Comment on Licht et al, page 584
Expression of a dominant-negative form of hypoxia-inducible factor 2α (HIF-2α) in endothelial cells (ECs) disrupts cardiovascular development in mouse embryos, providing further evidence that HIFs exert both non–cell-autonomous and cell-autonomous control over ECs.
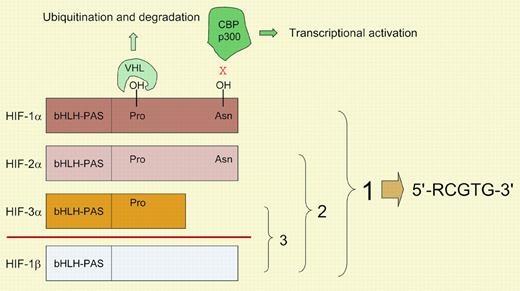
The developmental and physiologic processes that regulate O2 homeostasis in metazoans are directed by a family of hypoxia-inducible factors (HIFs), the prototype of which (HIF-1) was identified in 1992 as a mediator of erythropoietin gene transcription in hypoxic cells.1 Biochemical and molecular analyses revealed that HIF-1 was a heterodimer of constitutively expressed HIF-1β and O2-regulated HIF-1α subunits. Database searches identified HIF-2α, which dimerizes with HIF-1β and transactivates an overlapping but distinct set of genes from those regulated by HIF-1.2 In contrast to the ubiquitous expression of HIF-1α and HIF-1β, the expression of HIF-2α is tissue specific, most notably within vascular endothelial cells (ECs). HIF-3α is another family member that dimerizes with HIF-1β. HIF-1α and HIF-2α, but not HIF-3α, contain transactivation domains that interact with the coactivator proteins CBP and p300 (see figure). The half-life of HIF-1α, HIF-2α, and HIF-3α is regulated by O2-dependent hydroxylation of prolyl residues, which is necessary for binding of the von Hippel-Lindau protein, the recognition component of an E3-ubiquitin ligase that targets the proteins for proteasomal degradation.3 In addition, hydroxylation of an asparaginyl residue in HIF-1α and HIF-2α blocks their interaction with coactivators. Because O2 is a rate-limiting substrate for HIF asparaginyl and prolyl hydroxylases, changes in oxygenation are transduced into changes in transcription.FIG1
Mammalian hypoxia-inducible factors. HIF-1α, HIF-2α, HIF-3α, and HIF-1β are each encoded by a distinct gene, with alternative mRNA splicing generating multiple isoforms (not shown) of HIF-1α, HIF-3α, and HIF-1β. HIF-1α, HIF-2α, and HIF-3α dimerize with HIF-1β to form the functional proteins HIF-1, HIF-2, and HIF-3, respectively. The basic-helix-loop-helix (bHLH)–PAS domains mediate dimerization and DNA binding. All known HIF-1 binding sites contain the core DNA sequence 5′-RCGTG-3′ (R, A, or G). Hydroxylation of prolyl residues (402 and 564 in human HIF-1α) is required for the binding of the von Hippel-Lindau protein (VHL), which targets the proteins for ubiquitination and proteasomal degradation. Hydroxylation of an asparagine residue (803 in human HIF-1α) blocks the binding of coactivators p300 and CBP. The HIF prolyl and asparaginyl hydroxylases use O2 and α-ketoglutarate and generate CO2 and succinate as by-products.
Mammalian hypoxia-inducible factors. HIF-1α, HIF-2α, HIF-3α, and HIF-1β are each encoded by a distinct gene, with alternative mRNA splicing generating multiple isoforms (not shown) of HIF-1α, HIF-3α, and HIF-1β. HIF-1α, HIF-2α, and HIF-3α dimerize with HIF-1β to form the functional proteins HIF-1, HIF-2, and HIF-3, respectively. The basic-helix-loop-helix (bHLH)–PAS domains mediate dimerization and DNA binding. All known HIF-1 binding sites contain the core DNA sequence 5′-RCGTG-3′ (R, A, or G). Hydroxylation of prolyl residues (402 and 564 in human HIF-1α) is required for the binding of the von Hippel-Lindau protein (VHL), which targets the proteins for ubiquitination and proteasomal degradation. Hydroxylation of an asparagine residue (803 in human HIF-1α) blocks the binding of coactivators p300 and CBP. The HIF prolyl and asparaginyl hydroxylases use O2 and α-ketoglutarate and generate CO2 and succinate as by-products.
HIF-1 regulates genes encoding angiogenic cytokines, including vascular endothelial growth factor (VEGF), placental growth factor, angiopoietins 1 and 2, and stromal-derived factor 1, providing a mechanism by which cells are assured of adequate perfusion, as hypoxia-induced VEGF stimulates angiogenesis. Thus, HIF-1 was viewed as an extrinsic (non–cell-autonomous) regulator of ECs and their progenitors, which express VEGFR2 (Flk-1), VEGFR1 (Flt-1), Tie2, and CXCR4, the cell-surface receptors for VEGF, PLGF, angiopoietins, and SDF-1, respectively. HIF-2 was thought to function as an intrinsic (cell-autonomous) regulator of ECs. However, HIF-1 regulates the expression of hundreds of genes in ECs,4 and tumor angiogenesis is significantly impaired in mice with EC-specific loss of HIF-1α.5 Mice completely lacking HIF-2α expression have normal cardiovascular development in certain genetic backgrounds,6 whereas complete HIF-1α deficiency results in major defects in cardiovascular development.1
A logical conclusion is that HIF-1α and HIF-2α play critical and partially overlapping roles in ECs. To test this hypothesis, Licht and colleagues generated transgenic mice with EC-specific expression of a dominant-negative form of HIF-2α (HIFdn) directed by Flk1 gene promoter/enhancer elements. Overexpressed HIFdn competes with HIF-1α, HIF-2α, and HIF-3α for dimerization with HIF-1β, but the dimers cannot bind DNA or activate transcription. HIFdn transgenic embryos manifested cardiovascular defects similar to those of Tie2-deficient mice and the embryos completely lacked Tie2 expression. Thus, whereas loss of HIF-1 or HIF-2 activity in ECs is compatible with normal cardiovascular development, the combined loss of HIF-1 and HIF-2 activity is not. Overexpression of HIFdn may have confounding effects, such as competitive binding to proteins that interact with HIF-2α at residues not deleted in HIFdn, resulting in their cytoplasmic sequestration. It will be interesting to determine whether EC-specific HIF-1β deficiency phenocopies HIFdn and whether combined loss of HIF-1 and HIF-2 at earlier developmental stages (ie, prior to Flk1 expression) reveals essential roles for these proteins in hemangiopoiesis or vasculogenesis. Nevertheless, the results of Licht et al provide strong evidence that HIF-1 and HIF-2 play critical intrinsic roles in ECs. Because of their dual (extrinsic and intrinsic) effects, targeting these factors may represent a powerful approach to inhibiting angiogenesis in cancer and other disorders that are dependent upon neovascularization. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal