathways, with the possible contribution, at high doses of ATO, of the p53 pathway. These findings provide a rationale for an effective and relatively specific therapeutic strategy for AML.
Prepublished online as Blood First Edition Paper, February 7, 2006; DOI 10.1182/blood-2005-07-2829.
Supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC) (A.B., A.C., M.L., F.L.-C.), by the Ministero dell'Istruzione dell'Università e della Ricerca Scientifica (MIUR Fondo Interesse Regionale [FIN] [A.B.], Fondo Interesse Locale [FIL] [A.B.], Progetto Ricerca Interesse Regionale [PRIN] [M.L.], Fondo Investimenti Ricerca di Base [FIRB] [M.L.], and “Progetto Strategico Oncologia SP/4: Terapia preclinica molecolare in oncologia [A.B.]), by European Community grant LSHC-CT-2004-503576 (M.L.), and by Telethon (M.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal