Abstract
Chuvash polycythemia (MIM 263400) is an autosomal recessive disorder characterized by a high hemoglobin level, relatively high serum erythropoietin, and early death. It results from a Von Hippel-Lindau (VHL) gene mutation (C598T) that causes increased HIF-1α activity and erythrocyte production in the face of normoxia. This polycythemia is endemic in Chuvashia, whereas its worldwide frequency is very low. We investigated the incidence of the Chuvash-type VHL mutation in Campania (South Italy) and identified 14 affected subjects (5 families). Twelve live on the island of Ischia (Bay of Naples). From analysis of the mutated allele, we found that the disease was more frequent on Ischia (0.070) than in Chuvashia (0.057). The haplotype of all patients matched that identified in the Chuvash cluster, thereby supporting the single-founder hypothesis. We also found that nonaffected heterozygotes had increased HIF-1α activity, which might confer a biochemical advantage for mutation maintenance. In conclusion, we have identified the first large cluster of Chuvash erythrocytosis outside Chuvashia, which suggests that this familial polycythemia might be endemic in other regions of the world.
Introduction
The Chuvash variant of familial polycythemia was first described in more than 100 individuals from about 80 families living in the mid-Volga River region of European Russia.1 The disease is characterized by a high hemoglobin level, increased plasma erythropoietin (Epo) level, varicose veins, vertebral hemangiomas, low blood pressure, and an elevated serum concentration of vascular endothelial growth factor (VEGF).2 Patients affected by Chuvash polycythemia die early, mainly as a result of cerebral vascular events or peripheral thrombosis. These injuries seem to be linked to mechanisms other than blood hyperviscosity or serum Epo content.2 Indeed, the prevalence of low blood pressure in patients with Chuvash polycythemia contrasts with the hypertension frequently associated with polycythemia vera and other familial polycythemias resulting from excess Epo.
Genome-wide screening and candidate gene characterization demonstrated that the Arg200Trp mutation (C598T) of the Von Hippel-Lindau (VHL) gene causes the Chuvash form of polycythemia.3 Thereafter, the mutation was detected in homozygosity in patients with sporadic or familial congenital erythrocytosis from diverse ethnic groups.4-8 However, 19 homozygotes have been identified among the more than 150 known cases of non-Chuvash familial erythrocytosis.9 Furthermore, 8 other VHL mutations (Arg79Cys, Gly104Val, Asp126Tyr, Val130Leu, Gly144Arg, Tyr175Cys, Leu188Val, His191Asp, Pro192Ala) were detected in either homozygotes or compound heterozygotes.4,5,7,8,10 These mutations were detected in a total of 10 cases, which indicates that the C598T transition is the major cause of VHL-related erythrocytosis.
The C598T mutation likely originated from a single founder event because the VHL haplotype in non-Chuvash patients is identical to that in polycythemic patients from Chuvashia.6,11 The C598T allele is very rare outside the Chuvash population. In fact, its frequency in Chuvashia is about 0.057,3 whereas the worldwide frequency of the Chuvash-associated haplotype is about 0.001 377.11 The different haplotype recently identified in a patient of Turkish ancestry probably represents an independent mutational event.7
Together with other proteins (elongin B, C, Rbx1, and Cul2), the VHL protein participates in the hypoxia-sensing pathway, where it binds the proline-hydroxylated form of the hypoxia-inducible factor-1α (HIF-1α), thereby committing the transcription factor to polyubiquitination and proteasomal degradation.12-15 Under normoxic conditions, HIF-1α is hydroxylated and rapidly degraded, thereby resulting in down-regulation of the transcription of HIF-1α–regulated genes.13,16 Conversely, the C598T mutation impairs VHL function and causes an increase in the HIF-1 complex, which in turn could cause overexpression of its target genes.3 HIF-1α regulates such important genes as EPO, VEGF, SDF1, GLUT1, triosephosphate isomerase 1 (TP1) transferrin, and the transferrin receptor.3,17,18 Although an increased serum level of Epo is considered the major cause of polycythemia,1 other HIF-1α–modulated genes might be involved in the pathogenesis of erythrocytosis.
In this study, we investigated the frequency of the C598T VHL mutation in the Campania Region of South Italy. Unexpectedly, we discovered a cluster of the disease on the island of Ischia (Bay of Naples), which has a population of about 55 000. This is the first region other than Chuvashia where this congenital polycythemia is endemic.
Patients, materials, and methods
Patients
Twenty-two patients from 13 families with suspected Chuvash-like congenital polycythemia were included in the study. Diagnostic criteria included (1) persistent elevated hemoglobin level (> 180 g/L [18 g/dL] in males, > 165 g/L [16.5 g/dL] in females, or > 2 SD above the median of the sex- and age-specific normal range in children); (2) absence of splenomegaly; (3) normal leukocyte and platelet counts; (4) normal hemoglobin oxygen affinity; (5) high or inappropriately high serum Epo level,1,3,9,19 and (6) absence of known causes of secondary erythrocytosis. The median age of patients at diagnosis was 19 years (range, 1-34 years). Some patients had undergone sporadic or regular phlebotomy treatment. Eleven patients were members of 2 unrelated families: 8 from family A and 3 from family B. The other 11 subjects reported no affected relatives. All cases were recruited through the Department of Pediatrics (Second University of Naples) and the Division of Hematology (Federico II University of Naples). The study was approved by the Institutional Review Board of the Second University of Naples and performed in accordance with the World Medical Association Declaration of Helsinki of 1975, as revised in 2000. Written informed consent for molecular genetic analysis, data analysis, and publication was obtained from all participants.
Family A. Family A includes 8 polycythemic patients: a mother (P13), her 2 sons (P15 and P16), her brother (P12), her father (P05), her uncle (P04), and 2 cousins (P07 and P08) (Figure 1A). All lived on the island of Ischia. No patient had a history or evidence of thrombotic complications or cancer. There is no record of consanguinity in the family. Erythrocytosis was discovered in the mother when she was 10 years old, at which time the hemoglobin (Hb) was 165 g/L (16.5 g/dL) and packed cell volume (PCV) was .54 (54%). The serum Epo concentration was 35 IU/L (mIU/mL) (normal range, 11-30 IU/L [mIU/mL]) before phlebotomy therapy was initiated. Her husband was not affected by erythrocytosis. Polycythemia was diagnosed in her 2 sons shortly after birth, and they began a therapeutic phlebotomy program to maintain their hematocrit level below .45 (45%). Their Epo levels were 55 and 99 IU/L (mIU/mL) before phlebotomies.
Pedigrees of 2 families affected by VHL-dependent polycythemia living on the island of Ischia. The P code denotes individuals from whom DNA samples were obtained. Filled symbols denote polycythemic subjects who are homozygous for the C598T mutation; half-filled symbols, heterozygous subjects.
Pedigrees of 2 families affected by VHL-dependent polycythemia living on the island of Ischia. The P code denotes individuals from whom DNA samples were obtained. Filled symbols denote polycythemic subjects who are homozygous for the C598T mutation; half-filled symbols, heterozygous subjects.
Family B. Family B includes 3 polycythemic subjects. A 9-year-old girl from Ischia (P22) (Figure 1B) was diagnosed with erythrocytosis at the age of 3 months. Hb was 210 g/L (21.0 g/dL) and PCV was .59 (59%), whereas O2 P50 was normal. The Epo concentration was 41 IU/L (mIU/mL). Polycythemia was diagnosed in her mother (P19) and uncle (P21) when they were 10 and 12 years old, respectively. They had elevated levels of Hb (197 g/L [19.7 g/dL] and 225 g/L [22.5 g/dL], respectively) and Epo (34 IU/L [mIU/mL] and 75 IU/L [mIU/mL], respectively). There is no record of consanguinity in the family. The patients do not have a history of cerebrovascular complications or cancer.
Detection of gene mutations
Genomic DNA was extracted from peripheral blood leukocytes with the Flexigene DNA Kit (Qiagen GmbH, Hilden, Germany). To search for VHL mutations, we sequenced all 3 VHL exons and their intron-exon boundaries. Polymerase chain reaction (PCR) was performed essentially as reported in Ang et al,3 and the reaction products were purified using a QIAquick Gel Extraction Kit (Quiagen GmbH). The products were sequenced using the ABI 310 DNA Sequencer and the ABI PRISM Dye Terminator Cycle Sequencing Reaction Kit (Applied Biosystems, Milan, Italy), according to the manufacturer's instructions. The coding regions of the elongin B and elongin C genes and the HIF-1α sequences spanning the oxygen-dependent degradation/pVHL interaction domain (residues 417-698, exons 10-12) were screened for DNA sequence variations by PCR amplification and DNA sequencing using the oligonucleotide primers and PCR conditions described in Clifford et al.20 DNA sequencing of PCR products was carried out as reported.
Mutation screening for the C598T base change
The C598T mutation abolishes a Fnu4HI restriction endonuclease recognition site. Thus, to screen for the mutation, we digested 14 μL PCR-amplified exon 3 product with 0.5 U Fnu4HI (New England Biolabs, Hitchin, Herts, United Kingdom) for 3 hours at 37°C. The digested products were visualized by electrophoresis on 2% agarose.
Haplotype analysis
We used 8 single-nucleotide polymorphisms that span the VHL gene (rs1056286, rs722509, rs779805 A>G, rs779808, rs1678607, 1149A>G, rs696356, rs378630), which are known to be highly informative in the Chuvash population,11 to characterize polycythemic patients. The PCR products were sequenced as reported in “Detection of gene mutations.”
Reverse transcription polymerase chain reaction
We isolated RNA from 5 × 106 Epstein-Barr virus (EBV)–transformed lymphoblastoid cells using the Trizol Reagent Kit (Invitrogen, Carlsbad, CA). Total RNA was prepared from reticulocytes as described elsewhere.21 cDNA was synthesized using the Superscript II Kit (Invitrogen) with random hexamers and 1.5 μg total RNA. PCR amplification of the whole VHL coding region was carried out with primers cVHL1-3F1 5′-CAGCTCCGCCCCGCGTCCGAC-3′ (located at the 5′-untranslated region) and cVHL1-3R1 5′-AAGGAAGGAACCAGTCCTGT-3′ (located at the 3′-untranslated region). PCR conditions were as reported in Cario et al.7 The reaction products were analyzed by agarose electrophoresis. cDNA was amplified with a primer located in the second VHL exon (cVHL2-3F1 5′-CTCTTCAGAGATGCAGGGACAC-3′) and the cVHL1-3R1 primer in separate experiments. The reaction product (377 bp) was digested with 0.5 U Fnu4HI as described in “Mutation screening for the C598T base change.”
The expression of the EPO, VEGF, SDF1, and TP1 genes was evaluated by reverse transcriptase (RT)–PCR with cDNAs prepared as described in “Detection of gene mutations.” The primers and conditions were as follows: EPO (5′-CGCGCCCGCTCTGCTCCGACACC-3′ [forward] and 5′-GGAGCGACAGCAGGGACAGGAGA-3′ [reverse] for 32 cycles, each consisting in steps at 95°C for 45 seconds, 56°C for 45 seconds, and 68°C for 45 seconds), VEGF (5′-TCGGGCCTCCGAAACCATGA-3′ [forward] and 5′-CTCCTCCTTCTGCCATGGGT-3′ [reverse] for 32 cycles, each consisting in steps at 95°C for 45 seconds, 56°C for 45 seconds, and 68°C for 45 seconds), SDF-1 (5′-GTGTCACTGGCGACACGTAG-3′ [forward] and 5′-TCCCATCCCACAGAGAGAAG-3′ [reverse] for 32 cycles, each consisting in steps at 95°C for 45 seconds, 58°C for 45 seconds, and 68°C for 45 seconds), TP1 (5′-GTGAAGGACTGGAGCAAGGT-3′ [forward] and 5′-GGGCTCATTGTTTGGCATTG-3′ [reverse] for 28 cycles, each consisting in steps at 95°C for 45 seconds, 58°C for 45 seconds, and 68°C for 45 seconds). The PCR products were analyzed by electrophoresis on 1.8% agarose gel.
Before amplification with each specific primer pair, an aliquot of the cDNA preparation was amplified using primers for β-actin to determine the integrity of the generated cDNA (BD Biosciences Clontech, San Jose, CA). Moreover, we used 5 different cDNA concentrations to ensure that signals were proportional to input mRNA. Each experiment was performed at least in triplicate and, in several cases, in quadruplicate. The expression of β-actin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), EPO, and SDF-1 was also evaluated by real-time PCR as reported elsewhere.17,22,23 Human kidney RNA (BD Biosciences Clontech) served as a positive control for EPO expression.
Preparation of lymphoblastoid cell lines
We established EBV-transformed lymphoblastoid cell lines from the peripheral blood of 8 subjects: 2 homozygotes for the C598T mutation (P12 and P22), 2 polycythemic heterozygotes (P24 and P25), 2 nonpolycythemic heterozygotes (P09 and P11), and 2 control subjects. The EBV lines were produced with 0.2-nm filtered culture medium of the B-95.8 EBV-producing marmoset line24 and PHA-M. Cells (107) were pelleted and resuspended in 1 mL B-95.8 cell line supernatant. This preparation was incubated in a conical tube at 37°C and occasionally resuspended. After 60 to 90 minutes, the cells were pelleted and resuspended in RPMI 1640 supplemented with l-glutamine (2 mM), penicillin/streptomycin (100 μg/mL), gentamycin (100 μg/mL), and 10% heat-inactivated fetal bovine serum in the presence of 5 μg/mL PHA-M. Half the medium was replaced every 3 to 4 days. Outgrowth of EBV-transformed cells was evident after 4 to 6 weeks.
HIF-1α functional analysis and immunoblotting
HIF-1α transcription factor activity was determined with the TransAM HIF-1α Kit (Active Motif, Carlsbad, CA) according to the manufacturer's instructions. Briefly, in this enzyme-linked immunoadsorbent assay (ELISA) method, an oligonucleotide containing the hypoxia-response element is immobilized on a 96-well plate. HIF dimers in nuclear extracts specifically bind to the oligonucleotide and are identified by means of an anti–HIF-1α antibody. The procedures used for immunoblotting are reported elsewhere.25 The antibodies directed against aldolase and GAPDH were from Santa Cruz Biotechnology (Santa Cruz, CA); the antisera against HIF-1α is contained in the kit.
Results
The VHL gene C598T mutation in the Campania Region
We studied 22 patients from 13 families putatively affected by Chuvash-like erythrocytosis. We studied only patients whose hemoglobin-adjusted Epo serum concentration was higher than normal under nonphlebotomized conditions.3,9 All subjects live in the Campania region of southern Italy, which has a population of about 5 million. All declared that their family had resided in Campania for several generations. No patient is of Chuvash origin.
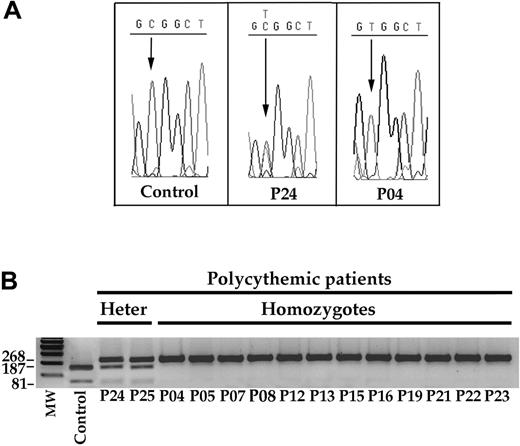
Detection of theC598Tmutation in DNA samples from 2 heterozygotes and 12 homozygotes. (A) Examples of sequences of the relevant region of the VHL gene in a control subject, in a heterozygote (P24), and in a homozygote (P04) patient. (B) FnU4HI digested the 268-bp PCR product of the wild-type VHL allele into 187- and 81-bp bands. Conversely, the C598T mutation resulted in an uncut 268-bp band. Patients P24 and P25 were heterozygotes for the mutation, whereas the other patients are homozygotes. MW indicates molecular weight standards; Heter, heterozygotes.
Detection of theC598Tmutation in DNA samples from 2 heterozygotes and 12 homozygotes. (A) Examples of sequences of the relevant region of the VHL gene in a control subject, in a heterozygote (P24), and in a homozygote (P04) patient. (B) FnU4HI digested the 268-bp PCR product of the wild-type VHL allele into 187- and 81-bp bands. Conversely, the C598T mutation resulted in an uncut 268-bp band. Patients P24 and P25 were heterozygotes for the mutation, whereas the other patients are homozygotes. MW indicates molecular weight standards; Heter, heterozygotes.
We first evaluated the occurrence of the C598T mutation by sequencing the 3 VHL exons (Figure 2A). We verified the results by amplifying the third VHL exon and digesting the PCR product with Fnu4HI (Figure 2B). Because the mutation abolishes the Fnu4HI restriction site, the amplified product containing C598T was not digested (Figure 2B). Identical results were obtained with the 2 approaches, thereby validating the second method in the screening of multiple DNA samples.
We identified the mutation in 14 patients (5 families) of the 22 examined (Figure 2B). Twelve patients were homozygotes for the C598T mutation and 2 were heterozygotes. A similar screening of putative Chuvash-like polycythemic patients living in other Italian regions revealed no patient with the C598T mutation (data not shown).
All the 12 homozygote patients (3 families) live on Ischia. Although the 2 heterozygotes do not live on Ischia, their parents who carried the VHL mutation live on the Naples coastline directly facing the island. No mutations were found in the 3 VHL exons of the other 8 polycythemic patients who did not carry the C598T mutation.
VHL mutation in heterozygotes
The 2 patients with a heterozygous C598T mutation were a 4-year-old boy (P24) and a 30-year-old man (P25). Erythrocytosis was discovered at the age of 2 years (P24) and 10 years (P25). Their present hemoglobin levels are 170 g/L (17 g/dL) and 205 g/L (20.5 g/dL), PCV of .54 (54%) and .65 (65%), and serum Epo 33 IU/L (mIU/mL) and 54 IU/L (mIU/mL), respectively. Thus far, they have no hyperviscosity symptoms or thromboembolic complications. These 2 patients (and their parents) declared that no other family member is affected by erythrocytosis. There was no consanguinity in the parents. In both the cases, the C598T mutation was inherited from the father, but neither father had any clinical or laboratory signs of polycythemia.
Subjects heterozygous for the C598T mutation do not usually manifest erythrocytosis. However, 2 independent cases of polycythemic patients heterozygous for the C598T mutation have been reported.6-8,10 We next carried out a series of analyses to look for other genetic aberrations (eg, mutations, deletions, and silencings) that could affect the apparently wild-type VHL allele.
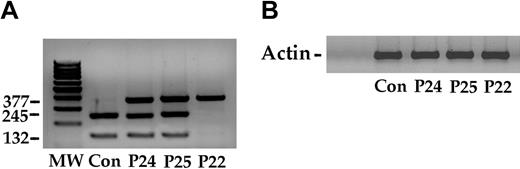
First, we sequenced the VHL gene promoter and did not find any mutations. However, this finding does not exclude abnormalities that may affect the expression of the wild-type VHL allele (eg, epigenetic events) or result in altered transcripts. Therefore, we analyzed the VHL transcripts. We retrotranscribed total RNA from EBV-transformed B lymphoblasts of the 2 patients and amplified the cDNA using primers localized in the 5′- and 3′-UTR of the VHL mRNA. This experiment revealed 2 full-length VHL transcripts, as previously reported.26 One transcript includes all 3 exons, and a smaller transcript results from splicing-induced skipping of the second exon. The 2 amplified products were purified and digested with Fnu4HI, and the assay mixtures were separated by electrophoresis on agarose gel. Both the wild-type and the mutated VHL allele expressed the 2 transcripts (data not shown). We also amplified the cDNAs using a forward primer localized in the second exon and a reverse primer at the 3′-UTR of the mRNA. In this case, only 1 amplified product was obtained. The PCR product was digested with Fnu4HI. Although these results are semiquantitative, they suggest that the 2 alleles are transcribed with a similar efficiency (Figure 3A).
Because the 2 heterozygote polycythemic patients have high Epo serum levels, it is conceivable that genetic alterations of components of the oxygen-sensing pathway other than VHL may contribute to erythrocytosis. Consequently, we looked for mutations in the HIF1A gene and in 2 genes (ie, elongin B and elongin C) that encode other components of the E3 complex that ubiquitinates HIF-1α. No mutations were found (data not shown).
VHL gene mutation is endemic on Ischia
The 12 patients, homozygous for the Chuvash-like mutation, come from 3 families that live on Ischia (Figures 1, 2). Greater than 50% of the patients are affected by hypotension and varicose veins. No cancer or other symptoms of Chuvash polycythemia were observed. In addition to families A and B (corresponding to 11 patients), the other homozygous subject (P23) was a 35-year-old man with a long-standing history of erythrocytosis. He is the only member of his family affected by polycythemia, and at present he has a hemoglobin level of 210 g/L (21 g/dL), PCV of .64 (64%), and a serum Epo level of 42 IU/L (mIU/mL). His heterozygous parents have no history of consanguinity.
Our 2 affected families included 6 homozygotes (P08, P12, P13, P15, P16, and P22) who had one homozygous parent (P04, P05, P13, and P19) (Figures 1, 2). Because the other parent was an obligate heterozygote (P03, P06, P14, and P20) and parental consanguinity was denied, we inferred a high frequency of the C598T mutation on Ischia. In this population, we determined that the mutation occurred with a frequency of 0.0703 (9 heterozygotes in 64 healthy subjects). None of the investigated subjects belonged to families that included homozygote patients. Conversely, we found no VHL mutations in 100 healthy subjects (200 chromosomes) from other Italian areas. The haplotype pattern in our 12 patients (data not shown) was identical to that previously reported in patients with Chuvash polycythemia.11
VHL expression in lymphoblastoid cells from a control subject (Con), 2 heterozygotes (P24 and P25), and a homozygote (P22). (A) Total RNA from each cell line was retrotranscribed to cDNA, which was amplified as reported in “Patients, materials, and methods,” and the product (377 bp) was digested with FnU4HI. The normal allele yielded 2 fragments of 245 bp and 132 bp. The C598T mutation abolished the restriction site and resulted in an uncut 377-bp band. The 2 heterozygote patients (P24 and P25) expressed the allele in roughly similar amounts. Conversely, the homozygote for the VHL mutation shows a single undigested band. (B) The expression of the actin gene served as control.
VHL expression in lymphoblastoid cells from a control subject (Con), 2 heterozygotes (P24 and P25), and a homozygote (P22). (A) Total RNA from each cell line was retrotranscribed to cDNA, which was amplified as reported in “Patients, materials, and methods,” and the product (377 bp) was digested with FnU4HI. The normal allele yielded 2 fragments of 245 bp and 132 bp. The C598T mutation abolished the restriction site and resulted in an uncut 377-bp band. The 2 heterozygote patients (P24 and P25) expressed the allele in roughly similar amounts. Conversely, the homozygote for the VHL mutation shows a single undigested band. (B) The expression of the actin gene served as control.
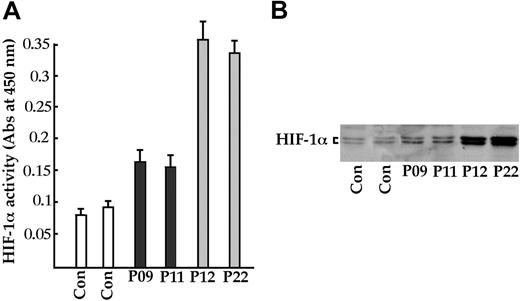
HIF-1α activity in lymphoblastoid cell lines. (A) Samples from 2 control subjects (Con), 2 healthy heterozygotes (P09 and P11), and 2 homozygotes (P12 and P22) were cultured, and nuclear extracts were assayed for HIF-1α activity with the TransAM HIF-1 kit (Active Motif) and (B) with immunoblotting with the antibody in the TransAM HIF-1 kit. Error bars indicate 2 SDs.
HIF-1α activity in lymphoblastoid cell lines. (A) Samples from 2 control subjects (Con), 2 healthy heterozygotes (P09 and P11), and 2 homozygotes (P12 and P22) were cultured, and nuclear extracts were assayed for HIF-1α activity with the TransAM HIF-1 kit (Active Motif) and (B) with immunoblotting with the antibody in the TransAM HIF-1 kit. Error bars indicate 2 SDs.
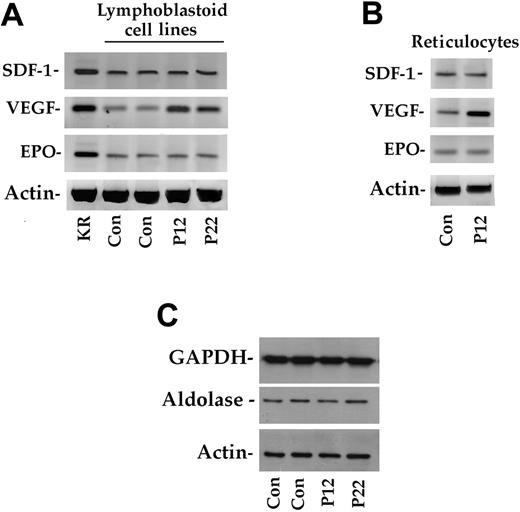
The data obtained in this novel VHL-dependent polycythemic cluster suggest that heterozygotes may have some selective advantages that favor the spread and maintenance of the mutated allele. To address this issue, we determined HIF-1α activity in EBV-transformed B lymphoblasts from healthy subjects, heterozygotes and homozygotes. As shown in Figure 4A, HIF-1α activity was 3-fold higher in homozygotes compared with controls. Surprisingly, activity was also increased in heterozygous subjects. These results were confirmed by a sensitive immunoblotting procedure (Figure 4B). Using RT-PCR, we also measured the expression of the EPO, VEGF, SDF1 (Figure 5A) and TP1 (data not shown) genes, which are targets of activated HIF-1α.27 In particular, SDF1 has recently been demonstrated to be modulated by HIF-1α.17 Because the SDF-1 protein is involved in angiogenesis, it could play a role in lowering blood pressure in homozygous and heterozygous subjects with Chuvash polycythemia. As shown in Figure 5A, VEGF expression was increased in homozygotes (by more than 3-fold as assessed by gel scanning). In contrast, EPO and SDF1 (Figure 5A) and TP1 (data not shown) gene transcription was identical in all samples.
HIF-1α–dependent expression in lymphoblastoid cell lines and reticulocytes. (A) Total RNA from lymphoblastoid cells of 2 control subjects (Con) and 2 homozygote patients (P12 and P22). Kidney total RNA (KR) was used as a positive control. Expression of the actin gene served as a control. (B) Total RNA from the reticulocytes of a control and a homozygote patient (P12) were used as starting material. (C) Immunoblotting analysis of aldolase and GAPDH in cellular extracts of 2 control subjects and 2 homozygotes (P12 and P22). See “Patients, materials, and methods” for further details.
HIF-1α–dependent expression in lymphoblastoid cell lines and reticulocytes. (A) Total RNA from lymphoblastoid cells of 2 control subjects (Con) and 2 homozygote patients (P12 and P22). Kidney total RNA (KR) was used as a positive control. Expression of the actin gene served as a control. (B) Total RNA from the reticulocytes of a control and a homozygote patient (P12) were used as starting material. (C) Immunoblotting analysis of aldolase and GAPDH in cellular extracts of 2 control subjects and 2 homozygotes (P12 and P22). See “Patients, materials, and methods” for further details.
We also evaluated the expression of SDF1, VEGF, and EPO in reticulocytes from a healthy subject and from a C598T homozygote. Only VEGF transcription was up-regulated, whereas EPO and SDF1 expression was unchanged, thereby confirming the findings obtained in lymphoblastoid cells (Figure 5B). Quantitative PCR confirmed the data on EPO and SDF1 expression. Immunoblotting experiments with 2 other HIF-1α targets—aldolase and GAPDH—revealed no variations (Figure 5C).
Discussion
This study demonstrates that Chuvash polycythemia is frequent in Campania and is endemic on the island of Ischia. This is the only cluster known besides the original Chuvash cluster. Our observation supports the notion that this erythrocytosis variant is spread throughout the world and demonstrates that it might be very frequent in some areas. A study carried out in other Italian regions (data not shown) suggests that the Ischia cluster is unique in Italy. Moreover, the finding that our 12 patients have the same haplotype as the Chuvash patients11 supports the single-founder hypothesis.
Although there is no proof of direct contact between the Chuvash and the inhabitants of Ischia, there is evidence, albeit weak, of a link between the 2 populations that might account for the high incidence of the disease on the island. The Chuvash derive from the Huns, who are thought to derive from the Middle East populations of the Sumerians and Scythians. The Huns also interacted with the Hungars and Vandals.28 Thus, the C598T VHL allele may have reached Ischia consequent to (1) the Vandals' invasion of Ischia that started from a Carthaginian harbor at the time of Attila the Hun, (2) the Hungars' conquest of central and south Italy, and (3) the pillaging by Turks of Ischia and surrounding areas.29
Irrespective of the route of transmission, we cannot explain the high incidence of the C598T mutation in Ischia and not in other Italian regions with a similar history. It is conceivable that a founder effect in a small isolated population within an island under social and environmental conditions that retard outbreeding may have led to the emergence of the mutation. Another possibility is that the altered gene might convey advantages in terms of iron metabolism, erythropoiesis, and embryonic development.2,3 For instance, improved erythropoiesis could compensate for iron deficiency consequent to a fish-based diet. Moreover, a slight increase of HIF-1α–regulated cytokines might be useful in such conditions as preeclampsia.
Our data on HIF target gene transcription in Chuvash polycythemia differ from those of a previous study3 in 2 aspects. First, EPO expression was not up-regulated in our EBV-transformed B-lymphocytes, which might reflect the low expression of EPO in these cells. Second, and more intriguing, is the observation that of the 5 genes expressed in lymphoblastoid cells, namely VEGF, SDF1, TPI, aldolase, and GAPDH,30,31 only VEGF appears to be up-regulated in the Chuvash-like polycythemic lymphoid cells. It is probable that, in an identical genetic background, different HIF-1α levels are required to express specific genes or sets of genes, which would explain, at least in part, the distinct phenotypes observed in subjects with different VHL mutations.
The mechanism underlying VHL-dependent polycythemia in patients with only one altered allele is not clear. In this context, it is noteworthy that we also found polycythemic patients who fulfilled the Chuvash-like erythrocytosis criteria9 and had high serum Epo, but who had no VHL mutations (S.P. and F.D.R., manuscript submitted). This raises the possibility of alterations at other steps of the HIF-1α–related pathway.
A clinical aspect of this study is that in regions, such as Chuvashia and Ischia, congenital polycythemia should be considered a “frequent” nonbenign hematologic disease. Awareness of this frequency may lead to early diagnosis and hence better patient management. Finally, because it is not strictly confined to Chuvashia and not solely a result of the C598T mutation, we suggest that “VHL-dependent polycythemia” would be a more accurate term for this condition.
Prepublished online as Blood First Edition Paper, October 6, 2005; DOI 10.1182/blood-2005-06-2422.
Supported by grants from “Progetto di Ricerca di Ateneo” Seconda Università di Napoli, Fondo per gli Investimenti della Ricerca di Base (FIRB), Progetti di Rilevante Interesse Nazionale (PRIN), Ministero dell'Istruzione dell'Università e della Ricerca (MIUR), and Associazione Italiana per la Ricerca sul Cancro (AIRC).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Marco Picardi, Rosanna Ciancia, and Fara Petruzziello for their invaluable assistance regarding the clinical aspects of the research, and Stefania Arciello for her technical help. We also thank Jean Gilder for text editing.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal