Abstract
Amphotericin B (AmB) is widely used for treating severe systemic fungal infections. However, long-term AmB treatment is invariably associated with adverse effects such as anemia. The erythropoietin (EPO) suppression by AmB has been proposed to contribute to the development of anemia. However, the mechanism whereby EPO is suppressed remains obscure. In this study, we investigated the possibility that AmB inhibits the transcription of the EPO gene by inactivating HIF-1, which is a known key transcription factor and regulator of EPO expression. EPO mRNA levels were markedly attenuated by AmB treatment both in rat kidneys and in Hep3B cells. AmB inactivated the transcriptional activity of HIF-1α, but did not affect the expression or localization of HIF-1 subunits. Moreover, AmB was found to specifically repress the C-terminal transactivation domain (CAD) of HIF-1α, and this repression by AmB required Asn803, a target site of the factor-inhibiting HIF-1 (FIH); moreover, this repressive effect was reversed by FIH inhibitors. Furthermore, AmB stimulated CAD-FIH interaction and inhibited the p300 recruitment by CAD. We propose that this mechanism underlies the unexplained anemia associated with AmB therapy.
Introduction
Amphotericin B (AmB) is a polyene macrolide antifungal agent with broad spectrum activity and has been popularly used as a therapeutic agent for treating systemic mycoses for more than 40 years.1 Currently, an increasing number of patients are being treated with this agent in immune-deficient conditions, such as AIDS, malignancy, anticancer treatment, or organ transplantation.2 The antifungal activity of AmB is due to its binding ergosterol, a sterol present in the fungal cell wall, which results in membrane disruption and cytoplasmic component leakage. At higher concentrations, AmB also binds to cholesterol in the plasma membrane of mammalian cells, which may be associated with its frequently encountered toxic effects.3 Despite these adverse effects and the recent development of new antifungal agents, AmB remains the drug of choice for treating severe systemic fungal infections.3
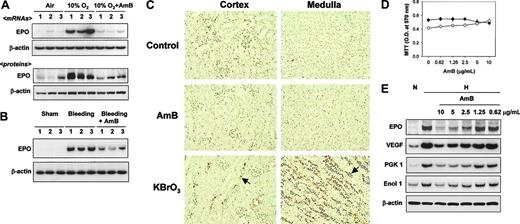
Amphotericin B inhibits EPO expression. (A) AmB inhibition of hypoxia-induced EPO production in rat kidneys. After rats were exposed to air or 10% O2 at 1 atm for 16 hours, kidneys were quickly frozen and stored at -70°C. The AmB stock solution (20 mg/mL) was dissolved in 35% (wt/wt) sodium deoxycholate and sodium phosphate provided by the manufacturer (Sigma-Aldrich) and stored at -20°C. Sodium deoxycholate for untreated animals or AmB (4 mg/kg in 4 divided doses) was injected into rats before rats were exposed to air or hypoxic atmosphere. After 16 hours of exposure, kidneys were excised to obtain RNA and protein samples. Tissue levels of EPO mRNA and protein were analyzed by semiquantitative RT-PCR and Western blotting, respectively, as described in “Materials and methods.” Results presented in lanes were obtained from individual animals. (B) AmB inhibition of anemia-induced EPO production in rat kidneys. After anemia was induced by removing blood, AmB solution (1 mg/kg) was once injected through the femoral vein. After 16 hours of incubation, kidneys were excised and quickly frozen. EPO and β-actin mRNAs were analyzed by the semiquantitative RT-PCR. Sham operations were performed in control rats in an identical manner, except for blood aspiration. (C) Renal toxicity of AmB. Rats were treated with sodium deoxycholate (Control) or AmB (4 mg/kg), or with KBrO3 (160 mg/kg) as a reference sample for renal injury. After 24 hours, the kidneys were excised and prepared for TUNEL staining, as described in “Materials and methods.” The stained slides were separately analyzed for the cortex (left panel) and medulla (right panel) parts. Arrows indicate examples of TUNEL-positive nuclei. Results are representative of 3 separate experiments. (D) Cytotoxicity of AmB. Cell viabilities were measured using an MTT-labeling method, as described in “Materials and methods.” Hep3B (•) and HEK293 (○) cells were treated with various concentrations of AmB for 16 hours. Points represent the means ± SD of 7 experiments. (E) AmB inhibition of HIF-1 target gene expression. RNAs were isolated from Hep3B cells subjected to normoxia (N) or 16 hours of hypoxia (H) with sodium deoxycholate (lanes 1-2) or AmB (lanes 3-7). The mRNAs of HIF-1 target genes and β-actin were analyzed by semiquantitative RT-PCR. Data are representative of 3 separate experiments. EPO indicates erythropoietin; VEGF, vascular endothelial growth factor; PGK 1, phosphoglycerate kinase 1; and Enol 1, enolase 1.
Amphotericin B inhibits EPO expression. (A) AmB inhibition of hypoxia-induced EPO production in rat kidneys. After rats were exposed to air or 10% O2 at 1 atm for 16 hours, kidneys were quickly frozen and stored at -70°C. The AmB stock solution (20 mg/mL) was dissolved in 35% (wt/wt) sodium deoxycholate and sodium phosphate provided by the manufacturer (Sigma-Aldrich) and stored at -20°C. Sodium deoxycholate for untreated animals or AmB (4 mg/kg in 4 divided doses) was injected into rats before rats were exposed to air or hypoxic atmosphere. After 16 hours of exposure, kidneys were excised to obtain RNA and protein samples. Tissue levels of EPO mRNA and protein were analyzed by semiquantitative RT-PCR and Western blotting, respectively, as described in “Materials and methods.” Results presented in lanes were obtained from individual animals. (B) AmB inhibition of anemia-induced EPO production in rat kidneys. After anemia was induced by removing blood, AmB solution (1 mg/kg) was once injected through the femoral vein. After 16 hours of incubation, kidneys were excised and quickly frozen. EPO and β-actin mRNAs were analyzed by the semiquantitative RT-PCR. Sham operations were performed in control rats in an identical manner, except for blood aspiration. (C) Renal toxicity of AmB. Rats were treated with sodium deoxycholate (Control) or AmB (4 mg/kg), or with KBrO3 (160 mg/kg) as a reference sample for renal injury. After 24 hours, the kidneys were excised and prepared for TUNEL staining, as described in “Materials and methods.” The stained slides were separately analyzed for the cortex (left panel) and medulla (right panel) parts. Arrows indicate examples of TUNEL-positive nuclei. Results are representative of 3 separate experiments. (D) Cytotoxicity of AmB. Cell viabilities were measured using an MTT-labeling method, as described in “Materials and methods.” Hep3B (•) and HEK293 (○) cells were treated with various concentrations of AmB for 16 hours. Points represent the means ± SD of 7 experiments. (E) AmB inhibition of HIF-1 target gene expression. RNAs were isolated from Hep3B cells subjected to normoxia (N) or 16 hours of hypoxia (H) with sodium deoxycholate (lanes 1-2) or AmB (lanes 3-7). The mRNAs of HIF-1 target genes and β-actin were analyzed by semiquantitative RT-PCR. Data are representative of 3 separate experiments. EPO indicates erythropoietin; VEGF, vascular endothelial growth factor; PGK 1, phosphoglycerate kinase 1; and Enol 1, enolase 1.
Since prolonged therapy is required to cure systemic mycoses, most patients experience the adverse effects of AmB. Nephrotoxicity is the most serious problem because it occurs in up to 80% of patients.4 Another serious problem is anemia, which occurs in 24% of total patients treated with AmB. In cases of prolonged therapy (ie, more than 1 month), anemia occurs in more than 90% of patients.3,5 The anemia produced is normocytic and normochromic, and reticulocyte numbers in anemic patients are below the normal range.5,6 It was concluded that this anemia is caused by a drug-induced suppression of erythrocyte production rather than by an increased rate of erythrocyte destruction. In spite of anemia, AmB-treated patients show low plasma levels of erythropoietin (EPO), which is markedly induced in case of anemia with other etiologies. Therefore, the reduced production of EPO is considered either a causative or an aggravating factor in AmB-induced anemia.6,7 In fact, supplementation with recombinant EPO protein has been attempted to correct this anemia.3 However, the mechanism underlying EPO suppression by AmB was unknown.
EPO is a glycoprotein hormone and a primary regulator of red blood cell production, and EPO production by the kidneys increases in response to anemia.8 This increase occurs primarily at the transcriptional level and involves transcriptional induction via the binding of a transcription factor, hypoxia-inducible factor-1 (HIF-1), to a conserved sequence, 5′-RCGTG-3′, in the hypoxia response element (HRE) located in the 3′-flanking region of the EPO gene.9 HIF-1 is a heterodimeric transcription factor composed of 2 basic helix-loop-helix (bHLH) proteins of the PAS family, namely HIF-1α and HIF-1β, the latter of which is also designated as aryl hydrocarbon receptor nuclear translocator (ARNT). Of these, HIF-1α is a key protein, as it determines the formation of HIF-1 and transactivates the EPO gene. Moreover, HIF-1 functions as a master regulator of oxygen homeostasis because it directly regulates the transcription of more than 40 kinds of hypoxia-inducible genes.10
The N-terminal portion of HIF-1α contains bHLH and PAS domains, which are essential for DNA binding and dimerization with ARNT.11,12 Its C-terminal portion contains 2 transactivation domains (TADs), which are localized at amino acids 531 to 575, N-terminal TAD (NAD), and at amino acids 786 to 826, C-terminal TAD (CAD).13,14 Its middle portion contains the Pro-Ser-Thr-rich oxygen-dependent degradation domain (ODDD; aa's 401-603), which determines its protein stability.15 Under normoxic conditions, HIF-1 prolyl hydroxylases (PHD1-3) hydroxylate 2 proline residues in the LXXLAP motif at the N-terminal end (Pro402) and in the C-terminal end (Pro564) within the ODDD.16-20 Von Hippel-Lindau tumor suppressor protein (pVHL) then binds to the modified motifs, which results in the ubiquitination and proteasomal degradation of HIF-1α.21,22 Since the enzymatic reaction of PHD requires molecular oxygen as a substrate, hypoxia limits this hydroxylation, thereby stabilizing HIF-1α and precluding its binding of pVHL. It was found recently that ARD1 acetylates Lys532 within the ODDD, and that this enhances the interaction between HIF-1α and pVHL.23 This lysyl acetylation, in addition to prolyl hydroxylation, is also viewed as a regulatory mechanism that underlies the stability of HIF-1α. In addition to its protein stabilization, the modulation of the transcriptional activity of HIF-1α regulates hypoxia-inducible gene expression. In an experiment using a fusion protein containing the Gal4 DNA-binding domain and CAD, although the protein stability was unaffected by hypoxia, the transcriptional activity of CAD was found to be enhanced under hypoxic conditions. It was also found that CAD is hydroxylated at an asparagine residue (803) under normoxic conditions, and that this results in a blockade of the recruitment of p300 coactivator.24 This hydroxylation is promoted by an asparagine hydroxylase, factor-inhibiting HIF1 (FIH).25-27 Under hypoxic conditions, asparagine hydroxylation is inhibited due to limited molecular oxygen, and CAD remains unmodified and activated.
To understand the mechanism underlying AmB-induced anemia, we first confirmed that AmB suppresses EPO production at the transcriptional level in vivo and in vitro. We also designed experiments to test the possibility that AmB inhibits HIF-1. AmB was found to attenuate the HIF-1-dependent stimulation of the EPO enhancer activity and to repress the transcriptional activation of HIF-1α, but it did not inhibit the expression or the nuclear translocation of HIF-1 subunits. Moreover, AmB deregulated the oxygen-sensing process to determine CAD activity, and this AmB effect was rescued by FIH inhibitors. Immunoprecipitation and mammalian 2-hybrid studies revealed that AmB enhanced FIH binding to CAD and blocked p300 recruitment by CAD. These results suggest that FIH repression of HIF-1 transcriptional activity is responsible for the AmB-induced suppression of EPO transcription.
Materials and methods
Reagents and antibodies
Amphotericin B (AmB), desferrioxamine (DFO), and other chemicals were purchased from Sigma-Aldrich (St Louis, MO). The FIH inhibitor, dimethyloxalylglycine (DMOG), was obtained from Frontier Scientific (Logan, UT) and [α-32P]CTP (18.5 TBq/mmol), from NEN Life Science (Boston, MA). Culture media and fetal calf serum were purchased from GIBCO/BRL (Grand Island, NY). Anti-HIF-1α antiserum was generated in rabbits against a bacterially expressed fragment encompassing amino acids 418 to 698 of human HIF-1α, as previously described.28 Antibodies, goat anti-ARNT, mouse anti-β-actin, rabbit anti-NF-κB P50, and rabbit anti-Gal4 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rat anti-HA and mouse anti-EPO monoclonal antibodies were obtained from Roche (Basel, Switzerland) and In2Gen (Seoul, Korea), respectively.
Procedures for hypoxia and anemia models
Specific pathogen-free adult male Sprague-Dawley rats were used throughout. For in vivo hypoxia, rats were exposed to 10% O2 at 1 atm in an air-tight plastic chamber for 16 hours. Humidified gas was supplied continuously to the chamber to minimize pCO2 changes. After death, kidneys were excised and quickly frozen in liquid nitrogen. Rats received injections of AmB (4 mg/kg in 4 divided doses) before hypoxic treatment. To induce anemia, rats were anesthetized with pentobarbital sodium (50 mg/kg) and mechanically ventilated. After incising the abdominal wall, we aspirated blood (20 mL/kg body weight) from the inferior vena cava and injected Tyrode solution to maintain the systemic circulation. After the abdomen was closed with 4-0 silk sutures, the animal was allowed to recover. Rats received injections of AmB (1 mg/kg, single intravenous injection) immediately after the surgery. Sham operations were performed in an identical manner, except for blood aspiration. All animal procedures were performed according to the established procedures described in the Seoul National University Laboratory Animal Maintenance Manual.29
Cell culture and treatment
Hep3B and HEK293 cell lines were obtained from ATCC (Manassas, VA) and cultured in α-modified Eagle medium or Dulbecco modified Eagle medium, supplemented with 10% heat-inactivated fetal calf serum, 100 units/mL penicillin, and 100 μg/mL streptomycin, in a humidified atmosphere containing 5% CO2 at 37°C. O2/CO2 levels in the incubator (Vision Sci, Seoul, Korea) were either 20%/5% (normoxic) or 1%/5% (hypoxic). Concentrated AmB (1 μL) was administered to 1 mL medium 1 hour prior to hypoxic incubation.
Preparation of expression plasmids and siRNA, and transfection
Hemagglutinin (HA)-tagged HIF-1α expression plasmid (pcDNA3) was constructed as described previously.30 The plasmid used to stably express hypoxia-activated HIF-1α mutant (sh-HIF-1α) was made by deleting 3 degradation motifs (aa's 397-405, 513-553, and 554-595) using a polymerase chain reaction (PCR)-based mutagenesis kit (Stratagene, Cedar Creek, TX).31 The plasmid used to stably express constitutively activated HIF-1α mutant (sc-HIF-1α) was made by substituting Ala803 for Asn803 in sh-HIF-1α using a QuickChange site-directed mutagenesis kit (Stratagene). The Gal4-CAD plasmid was constructed by inserting HIF-1α CAD (C-terminal transactivation domain, aa's 776-826) into EcoRI/BamHI-digested pCMX-G4(N), and the Gal4-CAD mutant plasmids were generated by site-directed mutagenesis.32 The (His)VP16-C/H1 plasmid was constructed by inserting the p300 C/H1 domain into EcoRI/XbaI-digested p(His)VP16 (Clontech, Palo Alto, CA).32 The HA-FIH plasmid was constructed by inserting full-length FIH cDNA (cloned by reverse-transcription-PCR) into pcDNA-HA. The nucleotide sequences of the primers used for RT-PCR were 5′-ATGGCGGCGACAGCGG-3′ and 5′-CTAGTTGTATCGGCCCTTGATC-3′. All constructs were verified by DNA sequencing. To suppress endogenous FIH-1 expression, a synthesized siRNA duplex was obtained from Invitrogen (Carlsbad, CA). The sequence targeting FIH1 (GenBank number NM_017902) corresponds to nucleotides 91 to 111 of the coding region.33 For transient transfection with plasmids or siRNA, about 40% of confluent cells in 60-mm cell-culture dishes were transfected with plasmid or siRNA using the calcium phosphate method. Cells were allowed to stabilize for 48 hours before being used in experiments.
Reporter assays
Luciferase reporter genes, containing the EPO enhancer region, the HRE-mutated EPO enhancer region, or the Gal4-binding motif, were constructed as previously described.32 Hep3B and HEK293 cells were cotransfected with 0.5 μg each of reporter gene and plasmid cytomegalovirus-β-gal and/or plasmids of HIF-1α or Gal4-CAD, using the calcium phosphate method. pcDNA was added to ensure that the final DNA concentrations in both the control and experimental groups were at similar levels. After being allowed to stabilize for 48 hours, cells were incubated under either normoxic or hypoxic conditions, in the absence or presence of AmB for 16 hours, and then lysed to determine luciferase and β-gal activities.
Immunoblotting of HIF-1 subunits and other proteins
Total cell lysates were separated on 8% or 10% SDS/polyacrylamide gels, and transferred to an Immobilon-P membrane (Millipore, Bedford, MA). Membranes were blocked with 5% nonfat milk in Tris-buffered saline containing 0.1% Tween-20 (TTBS) at room temperature for 1 hour and then incubated overnight at 4°C with a primary antibody diluted 1:1000 in 5% nonfat milk in TTBS. Membranes were incubated with a horseradish peroxidase-conjugated secondary antibody (diluted 1:5000 in 5% nonfat milk in TTBS) for 2 hours, and the antigen-antibody complexes were visualized using an Enhanced Chemiluminescence Plus kit (Amersham Biosciences, Piscataway, NJ).
Semiquantitative RT-PCR for EPO mRNA and other HIF-1-induced mRNAs
To quantify mRNA levels, we used a highly sensitive, semiquantitative RT-PCR, as previously described.28 Total RNAs were isolated from cultured cells or rat kidney tissues using TRIZOL (GIBCO/BRL). After verifying their qualities on a 1% denaturing agarose gel, 1 μg total RNAs was reverse-transcribed at 48°C for 1 hour, and the cDNAs were amplified over 18 PCR cycles (94°C for 30 seconds, 53°C for 30 seconds, and 68°C for 30 seconds) in 20 μL reaction mixture containing 0.185 MBq [α-32P]dCTP and 250 nM of each primer set. The PCR products (5 μL) were electrophoresed on a 4% polyacrylamide gel, and dried gels were autoradiographed. The nucleotide sequences of the primer pairs (5′ to 3′) for rat EPO and β-actin were GTCCCAGATACCAAAGTCAA and CGGAAGAGCTTGCAGAAAGTA; and GGCACCACACTTTCTACAAT and TCTCTTGCTCGAAGTCTAGG, respectively. Primers for human EPO, VEGF, PGK 1, enolase 1, and β-actin were constructed as previously described.30
TUNEL and MTT assays
To evaluate renal toxicity, the ApopTag in situ apoptosis detection kit (Oncor, Gaithersburg, MD) was used. Rat kidneys were fixed with formalin and embedded in paraffin. Sections (6 μm) were dewaxed and treated with proteinase K, then incubated with equilibration buffer for 10 minutes, followed by incubation with working-strength TdT enzyme solution at 37°C for 2 hours. The reaction was terminated by incubation in stop/wash buffer. Sections were then incubated with antidigoxigenin peroxidase and then incubated with diaminobenzidine and 0.01% H2O2 for 5 minutes. The sections were counterstained with hematoxylin and examined by microscopy (Nikon E200 microscope, Melville, NY). TUNEL-positive cells were identified at a magnification of ×100 (objective, 10×/__) and were examined using a Microcomputer Imaging Device model 4 (MCID-M4) image analysis system (Imaging Research, St. Catherines, ON, Canada). To evaluate cytotoxicity, the Sigma-Aldrich MTT labeling kit was used. Cells were grown in 12-well plates in 1 mL medium per well. After incubating with AmB for 16 hours, 100 μL of an MTT labeling reagent (5 mg/mL) was added and incubation was continued in the CO2 chamber for 3 hours. After solubilizing the blue formazan crystal with acidified isopropanol, formazan levels were determined at 570 nm.
Statistical analysis
All data were analyzed using Microsoft Excel 2000 software (Microsoft, Redmond, WA), and results are expressed as means and standard deviations. We used the unpaired Student t test (SPSS 10.0 for Windows software; SPSS, Chicago, IL) to compare reporter activities between control and AmB-treated groups. Differences were considered significant when P values were less than .05. All statistical tests were 2-sided.
Results
AmB blunts EPO response to hypoxia in vivo and in vitro
Although the suppression of EPO production by AmB has been well defined clinically, it has not been determined whether AmB suppresses EPO production in an experimental situation. When rats had been exposed under 10% O2, EPO mRNA and protein levels increased in the kidneys, but this EPO induction was markedly diminished in AmB-treated rats (Figure 1A). We also examined the EPO suppression effect of AmB in anemic rats. The EPO mRNA expression was induced after bleeding, and this induction was reduced by AmB (Figure 1B). In such conditions, the microscopic structures of the kidneys were not changed (data not shown) and apoptotic death was not observed in AmB-treated kidneys, whereas massive cell death occurred in the renal medulla of rats treated with a renal toxin, KBrO3 (Figure 1C). To examine the effect of AmB on EPO response to hypoxia in vitro, we first determined the experimental AmB concentrations. Since 2.5 μg/mL AmB is recommended for preventing fungal contamination, we reasoned that AmB can be safely used at around this concentration. Moreover, Hep3B and HEK293 cells were not obviously injured by treatment with AmB at up to 10 μg/mL for 16 hours (Figure 1D). Thus we used AmB at less than 10 μg/mL throughout the experiments. After exposing EPO-producing Hep3B cells to hypoxia, EPO mRNA was markedly enhanced versus the normoxic control (Figure 1E). To examine the effect of AmB on EPO expression, cells were treated with AmB under hypoxic conditions. AmB at 2.5 μg/mL or more significantly reduced EPO mRNA levels in a dose-dependent manner. In addition to EPO, other HIF-1 target gene expression was also dose-dependently reduced by AmB (Figure 1E). These results indicate that as occurs in patients, EPO production is suppressed by AmB both in rats and in Hep3B cells, and suggest that HIF-1 inhibition is responsible for the EPO suppression.
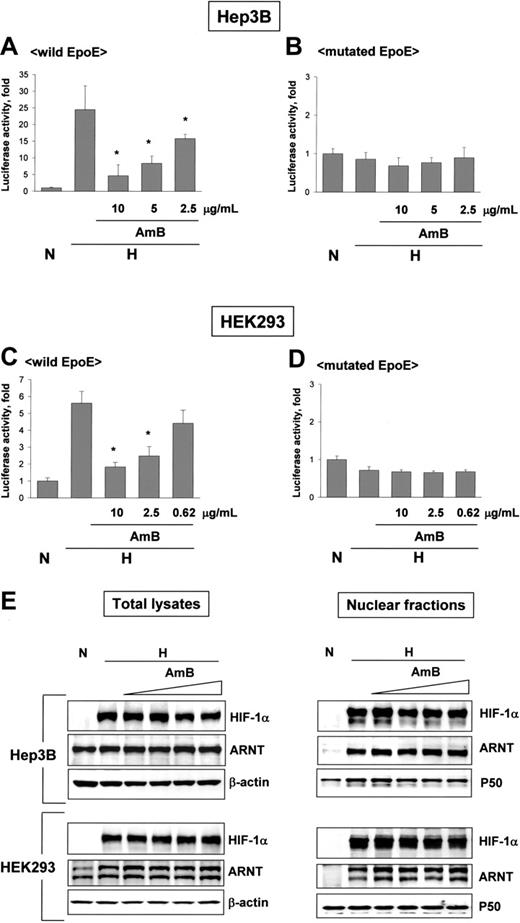
Amphotericin B inactivates HIF-1 without changing protein levels of HIF-1 subunits. (A-D) AmB-induced HIF-1 inhibition. Luciferase reporter plasmids containing wild EpoE or mutated EpoE were transfected into Hep3B and HEK293 cells. After incubation under normoxic or hypoxic conditions for 16 hours with sodium deoxycholate or AmB, luciferase activities were measured. Results are relative values versus the normoxic control and are plotted as the means ± SD of 12 experiments. *P < .01 versus the hypoxic control. (E) No changes in expression and nuclear translocation of HIF-1 subunits. Hep3B and HEK293 cells were incubated under normoxic or hypoxic conditions for 16 hours with sodium deoxycholate (10 μg/mL) or AmB (1.25 to 10 μg/mL). HIF-1α and ARNT proteins in total cell lysates (left panel) or in nuclear fractions (right panel) were analyzed by Western blotting. β-actin or NF-κB p50 protein was used as a loading control for total cell lysates or nuclear fractions, respectively. The data shown are representative of 3 separate experiments.
Amphotericin B inactivates HIF-1 without changing protein levels of HIF-1 subunits. (A-D) AmB-induced HIF-1 inhibition. Luciferase reporter plasmids containing wild EpoE or mutated EpoE were transfected into Hep3B and HEK293 cells. After incubation under normoxic or hypoxic conditions for 16 hours with sodium deoxycholate or AmB, luciferase activities were measured. Results are relative values versus the normoxic control and are plotted as the means ± SD of 12 experiments. *P < .01 versus the hypoxic control. (E) No changes in expression and nuclear translocation of HIF-1 subunits. Hep3B and HEK293 cells were incubated under normoxic or hypoxic conditions for 16 hours with sodium deoxycholate (10 μg/mL) or AmB (1.25 to 10 μg/mL). HIF-1α and ARNT proteins in total cell lysates (left panel) or in nuclear fractions (right panel) were analyzed by Western blotting. β-actin or NF-κB p50 protein was used as a loading control for total cell lysates or nuclear fractions, respectively. The data shown are representative of 3 separate experiments.
AmB attenuates the HIF-1-dependent stimulation of EPO enhancer
Since hypoxia induces EPO gene transcription due to HIF-1 targeting its 3′-enhancer segment, we examined the effect of AmB on the activity of an EPO enhancer reporter. In hypoxic Hep3B and 293 cells, this reporter activity increased 25- and 5.5-fold, respectively, versus that of normoxic cells. However, AmB reduced this activity in a dose-dependent manner (Figure 2A,C). In contrast, in cells transfected with an EPO enhancer reporter plasmid mutated at the HIF-1-binding site, reporter activity was unaffected by either hypoxia or AmB (Figure 2B,D), suggesting that AmB does not influence the HIF-1-independent transcription of the reporter gene. These results indicate that AmB blocks the interaction between HIF-1 and the EPO enhancer, thus reducing EPO mRNA levels. Basically, HIF-1 activation requires that both HIF-1 subunits, HIF-1α and ARNT, should be present and translocated to the nucleus. Thus, we examined whether AmB reduces the levels of HIF-1 subunits in hypoxic cells. AmB did not reduce the cellular levels of either HIF-1α or ARNT in Hep3B or HEK293 cells (Figure 2E, left panel). In the nuclear fractions, HIF-1α levels were not affected by AmB in Hep3B cells, but slightly reduced in HEK293 cells (Figure 2E, right panel). However, the reduction in nuclear HIF-1α occurred only in HEK293 cells, and the reduction degree was not significant compared with that in HIF-1 activity. Therefore, either HIF-1 expression or nuclear translocation is unlikely to be responsible for the HIF-1 inhibition by AmB.
HIF-1α repression by AmB requires Asn803 and FIH
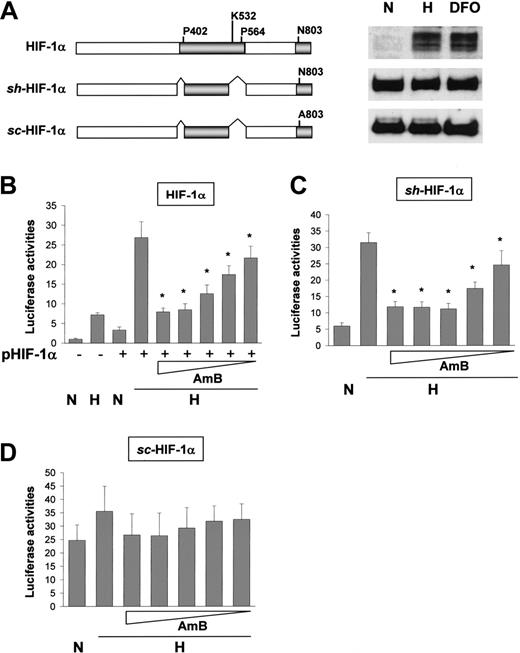
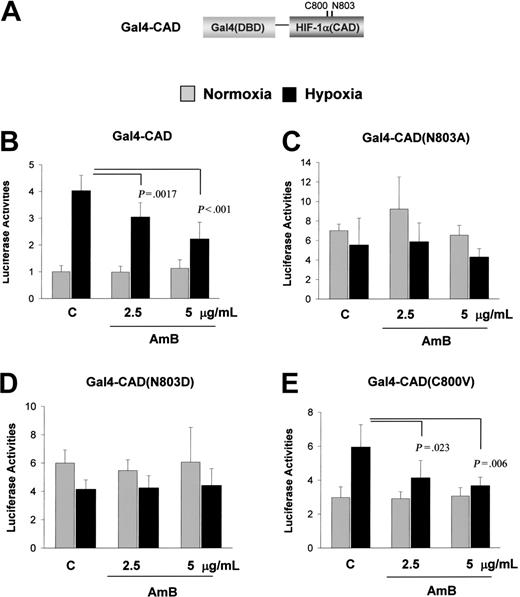
To test the possibility that AmB inhibits HIF-1 at the transcriptional level, we expressed HIF-1α or its stable mutants, structures of which are illustrated in Figure 3A. Stable, hypoxia-activated (sh)-HIF-1α is expressed stably under normoxic conditions, but its transcriptional activity is O2-dependently regulated. In contrast, stable, constitutively activated (sc)-HIF-1α lacks theAsn803 residue responsible for hypoxia-activated transcription and is active, regardless of O2 tension. As expected, the EPO reporter activity was enhanced by wild-type HIF-1α and sensitively repressed by AmB (Figure 3B). sh-HIF-1α transcriptional activity was also inhibited by AmB (Figure 3C). However, sc-HIF-1α showed no significant change in transcriptional activity due to AmB (Figure 3D). The slight increase of reporter activity by hypoxia (the second bar in Figure 3D) may be due to the reporter activation by endogenous HIF-1α. These results suggest that AmB inhibits the hypoxic activation of CAD by targeting Asn803. To confirm that AmB specifically blocks the hypoxic activation of CAD, we coexpressed a Gal4-CAD fusion protein (illustrated in Figure 4A) with a Gal4-Luc reporter plasmid in HEK293 cells. This fusion protein was activated under hypoxic conditions and this activation was significantly diminished by AmB (Figure 4B). However, when Asn803 in CAD had been replaced with alanine or aspartate, AmB showed no effect on the Gal4-CAD mutants (Figure 4C-D). When Cys800, which is responsible for the redox-dependent regulation of CAD, was mutated, the induction fold of CAD activity under hypoxic conditions reduced slightly, but was significantly reduced by AmB treatment (Figure 4E). These results suggest that AmB represses CAD activity by targeting Asn803 and that this AmB action is possible when O2, even at low levels, is available for an O2-sensing system to regulate CAD activity.
Since Asn803 hydroxylation by FIH is a key step in the inactivation of CAD, we hypothesized that AmB stimulates this inhibitory process, and therefore we examined the inhibitory effect of AmB in cells in which FIH was overexpressed or knocked down. The HIF-1α repression by AmB was significantly augmented in FIH-overexpressing cells (Figure 5C) and inversely diminished in FIH-silenced cells (Figure 5E). In cells cotransfected with control DNA (Figure 5B) or siRNA (Figure 5D), the AmB effect was not different from that in untransfected cells (Figure 4B). These results suggest that AmB augments the CAD inhibition by FIH under hypoxic conditions. However, it is uncertain that FIH can be activated under hypoxic conditions because oxygen is essentially required for the enzymatic action of FIH.27 To examine whether FIH is functional under hypoxic (1% O2) conditions, we expressed or knocked down FIH. The hypoxic CAD activity was significantly enhanced by FIH siRNA and suppressed by FIH expression (Figure 5F, right panel). This result suggests that endogenous FIH is not completely inactivated and expressed FIH has activity under hypoxic conditions. FIH activity is limited in such O2 tension, but remains in part. Thus, it is possible that AmB stimulates the FIH activity remaining under hypoxic conditions. On the other hand, FIH expression showed no further inhibition of CAD activity under normoxic conditions (Figure 5F, left panel), which suggests that FIH activity is already saturated. Therefore, CAD activity could not be inhibited by AmB, as shown in Figure 4B.
The inhibition of FIH recovers HIF-1α activity and EPO expression in vitro and in vivo
To confirm that FIH is involved in the AmB-induced repression of CAD, we treated cells with FIH inhibitors. The iron chelator, desferrioxamine, and the 2-oxoglutarate analog, DMOG, inhibit FIH because iron and 2-oxoglutarate are cofactors essential for the enzymatic reaction.27,34 Both FIH inhibitors at low concentrations effectively reversed CAD inhibition by AmB (Figure 6A). Moreover, DMOG at these low concentrations recovered the hypoxic expression of the EPO and other HIF-1 target genes in the presence of AmB (Figure 6B). In general, 100 μM desferrioxamine and 1 mM DMOG are required to block the oxygen-sensing process. However, AmB action was sensitively inhibited by 10 μM desferrioxamine and 1 μM DMOG. Considering that Gal4-CAD protein is not regulated by PHDs, which can be also inhibited by these molecules, the recovery of CAD activity is likely to be a consequence of FIH inhibition.
AmB enhances HIF-1α-FIH binding and inhibits the recruitment of p300
To determine whether AmB stimulates binding between FIH and CAD, we coexpressed Gal4-CAD and HA-tagged FIH and performed a coimmunoprecipitation experiment using anti-Gal4 and anti-HA antibodies. The input data revealed that the amounts of FIH protein were not changed by AmB, and neither was the Gal4 fusion (Figure 7A, upper and middle panels). However, FIH binding to CAD was significantly increased in AmB-treated cells, but FIH binding to Gal4 was not (Figure 7A, lower panel). FIH is known to inactivate CAD by preventing the interaction between CAD and the C/H1 domain of p300 coactivator. Thus, we examined the CAD-p300 interaction by using a mammalian 2-hybrid assay, in which reporter activity was determined by the interaction between Gal4-CAD and C/H1-VP16. Figure 7B shows that the CAD-p300 interaction was enhanced by hypoxia and that this interaction was significantly inhibited by AmB. These results suggest that AmB enhances the binding activity of FIH to CAD and inhibits the recruitment of p300, thus resulting in a repression of CAD activity under hypoxic conditions.
Amphotericin B represses the CAD of HIF-1α. (A) Structures of HIF-1α mutants. The expressions of HIF-1α mutants were verified by Western blotting. N indicates normoxia; H, hypoxia; and DFO, 130 μM desferrioxamine. (B) AmB repression of expressed wild-type HIF-1α. The plasmid for HIF-1α was cotransfected with the EpoE reporter into HEK293 cells. After incubation under normoxic (N) or hypoxic (H) conditions for 16 hours with sodium deoxycholate or AmB, luciferase activities were determined. (C) AmB repression of stably expressed, hypoxia-activated HIF-1α mutant. The plasmid for a stable, hypoxia-activated HIF-1α mutant (sh-HIF-1α) was cotransfected with the EpoE reporter into HEK293 cells. The cells were incubated under normoxic or hypoxic conditions for 16 hours with sodium deoxycholate or AmB. (D) Asn803 is required for AmB repression. The plasmid for a stable, constitutively activated HIF-1α mutant (sc-HIF-1α, Asn803Ala) was cotransfected with the EpoE reporter into HEK293 cells. The cells were incubated under normoxic or hypoxic conditions for 16 hours with sodium deoxycholate or AmB. Results are expressed as relative values versus the normoxic activity in the untransfected cells and are plotted as the means ± SD of 12 experiments. *P < .05 versus the hypoxic control.
Amphotericin B represses the CAD of HIF-1α. (A) Structures of HIF-1α mutants. The expressions of HIF-1α mutants were verified by Western blotting. N indicates normoxia; H, hypoxia; and DFO, 130 μM desferrioxamine. (B) AmB repression of expressed wild-type HIF-1α. The plasmid for HIF-1α was cotransfected with the EpoE reporter into HEK293 cells. After incubation under normoxic (N) or hypoxic (H) conditions for 16 hours with sodium deoxycholate or AmB, luciferase activities were determined. (C) AmB repression of stably expressed, hypoxia-activated HIF-1α mutant. The plasmid for a stable, hypoxia-activated HIF-1α mutant (sh-HIF-1α) was cotransfected with the EpoE reporter into HEK293 cells. The cells were incubated under normoxic or hypoxic conditions for 16 hours with sodium deoxycholate or AmB. (D) Asn803 is required for AmB repression. The plasmid for a stable, constitutively activated HIF-1α mutant (sc-HIF-1α, Asn803Ala) was cotransfected with the EpoE reporter into HEK293 cells. The cells were incubated under normoxic or hypoxic conditions for 16 hours with sodium deoxycholate or AmB. Results are expressed as relative values versus the normoxic activity in the untransfected cells and are plotted as the means ± SD of 12 experiments. *P < .05 versus the hypoxic control.
Asn803 is required for amphotericin B-induced CAD repression. (A) Structure of Gal4-CAD. (B) AmB repression of CAD of HIF-1α. The plasmid for Gal4-CAD was cotransfected with a Gal4-luc reporter plasmid into Hep3B cells. After incubation under normoxic (21% O2,  ) or hypoxic (1% O2, ▪) conditions for 16 hours with sodium deoxycholate or AmB, luciferase activities were measured. (C-D) Requirement of Asn803 in the AmB-induced CAD repression. The plasmid for Gal4-CAD mutant, in which Asn803 was substituted with Ala (N803A) or with Asp (N803D), was cotransfected with a Gal4-luc reporter plasmid. (E) No involvement of Cys800 in the AmB repression. The plasmid for Gal4-CAD mutant having Val800 for Cys800 (C800V) was cotransfected with a Gal4-luc reporter plasmid. Results are quoted as relative values versus the normoxic activity of Gal4-CAD and are the means ± SD of 16 experiments. P values versus the hypoxic control are presented in the figures.
) or hypoxic (1% O2, ▪) conditions for 16 hours with sodium deoxycholate or AmB, luciferase activities were measured. (C-D) Requirement of Asn803 in the AmB-induced CAD repression. The plasmid for Gal4-CAD mutant, in which Asn803 was substituted with Ala (N803A) or with Asp (N803D), was cotransfected with a Gal4-luc reporter plasmid. (E) No involvement of Cys800 in the AmB repression. The plasmid for Gal4-CAD mutant having Val800 for Cys800 (C800V) was cotransfected with a Gal4-luc reporter plasmid. Results are quoted as relative values versus the normoxic activity of Gal4-CAD and are the means ± SD of 16 experiments. P values versus the hypoxic control are presented in the figures.
Asn803 is required for amphotericin B-induced CAD repression. (A) Structure of Gal4-CAD. (B) AmB repression of CAD of HIF-1α. The plasmid for Gal4-CAD was cotransfected with a Gal4-luc reporter plasmid into Hep3B cells. After incubation under normoxic (21% O2,  ) or hypoxic (1% O2, ▪) conditions for 16 hours with sodium deoxycholate or AmB, luciferase activities were measured. (C-D) Requirement of Asn803 in the AmB-induced CAD repression. The plasmid for Gal4-CAD mutant, in which Asn803 was substituted with Ala (N803A) or with Asp (N803D), was cotransfected with a Gal4-luc reporter plasmid. (E) No involvement of Cys800 in the AmB repression. The plasmid for Gal4-CAD mutant having Val800 for Cys800 (C800V) was cotransfected with a Gal4-luc reporter plasmid. Results are quoted as relative values versus the normoxic activity of Gal4-CAD and are the means ± SD of 16 experiments. P values versus the hypoxic control are presented in the figures.
) or hypoxic (1% O2, ▪) conditions for 16 hours with sodium deoxycholate or AmB, luciferase activities were measured. (C-D) Requirement of Asn803 in the AmB-induced CAD repression. The plasmid for Gal4-CAD mutant, in which Asn803 was substituted with Ala (N803A) or with Asp (N803D), was cotransfected with a Gal4-luc reporter plasmid. (E) No involvement of Cys800 in the AmB repression. The plasmid for Gal4-CAD mutant having Val800 for Cys800 (C800V) was cotransfected with a Gal4-luc reporter plasmid. Results are quoted as relative values versus the normoxic activity of Gal4-CAD and are the means ± SD of 16 experiments. P values versus the hypoxic control are presented in the figures.
Discussion
Hypoproliferative anemia, which is due to impairment of erythropoiesis, is secondarily developed in patients who have chronic inflammatory disorders persisting more than a month. These disorders include bacterial or fungal infections, autoimmune diseases, and tumors. In most cases of secondary anemia, correction of the underlying disorder suffices to alleviate the blood abnormality. In this respect, patients infected by fungi could undergo anemic process already before AmB therapy. However, the important thing is the fact that the anemia is aggravated after AmB therapy even though infection is improved. In a clinical study, mean hematocrit fell from 41 before AmB therapy to 27 at therapy completion.5 This AmB-induced anemia has also been clinically demonstrated by other research groups.6,7 Furthermore, serum EPO levels fall significantly in parallel with hematocrit and hemoglobin reduction during therapy. Therefore, AmB-induced EPO suppression is considered to aggravate the hypoproliferative anemia.
To date, the mechanism underlying EPO suppression is not understood. It is possible that EPO suppression occurs secondary to the nephrotoxicity induced by AmB, as occurs in chronic renal failure. If so, degrees of anemia and EPO level reduction should be correlated with increased levels of blood urea nitrogen (BUN), which is a marker for nephrotoxicity. However, Brandriss et al5 and MacGregor et al6 reported no such correlation between BUN levels and anemia degree or EPO levels. We also demonstrated that EPO production was suppressed by AmB without microscopic changes and apoptotic cell death in rat kidneys. Therefore, it is believed that EPO suppression cannot be explained as being secondary to nephrotoxicity, rather it may be mediated by AmB's specific action on kidney cells. In the present study, we provide an answer to this mystery, namely, that AmB enhances the interaction between FIH and the CAD of HIF-1α, and thus represses CAD activity, which results in an inhibition of EPO gene transcription. Although we cannot fully understand the complicated anemia occurring in patients, HIF-1α repression by FIH is likely to contribute to anemia aggravated after AmB therapy.
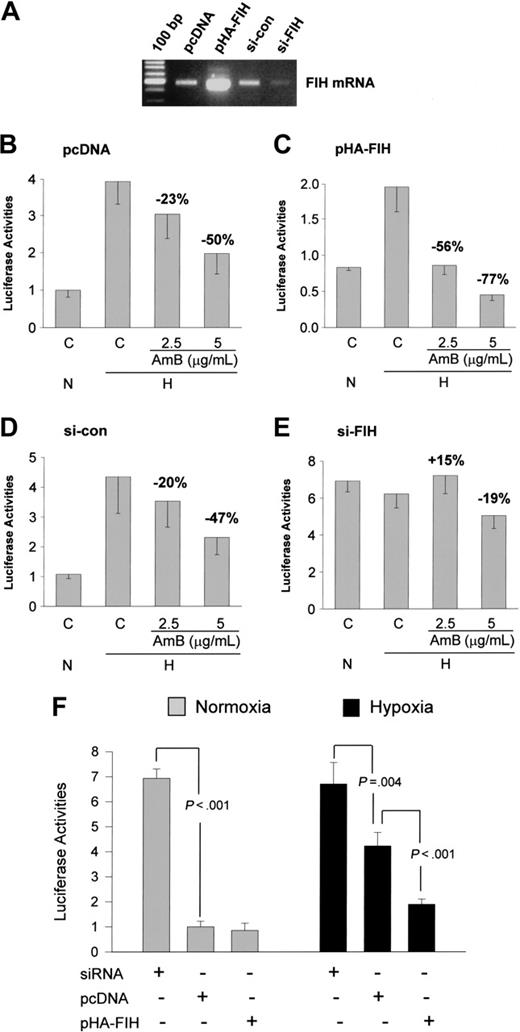
HIF-1α repression by amphotericin B requires FIH. (A) FIH mRNA levels. FIH overexpression and suppression by siRNA were evaluated by RT-PCR method. (B-C) Augmentation of the AmB-induced CAD repression by FIH expression. The plasmid (0.2 μg) for pcDNA or HA-tagged FIH was cotransfected with Gal4-CAD and Gal4-luc reporter plasmids into Hep3B cells. After incubation under normoxic (N) or hypoxic (H) conditions for 16 hours with AmB, luciferase activities were measured. (D-E) Abolishment of the AmB-induced CAD repression by knocking down FIH. siRNA (20 nM) of control (si-con) or FIH (si-FIH) was cotransfected with Gal4-CAD and Gal4-luc reporter plasmids into Hep3B cells. After normoxic or hypoxic incubation with AmB for 16 hours, luciferase activities were measured. (F) Status of FIH activity under normoxic or hypoxic conditions. FIH siRNA (20 nM), pcDNA (0.2 μg), or pHA-FIH (0.2 μg) was cotransfected with Gal4-CAD and Gal4-luc reporter plasmids into Hep3B cells. After normoxic or hypoxic incubation for 16 hours, luciferase activities were measured. Results are quoted as relative values versus the normoxic value in pcDNA-transfected cells and are the means ± SD of 8 experiments. The averages of inhibition percent versus the hypoxic control or P values versus the pcDNA control are presented in the figures.
HIF-1α repression by amphotericin B requires FIH. (A) FIH mRNA levels. FIH overexpression and suppression by siRNA were evaluated by RT-PCR method. (B-C) Augmentation of the AmB-induced CAD repression by FIH expression. The plasmid (0.2 μg) for pcDNA or HA-tagged FIH was cotransfected with Gal4-CAD and Gal4-luc reporter plasmids into Hep3B cells. After incubation under normoxic (N) or hypoxic (H) conditions for 16 hours with AmB, luciferase activities were measured. (D-E) Abolishment of the AmB-induced CAD repression by knocking down FIH. siRNA (20 nM) of control (si-con) or FIH (si-FIH) was cotransfected with Gal4-CAD and Gal4-luc reporter plasmids into Hep3B cells. After normoxic or hypoxic incubation with AmB for 16 hours, luciferase activities were measured. (F) Status of FIH activity under normoxic or hypoxic conditions. FIH siRNA (20 nM), pcDNA (0.2 μg), or pHA-FIH (0.2 μg) was cotransfected with Gal4-CAD and Gal4-luc reporter plasmids into Hep3B cells. After normoxic or hypoxic incubation for 16 hours, luciferase activities were measured. Results are quoted as relative values versus the normoxic value in pcDNA-transfected cells and are the means ± SD of 8 experiments. The averages of inhibition percent versus the hypoxic control or P values versus the pcDNA control are presented in the figures.
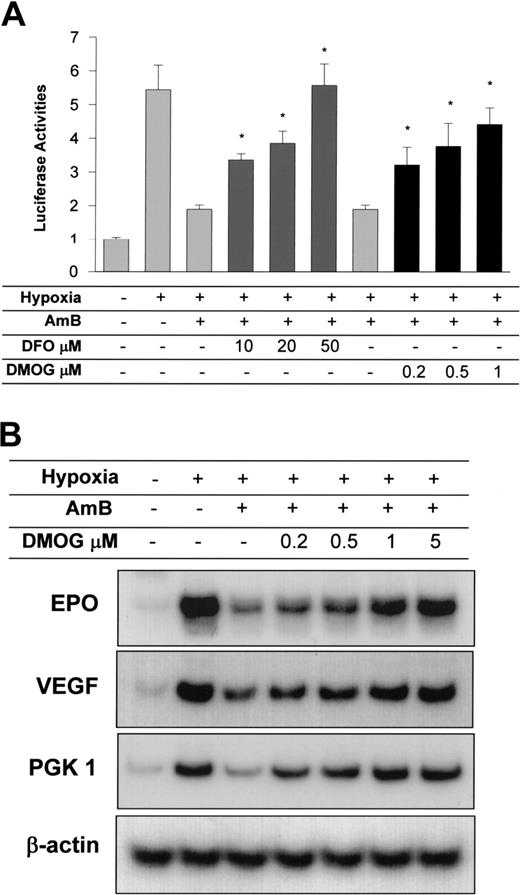
HIF-1α repression by amphotericin B is recovered by inhibition of FIH. (A) Recovery of AmB-repressed HIF-1α activity by FIH inhibitors. Hep3B cells transfected with Gal4-CAD plasmid were treated with desferrioxamine (DFO) or DMOG at various concentrations and incubated under hypoxic conditions in the presence of AmB (5 μg/mL) for 16 hours. Results are plotted as the means ± SD of 12 experiments. *P < .05 versus the hypoxic, AmB-treated group. (B) Recovery of AmB-inhibited HIF-1 target gene expression by FIH inhibitors. Hep3B cells were subjected to normoxia (N, lane 1) or 16 hours of hypoxia (H, lanes 2-7) in the presence of sodium deoxycholate (lanes 1-2) or 10 μg/mL AmB (lane 3). Cells were cotreated with AmB and various DMOG concentrations (lanes 4-7). mRNAs of HIF-1 target genes and β-actin were isolated and analyzed by semiquantitative RT-PCR. The data shown are representative of 3 separate experiments.
HIF-1α repression by amphotericin B is recovered by inhibition of FIH. (A) Recovery of AmB-repressed HIF-1α activity by FIH inhibitors. Hep3B cells transfected with Gal4-CAD plasmid were treated with desferrioxamine (DFO) or DMOG at various concentrations and incubated under hypoxic conditions in the presence of AmB (5 μg/mL) for 16 hours. Results are plotted as the means ± SD of 12 experiments. *P < .05 versus the hypoxic, AmB-treated group. (B) Recovery of AmB-inhibited HIF-1 target gene expression by FIH inhibitors. Hep3B cells were subjected to normoxia (N, lane 1) or 16 hours of hypoxia (H, lanes 2-7) in the presence of sodium deoxycholate (lanes 1-2) or 10 μg/mL AmB (lane 3). Cells were cotreated with AmB and various DMOG concentrations (lanes 4-7). mRNAs of HIF-1 target genes and β-actin were isolated and analyzed by semiquantitative RT-PCR. The data shown are representative of 3 separate experiments.
Circulating EPO in the adult is produced mainly from cells in and around the proximal tubules of the kidney. Hybridization experiments have demonstrated that EPO mRNA is present in fibroblast-like interstitial cells in the vicinity of the proximal tubules in humans, monkeys, mice, and sheep.35 EPO mRNA was also found in epithelial cells of the proximal renal tubules.36 Therefore, toxic drugs that are accumulated around the proximal tubules may perturb these EPO-producing cells, causing impairment of EPO induction in response to hypoxia. AmB could be one such drug. Because of its high hydrophobicity, AmB is reabsorbed through the proximal tubule and is accumulated in the kidney.37 A pharmacokinetic study revealed that the average AmB level was 12.7 μg/mL in the kidneys of rabbits treated with AmB (1 mg/kg per day) for 28 days.38 It is noteworthy that this renal concentration is higher than the effective concentrations (2.5-10 μg/mL) of AmB in the present study. Therefore, the AmB inhibition of HIF-1α activity or EPO expression might occur in the kidney where AmB is present at a level higher than 2.5 μg/mL.
To examine the effect of AmB on EPO expression in the kidney, rats were incubated in the chamber containing 10% oxygen, which is a commonly used condition for whole-body hypoxia. On the other hand, the mechanism of AmB-induced HIF-1α repression was studied in cultured cells under 1% oxygen conditions, which is also well established as activating HIF-1α. To assume that the in vitro effect of AmB occurs in rat kidneys, it is necessary to consider what the renal oxygen tension is in rats subjected to 10% oxygen. Recently, Mik et al39 measured the renal oxygen tension in anesthetized rats by detecting light emitted from Pd-porphyrin. In rats breathing 37% oxygen, the renal oxygen tension was 43 Torr (5.66%). Surprisingly, it was found that the oxygen tension dropped extremely to 3.6 Torr (0.47%) in rats breathing 10% oxygen. However, because rats hyperventilated in our experimental settings, the renal oxygen tension may be somehow higher than 0.47%, but not be out of the oxygen range to activate HIF-1α. Therefore, it is expected that the AmB-induced HIF-1 repression in cultured cells could also occur in the hypoxic kidney.
FIH was first identified in the yeast 2-hybrid assay by screening for proteins that interact with CAD and was characterized as a negative regulator of CAD function.25 Like PHD enzymes, FIH-1 also belongs to the 2-oxoglutarate (2-OG)-dependent dioxygenase superfamily.40 Moreover, hydroxylation catalyzed by FIH requires ferrous iron and consumes molecular oxygen and 2-oxoglutarate (2-OG). Here, iron functions as a reaction center, whereas molecular oxygen facilitates the hydroxylation of the β-carbon on Asn-803, and 2-OG functions as a substrate that accepts the remaining oxygen atom from molecular oxygen, and subsequently undergoes decarboxylation to produce succinate.40 Thus DFO (iron chelator) and DMOG (2-OG analog) are used to inhibit the enzymatic reaction of FIH. However, although the enzymatic reaction and the x-ray crystal structure of FIH have been extensively examined, the FIH regulatory mechanism has not been elucidated. Here, we demonstrated that AmB stimulated the inactivation of CAD by FIH without altering FIH expression in the 1% O2 tension. However, we did not determine how AmB stimulates the action of FIH. To understand this mechanism, we first need to ask: does AmB regulate FIH activity, or does it modify the CAD of HIF-1α such that it is more accessible to FIH? These questions remain to be addressed by future studies.
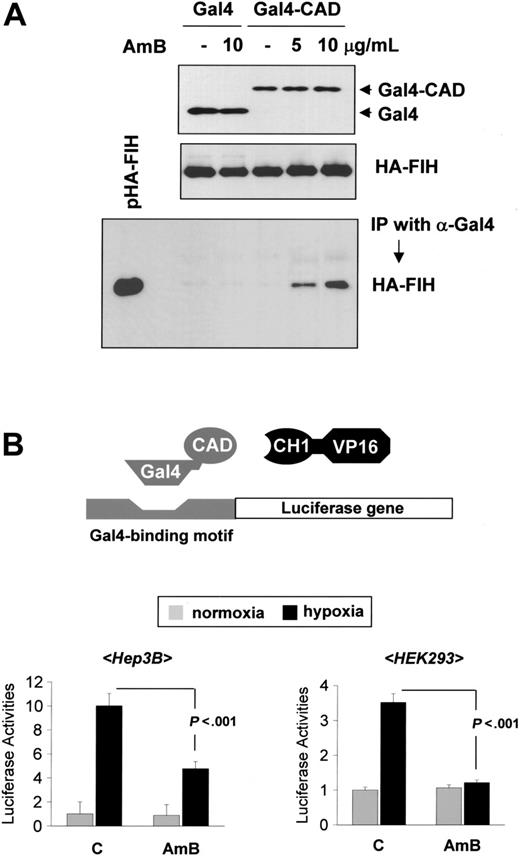
Amphotericin B blocks p300 recruitment by binding FIH to CAD. (A) Coimmunoprecipitation assay for FIH-CAD binding. HEK293 cells were cotransfected with pHA-FIH and either pGal4-CAD or pGal4, and incubated with sodium deoxycholate (-) or AmB under hypoxic conditions for 16 hours. Lysates were prepared and immunoprecipitations were performed using anti-Gal4 antibody. The coimmunoprecipitation of HA-FIH and Gal4-CAD was identified by Western blotting using anti-HA antibody (bottom panel). Protein quantities in the samples were verified using anti-Gal4 and anti-HA antibodies (top and middle panels). The data shown are representative of 3 separate experiments. (B) Mammalian 2-hybrid assay for CAD-p300 binding. Hep3B (left panel) and HEK293 (right panel) cells were cotransfected with 1 μg of a Gal4-luciferase reporter, 1 μg pGal4-CAD, and 500 ng p(His)VP16-C/H1. After a stabilizing period of 48 hours, cells were incubated under either normoxic or hypoxic conditions, in the absence or presence of AmB (5 μg/mL) for 16 hours, and then lysed to determine luciferase and β-gal activities. The results shown are relative values versus the normoxic control and are plotted as the means ± SD of 8 experiments.
Amphotericin B blocks p300 recruitment by binding FIH to CAD. (A) Coimmunoprecipitation assay for FIH-CAD binding. HEK293 cells were cotransfected with pHA-FIH and either pGal4-CAD or pGal4, and incubated with sodium deoxycholate (-) or AmB under hypoxic conditions for 16 hours. Lysates were prepared and immunoprecipitations were performed using anti-Gal4 antibody. The coimmunoprecipitation of HA-FIH and Gal4-CAD was identified by Western blotting using anti-HA antibody (bottom panel). Protein quantities in the samples were verified using anti-Gal4 and anti-HA antibodies (top and middle panels). The data shown are representative of 3 separate experiments. (B) Mammalian 2-hybrid assay for CAD-p300 binding. Hep3B (left panel) and HEK293 (right panel) cells were cotransfected with 1 μg of a Gal4-luciferase reporter, 1 μg pGal4-CAD, and 500 ng p(His)VP16-C/H1. After a stabilizing period of 48 hours, cells were incubated under either normoxic or hypoxic conditions, in the absence or presence of AmB (5 μg/mL) for 16 hours, and then lysed to determine luciferase and β-gal activities. The results shown are relative values versus the normoxic control and are plotted as the means ± SD of 8 experiments.
The present study demonstrates that 2 FIH inhibitors effectively recovered CAD repression by AmB. Of interest, FIH was inhibited by DFO and DMOG at concentrations as low as 10 to 50 μM and 0.2 to 1 μM, respectively. Considering that, in general, much higher concentrations of DFO and DMOG are needed to inhibit FIH in vitro or in cultured cells, FIH in AmB-treated cells proved highly sensitive to these inhibitors. We speculate that AmB increases this sensitivity. With respect to hydrophilic agents, such as DFO and DMOG, their concentrations in the cytoplasm are probably largely affected by membrane permeability. Since AmB binds to cholesterol in the plasma membrane, it may disturb membrane integrity and facilitate DFO or DMOG entry. However, we cannot rule out the possibility that AmB directly enhances drug sensitivity of FIH.
Currently, AmB is the most widely used antibiotic for preventing accidental fungal infections in mammalian cells and tissue cultures, at a recommended level of 2.5 μg/mL. Moreover, during the isolation of cells or tissues from animals, a higher concentration of AmB is added to media. However, as shown by this study, AmB effectively suppresses HIF-1 activity at such concentrations. Therefore, when investigating hypoxic response, especially concerning HIF-1-related gene expression, we suggest that this antibiotic be omitted from media.
Prepublished online as Blood First Edition Paper, September 27, 2005; DOI 10.1182/blood-2005-06-2564.
E.-J.Y. and J.-H.R. contributed equally to this work.
Supported by a grant from the Seoul National University College of Medicine Research Fund 2004 and by a grant from the Korean Ministry of Health and Welfare Research Fund 2005.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal