Abstract
Recent advances have increased the purity of hematopoietic stem cells (HSCs) isolated from young mouse bone marrow. However, little attention has been paid to the purity of HSCs from other contexts. Although Thy-1lowSca-1+Lineage-c-kit+ cells from young bone marrow are highly enriched for HSCs (1 in 5 cells gives long-term multilineage reconstitution after transplantation into irradiated mice), the same population from old, reconstituted, or cytokine-mobilized mice engrafts much less efficiently (1 in 78 to 1 in 185 cells gives long-term multilineage reconstitution). To test whether we could increase the purity of HSCs isolated from these contexts, we examined the SLAM family markers CD150 and CD48. All detectable HSCs from old, reconstituted, and cyclophosphamide/G-CSF-mobilized mice were CD150+CD48-, just as in normal young bone marrow. Thy-1lowSca-1+Lineage-c-kit+ cells from old, reconstituted, or mobilized mice included mainly CD48+ and/or CD150- cells that lacked reconstituting ability. CD150+CD48-Sca-1+Lineage-c-kit+ cells from old, reconstituted, or mobilized mice were much more highly enriched for HSCs, with 1 in 3 to 1 in 7 cells giving long-term multilineage reconstitution. SLAM family receptor expression is conserved among HSCs from diverse contexts, and HSCs from old, reconstituted, and mobilized mice engraft relatively efficiently after transplantation when contaminating cells are eliminated.
Introduction
Hematopoietic stem cells (HSCs) are self-renewing, multipotent progenitors that give rise to all types of blood and immune system cells.1,2 Recent studies have improved the purity with which HSCs can be isolated from young adult mouse bone marrow, to the point where 40% to 96% of single cells from various populations can give long-term multilineage reconstitution in irradiated mice.3-5 This proves that HSCs from normal young bone marrow can engraft highly efficiently after intravenous transplantation into irradiated mice.6
Comparatively little attention has been paid to the purity of HSCs isolated from other contexts and it remains to be determined whether HSCs from other contexts can also engraft efficiently after transplantation. One (20%) of every 5 Thy-1lowSca-1+Lineage-c-kit+ cells isolated from young adult mouse bone marrow gives long-term multilineage reconstitution upon transplantation into irradiated mice.7-10 However, HSCs isolated from other contexts have exhibited much poorer reconstituting efficiencies. One (1.3%) in 78 Thy-1lowSca-1+Lineage-c-kit+ cells isolated from old mouse bone marrow gave long-term multilineage reconstitution in irradiated mice.11 One (0.7%) in 150 to 1 (0.5%) in 185 Thy-1lowSca-1+Lineage-c-kit+ cells isolated from reconstituted or cyclophosphamide/G-CSF-mobilized mice gave long-term multilineage reconstitution in irradiated mice.12,13 Others have also reported the reduced engraftment efficiency of HSCs isolated from old or cytokine-mobilized mice.14-16 These observations raise the question of whether HSCs from old, reconstituted, and mobilized mice exhibit large defects in their ability to home/engraft after transplantation, or whether Thy-1lowSca-1+Lineage-c-kit+ cells from these contexts contain more contaminating non-HSCs. If HSCs from old, reconstituted, and mobilized donors are intrinsically less able to engraft after transplantation, it could have important implications for clinical transplants in these settings.
We recently discovered that HSCs from young adult mice and cytokine-mobilized mice can be more highly purified with the use of SLAM family markers, either by themselves or in combination with other HSC markers.3 In particular, the SLAM family receptors CD150 and CD48 enhance HSC purity, as 47% of single CD150+CD48-Sca-1+Lineage-c-kit+ bone marrow cells (1 in 2.1) give long-term multilineage reconstitution. The fact that SLAM family members are differentially expressed among young adult bone marrow hematopoietic progenitors in a way that correlates with primitiveness, and that HSCs can be highly enriched using only CD150 and CD48, suggested that HSCs can be identified based on a SLAM code (CD150+CD48-).3
This raises the question of whether these markers also enhance the purity of HSCs in other contexts. Cyclophosphamide/G-CSF-mobilized HSCs are also highly enriched within the CD150+CD48- population,3 though we did not test whether all mobilized HSCs fell within this population and therefore could not assess the overall engraftment efficiency of mobilized HSCs. It has not been tested whether HSCs from old or reconstituted mice are CD150+ or CD48-. The conservation of these markers between HSCs in different mouse strains suggests they might be robust stem cell markers.3 However, gene expression profiling showed that CD48 appeared to be up-regulated in an enriched HSC population after 5-fluorouracil treatment.17 Although this study did not functionally test whether the CD48+ cells were HSCs or contaminating cells, these results raised the possibility that CD48 may change its expression on HSCs under certain circumstances.
To test whether the CD150+CD48- markers that identify young adult bone marrow HSCs3 are conserved among HSCs in other contexts, we examined HSCs from old, mobilized, and reconstituted mice. In each case, all detectable HSCs were CD150+ and CD48-, emphasizing the robustness of these markers. The use of these new markers substantially increased the purity of HSCs from old, mobilized, and reconstituted mice, such that relatively efficient engraftment was observed from CD150+CD48-Sca-1+Lineage- c-kit+ cells in each case (on average 1 in 3.6 to 1 in 6.9 cells engrafted and gave long-term multilineage reconstitution in each case). These data are consistent with our previous analysis of old HSCs11 and with the recent functional analysis of old HSC engraftment by Liang et al,14 in suggesting that old HSCs may exhibit a 3-fold engraftment defect relative to young adult HSCs. However, the data do not support the possibility of more profound engraftment defects. By substantially increasing the purity with which HSCs can be isolated from old, cytokine-mobilized, and reconstituted mice, SLAM family markers enhance the precision with which HSCs can be studied.
Materials and methods
All mice used in this study were housed in the Unit for Laboratory Animal Medicine at the University of Michigan. Donor hematopoietic progenitors were obtained from adult C57BL/Ka-CD45.2:Thy-1.1 mice or C57BL/Ka-CD45.1:Thy-1.2 mice as specified. Old donor mice were at least 22 months of age and had normal spleen size. Young adult mice were 6 to 8 weeks of age. Prior to being used in these experiments, reconstituted mice were long-term multilineage reconstituted for at least 20 weeks after receiving transplants with either donor whole bone marrow cells or enriched donor HSCs. When donor cells were retransplanted from these mice into secondary recipients, CD45.1 (clone A20.1) or CD45.2 (clone 104) was included in the lineage cocktail to exclude recipient-type cells. As described previously,13 mobilized mice were injected with cyclophosphamide (200 mg/kg; Bristol-Myers Squibb, New York, NY) and then on each of 7 subsequent days with 5 μg human G-CSF (250 μg/kg per day; Amgen, Thousand Oaks, CA) before being killed to obtain splenocytes for analysis. Throughout the paper, these “mobilized” cells are referred to as day-7 cyclophosphamide/G-CSF-mobilized splenocytes. Recipient mice in reconstitution assays were adult C57BL/Ka-CD45.2:Thy-1.1 mice or C57BL/Ka-CD45.1:Thy-1.2 mice as specified.
Flow-cytometric isolation of stem and progenitor cells
Bone marrow cells were flushed from femurs and tibias with Hanks buffered salt solution without calcium or magnesium, supplemented with 2% heat-inactivated calf serum (HBSS+; Gibco, Grand Island, NY). Cells were triturated and filtered through nylon screen (45 μm; Sefar America, Kansas City, MO) to obtain single-cell suspensions.
Thy-1lowSca-1+Lineage-c-kit+ HSCs were isolated as previously described.12,18 Briefly, whole bone marrow cells were incubated with unconjugated monoclonal antibodies to lineage markers including B220 (6B2), CD3 (KT31.1), CD5 (53-7.3), CD8 (53-6.7), Gr-1 (8C5), and Ter119. Following dilution, pelleted cells were resuspended in anti-rat IgG specific F(ab)2 fragment conjugated to phycoerythrin (PE; Jackson ImmunoResearch, West Grove, PA). Cells were subsequently stained with directly conjugated antibodies to Sca-1 (Ly6A/E-APC), c-kit (2B8-biotin), Thy-1.1 (19XE5-FITC), Mac-1 (M1/70-PE), CD4 (GK1.5-PE), and, when specified, CD48 (HM48-1-PE; Pharmingen, San Diego, CA). Progenitors were often enriched by preselecting for Sca-1+ or c-kit+ cells using paramagnetic microbeads (Miltenyi Biotec, Auburn, CA) and selecting on a Miltenyi autoMACS prior to flow cytometry.
Cells sorted based on CD150 expression were incubated with unconjugated antibody to CD150 (26D12; a gift of DNAX, Palo Alto, CA), and subsequently stained with goat anti-rat IgG F(ab)2 fragment conjugated to FITC (Jackson ImmunoResearch). Cells sorted according to CD48 were stained with directly conjugated anti-CD48 (FITC or PE). Cells were resuspended in 2 μg/mL 7-AAD (Molecular Probes, Eugene, OR) to discriminate live from dead cells. Only live (7-AAD-) cells were included in analyses and sorts. All flow cytometry was performed on a FACS Vantage dual laser flow-cytometer (Becton Dickinson, San Jose, CA).
Methylcellulose culture
Methylcellulose cultures were performed as previously described.11 Briefly, unfractionated bone marrow cells, or single resorted hematopoietic progenitors were plated in wells of 96-well plates (Corning, Corning, NY) containing 100 μL 1.0% methylcellulose (Stem Cell Technologies, Vancouver, BC, Canada). The methylcellulose was supplemented with 20% charcoal-absorbed fetal bovine serum (Cocalico, Reamstown, PA), 1% BSA (Sigma), 1% penicillin/streptomycin (Gibco), 50 ng/mL stem cell factor (SCF), 10 ng/mL interleukin-3 (IL-3), 10 ng/mL interleukin-6 (IL-6), 3 U/mL erythropoietin (Epo), 10 ng/mL Flt-3, and 10 ng/mL thrombopoietin (Tpo). All cytokines were obtained from R&D Systems (Minneapolis MN). Colonies were maintained at 37°C in fully humidified chambers containing 6% CO2. Colony formation was scored after 10 to 14 days of culture.
Long-term competitive reconstitution assays
Adult recipient mice were lethally irradiated with an Orthovoltage x-ray source delivering approximately 3 Gy/min. The mice received 2 doses of 5.5 to 5.7 Gy, delivered at least 2 hours apart. For transplantation of sorted cell populations, CD45.2+ stem or progenitor cells were sorted and then resorted into individual wells of a 96-well plate containing 200 000 CD45.1+ whole bone marrow cells in 100 μL HBSS+. In some experiments, 300 000 recipient-type CD150- bone marrow cells were used for radioprotection. The contents of individual wells were drawn into a 500-μL insulin syringe (Becton Dickinson) and injected into the retro-orbital venous sinus of lethally irradiated, anesthetized CD45.1 recipients. Mice were maintained on antibiotic water (1.1 g neomycin sulfate and 106 U/L polymixin B sulfate; Sigma) ad libitum. Starting at 3.5 weeks after transplantation and continuing for at least 16 weeks after transplantation, peripheral blood was obtained from the tail veins of individual recipient mice, subjected to ammonium-chloride potassium red cell lysis,18 and stained with antibodies to CD45.2 (clone 104) or CD45.1 (clone A20.1), and B220 (6B2), Mac-1 (M1/70), CD3 (KT31.1), and Gr-1 (8C5).
Results
ThylowSca-1+Lineage-c-kit+ cells from old, reconstituted, and mobilized mice contain CD48+ cells with no multilineage-reconstituting activity
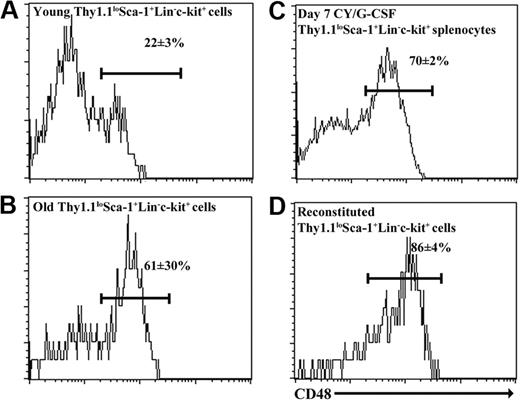
To determine whether CD48 might enhance the purity of HSCs from old, reconstituted, or mobilized mice, we examined CD48 expression on ThylowSca-1+Lineage-c-kit+ cells from each of these contexts. While only 22 ± 3% of young adult ThylowSca-1+Lineage-c-kit+ bone marrow cells expressed CD48, 61 ± 30% of ThylowSca-1+Lineage-c-kit+ bone marrow cells from 26- to 28-month-old mice, 70 ± 2% of ThylowSca-1+Lineage-c-kit+ splenocytes from day-7 cyclophosphamide/G-CSF-treated mice, and 86 ± 4% of ThylowSca-1+Lineage-c-kit+ bone marrow cells from reconstituted mice expressed CD48 (Figure 1). The observation that most ThylowSca-1+Lineage-c-kit+ cells from old, reconstituted, or mobilized mice expressed CD48 was reminiscent of the increase in CD48 expression by an enriched HSC population after 5-FU treatment.17 This raised the question of whether HSCs gain CD48 expression in old, reconstituted, and mobilized mice, or whether the CD48+ cells are contaminating non-HSCs, just as they were previously observed to lack HSC activity in young adult bone marrow.3
To test this, we performed competitive reconstitution assays in which CD48+ or CD48- cells from old, reconstituted, or mobilized mice were transplanted into lethally irradiated recipients along with a radioprotective dose of 200 000 recipient-type whole bone marrow cells (Table 1). In each context, recipients that received CD48- cells were usually long-term multilineage reconstituted by donor cells (11/11 from old mice, 4/7 from reconstituted mice, and 5/5 from mobilized mice), but recipients that received CD48+ cells were rarely or never long-term multilineage reconstituted by donor cells (1/11 from old mice, 0/5 from reconstituted mice, and 0/5 from day-7 Cy/G-CSF-treated mice). These data indicate that most or all of the detectable HSC activity is in the CD48- fractions of old bone marrow cells, reconstituted bone marrow cells, and mobilized splenocytes.
HSCs are enriched within the CD48- fraction but not the CD48+ fraction of old bone marrow, reconstituted bone marrow, and cyclophosphamide/G-CSF-mobilized spleen
Source of cells . | No. donor-type cells transplanted . | No. mice with long-term multilineage engraftment/no. mice total . |
|---|---|---|
| Old bone marrow | ||
| CD48+ | 60 000 | 1/11 |
| CD48– | 140 000 | 11/11 |
| Mobilized splenocytes | ||
| CD48+ | 30 000 | 0/5 |
| CD48– | 170 000 | 5/5 |
| Reconstituted bone marrow | ||
| CD48+ | 35 000 | 0/5 |
| CD48– | 165 000 | 4/7 |
Source of cells . | No. donor-type cells transplanted . | No. mice with long-term multilineage engraftment/no. mice total . |
|---|---|---|
| Old bone marrow | ||
| CD48+ | 60 000 | 1/11 |
| CD48– | 140 000 | 11/11 |
| Mobilized splenocytes | ||
| CD48+ | 30 000 | 0/5 |
| CD48– | 170 000 | 5/5 |
| Reconstituted bone marrow | ||
| CD48+ | 35 000 | 0/5 |
| CD48– | 165 000 | 4/7 |
Old bone marrow cells were obtained from 26- to 28-month-old C57BL mice. Mobilized splenocytes were obtained from mice that had been treated with cyclophosphamide followed by 7 daily injections of G-CSF.13 Reconstituted bone marrow cells were obtained from mice that had been long-term multilineage reconstituted for 20 to 24 weeks by highly enriched HSCs. Donor cells from reconstituted mice were selected for donor cell origin (CD45.1+) in addition to CD48. The indicated number of donor-type (CD45.1+) cells was transplanted intravenously into lethally irradiated recipients (CD45.2+) along with 200 000 recipient-type (CD45.2+) whole bone marrow cells. The dose of CD48+ or CD48– donor cells was based on the number of cells from each population contained in 200 000 old bone marrow, mobilized spleen, or reconstituted bone marrow cells as done in previous studies of marker expression on HSCs.3,10,13,19 Mice were considered long-term multilineage reconstituted if donor-type myeloid, B, and T cells were present for at least 16 weeks after transplantation.
This suggested that it might be possible to significantly improve HSC enrichment in each of these contexts by excluding the CD48+ cells from the ThylowSca-1+Lineage-c-kit+ population. To further test this possibility using highly enriched HSCs, we isolated the CD48+ and CD48- subsets of Flk2-Sca-1+Lineage-c-kit+ cells. Flk2 was used in place of Thy1.1 because Flk2-Sca-1+Lineage-c-kit+ cells are highly enriched for HSC activity,20 and Flk2 could be added to the lineage panel, freeing up a channel to sort based on CD48. We transplanted 15 Flk2-Sca-1+Lineage-c-kit+CD48- cells or 15 Flk2-Sca-1+Lineage-c-kit+CD48+ cells from old bone marrow, mobilized spleen, or reconstituted bone marrow into lethally irradiated recipient mice along with 200 000 recipient-type whole bone marrow cells (Table 2). While virtually all recipients of Flk2-Sca-1+Lineage-c-kit+CD48- cells became reconstituted and most of these mice exhibited long-term multilineage reconstitution, none of the recipients of Flk2-Sca-1+Lineage-c-kit+CD48+ cells showed any engraftment by donor-type cells. These data further support the conclusion that HSCs from old, mobilized, and reconstituted mice are CD48- and that HSCs are further enriched by excluding the contaminating CD48+ cells.
All HSC activity from the Flk2-Sca-1+Lineage-c-kit+ population is contained within the CD48- subset of cells
Donor . | Cell dose . | No. mice that engrafted/no. mice total . | No. mice with long-term multilineage reconstitution/no. mice total . | Frequency of cells that long-term multilineage reconstituted . |
|---|---|---|---|---|
| Young | ||||
| FSLK48– | 10 | 5/5 | 5/5 | CNBC |
| FSLK48+ | 10 | 0/6 | 0/6 | NA |
| Old | ||||
| FSLK48– | 15 | 8/8 | 6/8 | 1 in 11.0 |
| FSLK48+ | 15 | 0/8 | 0/8 | NA |
| Reconstituted | ||||
| FSLK48– | 15 | 5/5 | 2/5 | 1 in 29.9 |
| FSLK48+ | 15 | 0/7 | 0/7 | NA |
| Mobilized | ||||
| FSLK48– | 15 | 3/4 | 2/4 | 1 in 22.1 |
| FSLK48+ | 15 | 0/8 | 0/8 | NA |
Donor . | Cell dose . | No. mice that engrafted/no. mice total . | No. mice with long-term multilineage reconstitution/no. mice total . | Frequency of cells that long-term multilineage reconstituted . |
|---|---|---|---|---|
| Young | ||||
| FSLK48– | 10 | 5/5 | 5/5 | CNBC |
| FSLK48+ | 10 | 0/6 | 0/6 | NA |
| Old | ||||
| FSLK48– | 15 | 8/8 | 6/8 | 1 in 11.0 |
| FSLK48+ | 15 | 0/8 | 0/8 | NA |
| Reconstituted | ||||
| FSLK48– | 15 | 5/5 | 2/5 | 1 in 29.9 |
| FSLK48+ | 15 | 0/7 | 0/7 | NA |
| Mobilized | ||||
| FSLK48– | 15 | 3/4 | 2/4 | 1 in 22.1 |
| FSLK48+ | 15 | 0/8 | 0/8 | NA |
The indicated number of donor-type (CD45.2+) FLK2–Sca-1+Lineage–c-kit+ cells from young adult bone marrow, old adult bone marrow, reconstituted bone marrow, and day-7 cyclophosphanide/G-CSF–mobilized splenocytes were transplanted intravenously into lethally irradiated recipients (CD45.1+) along with recipient-type 200 000 whole bone marrow cells. Recipients were considered engrafted by donor cells if any CD45.2+ cells were detected in their peripheral blood (above background: > 0.3 of myeloid cells or > 0.1-0.15 of lymphoid cells). Mice were considered long-term multilineage reconstituted if donor-type myeloid, B, and T cells were present for at least 16 weeks after reconstitution. The frequency of cells that gave long-term multilineage reconstitution (HSCs) was calculated based on limitdilution Poisson statistics.21 NA indicates not applicable because no HSC activity was detected; CNBC, could not be calculated because all mice were LTMR.
CD48 is heterogeneously expressed by Thy-1lowSca-1+Lineage-c-kit+ cells from young, old, cyclophosphamide/G-CSF-mobilized, and reconstituted mice. Cells were derived from bone marrow in each case except mobilized mice in which they were obtained from the spleen.
CD48 is heterogeneously expressed by Thy-1lowSca-1+Lineage-c-kit+ cells from young, old, cyclophosphamide/G-CSF-mobilized, and reconstituted mice. Cells were derived from bone marrow in each case except mobilized mice in which they were obtained from the spleen.
Selecting CD48- cells improves HSC purity from old, mobilized, and reconstituted mice
To test the extent to which HSCs were enriched by excluding CD48+ cells, we transplanted varying doses of either ThylowSca-1+Lineage-c-kit+ cells or ThylowSca-1+Lineage-c-kit+CD48- cells from old, reconstituted, and mobilized mice into lethally irradiated recipients along with a radioprotective dose of 200 000 recipient-type whole bone marrow cells. In each case, the exclusion of CD48+ cells by adding CD48 to the lineage panel led to an increase in the frequency of HSCs based on limit-dilution analysis.21 The frequency of long-term multilineage-reconstituting HSCs from old donors appeared to increase about 2-fold by excluding CD48+ cells, while the frequency of long-term multilineage-reconstituting HSCs from reconstituted or mobilized donors appeared to increase 3- to 8-fold (Table 3). The precise magnitude of the increases in HSC purity cannot be calculated solely from the experiments in Table 3 because the confidence intervals around the calculated HSC frequencies remain large. Nonetheless, when combined with the observation that most ThylowSca-1+Lineage-c-kit+ cells in old, mobilized, and reconstituted mice are CD48+ (Figure 1), and that these CD48+ cells lack reconstituting potential (Tables 1, 2), the data in Table 3 indicate that HSC purity can be significantly increased in each of these contexts by excluding CD48+ cells.
The CD48- subset of Thy-1lowSca-1+Lineage-c-kit+ (TSLK) cells is further enriched for long-term multilineage-reconstituting HSCs
Donor . | Cell dose . | No. mice that engrafted/no. mice total . | No. mice with long-term multilineage reconstitution/no. mice total . | Frequency of cells that long-term multilineage reconstituted . |
|---|---|---|---|---|
| Old | ||||
| TSLK | 5 | 6/9 | 4/9 | 1 in 9.0 |
| TSLK | 5 | 3/7 | 2/7 | 1 in 15.4 |
| TSLK48– | 5 | 8/9 | 7/9 | 1 in 3.8 |
| Reconstituted | ||||
| TSLK | 10 | 9/11 | 1/11 | 1 in 105 |
| TSLK | 20 | 2/4 | 1/4 | 1 in 70.0 |
| TSLK48– | 5 | 6/9 | 2/9 | 1 in 20.4 |
| Mobilized | ||||
| TSLK | 30 | 8/9 | 3/9 | 1 in 74.5 |
| TSLK48– | 5 | 14/18 | 8/18 | 1 in 9.0 |
Donor . | Cell dose . | No. mice that engrafted/no. mice total . | No. mice with long-term multilineage reconstitution/no. mice total . | Frequency of cells that long-term multilineage reconstituted . |
|---|---|---|---|---|
| Old | ||||
| TSLK | 5 | 6/9 | 4/9 | 1 in 9.0 |
| TSLK | 5 | 3/7 | 2/7 | 1 in 15.4 |
| TSLK48– | 5 | 8/9 | 7/9 | 1 in 3.8 |
| Reconstituted | ||||
| TSLK | 10 | 9/11 | 1/11 | 1 in 105 |
| TSLK | 20 | 2/4 | 1/4 | 1 in 70.0 |
| TSLK48– | 5 | 6/9 | 2/9 | 1 in 20.4 |
| Mobilized | ||||
| TSLK | 30 | 8/9 | 3/9 | 1 in 74.5 |
| TSLK48– | 5 | 14/18 | 8/18 | 1 in 9.0 |
The indicated number of donor-type (CD45.2+) Thy-1lowSca-1+Lineage–c-kit+ cells from old bone marrow, reconstituted bone marrow, or day-7 cyclophosphanide/G-CSF–mobilized splenocytes were transplanted intravenously into lethally irradiated recipients (CD45.1+) along with 200 000 recipient-type whole bone marrow cells. The frequency of cells that gave long-term multilineage reconstitution (HSCs) was calculated based on limit-dilution (Poisson) statistics.21 Mice were considered long-term multilineage reconstituted if donor-type myeloid, B, and T cells were present for at least 16 weeks after reconstitution.
HSCs from old, reconstituted, and mobilized mice are CD150+ and not CD150-
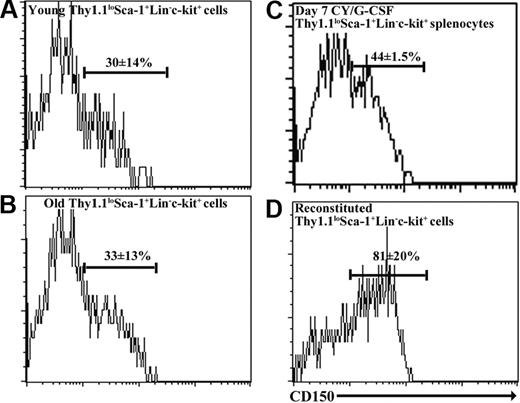
The other SLAM family member that facilitated the purification of young adult HSCs and that was expressed by at least some cytokine-mobilized HSCs was CD150.3 ThylowSca-1+Lineage-c-kit+ cells from old bone marrow, reconstituted bone marrow, and mobilized spleen were heterogeneous for CD150 expression. While 30% ± 14% of young adult ThylowSca-1+Lineage-c-kit+ bone marrow cells expressed CD150 in these experiments, 33% ± 13% of ThylowSca-1+Lineage-c-kit+ bone marrow cells from 26- to 28-month-old mice, 44% ± 2% of ThylowSca-1+Lineage-c-kit+ splenocytes from day-7 cyclophosphamide/G-CSF-treated mice, and 81% ± 20% of ThylowSca-1+Lineage-c-kit+ bone marrow cells from reconstituted mice expressed CD150 (Figure 2). If HSCs are CD150+ and not CD150- in each of these contexts, as was previously observed in young adult bone marrow,3 then CD150 might further enhance the purification of these HSCs.
CD150 is heterogeneously expressed by Thy-1lowSca-1+Lineage-c-kit+ cells from young, old, cyclophosphamide/G-CSF-mobilized, and reconstituted mice. Cells were derived from bone marrow in each case, except mobilized mice in which they were obtained from the spleen.
CD150 is heterogeneously expressed by Thy-1lowSca-1+Lineage-c-kit+ cells from young, old, cyclophosphamide/G-CSF-mobilized, and reconstituted mice. Cells were derived from bone marrow in each case, except mobilized mice in which they were obtained from the spleen.
To test whether HSCs from old and reconstituted mice were also CD150+, and whether all mobilized HSCs were CD150+, we performed competitive reconstitution assays in which CD150+ or CD150- donor cells from each of these contexts were transplanted into lethally irradiated mice along with 200 000 recipient-type whole bone marrow cells. CD150+ cells accounted for about 10% of cells in the bone marrow of old and reconstituted mice, as well as around 10% of splenocytes in cytokine-mobilized mice (data not shown). These frequencies of CD150+ cells were similar to that observed in the bone marrow of young adult mice (7%).3 Irrespective of the source of cells, recipients of CD150+ cells were almost always long-term multilineage reconstituted (7/8 for old donors, 3/4 for reconstituted donors, and 8/9 for mobilized donors), while recipients of CD150- cells were never long-term multilineage reconstituted (0/9 for old donors, 0/5 for reconstituted donors, 0/10 for mobilized donors) (Table 4). Especially in view of the fact that CD150- cells represent around 90% of all cells in old bone marrow, reconstituted bone marrow, and cytokine-mobilized spleen, these data indicate that HSCs are enriched in the CD150+ fraction and depleted in the CD150- fraction in each of these contexts.
All HSC activity is in the CD150+ fraction of old bone marrow, reconstituted bone marrow, and day-7 cyclophosphamide/G-CSF-treated splenocytes
Source of cells . | No. donor-type cells transplanted . | No. mice with long-term multilineage engraftment/no. mice total . |
|---|---|---|
| Old bone marrow | ||
| CD150+ | 20 000 | 7/8 |
| CD150– | 180 000 | 0/9 |
| Mobilized splenocytes | ||
| CD150+ | 20 000 | 8/9 |
| CD150– | 180 000 | 0/10 |
| Reconstituted bone marrow | ||
| CD150+ | 20 000 | 3/4 |
| CD150– | 180 000 | 0/5 |
Source of cells . | No. donor-type cells transplanted . | No. mice with long-term multilineage engraftment/no. mice total . |
|---|---|---|
| Old bone marrow | ||
| CD150+ | 20 000 | 7/8 |
| CD150– | 180 000 | 0/9 |
| Mobilized splenocytes | ||
| CD150+ | 20 000 | 8/9 |
| CD150– | 180 000 | 0/10 |
| Reconstituted bone marrow | ||
| CD150+ | 20 000 | 3/4 |
| CD150– | 180 000 | 0/5 |
The indicated number of donor-type (CD45.1+) cells was transplanted intravenously into lethally irradiated recipients (CD45.2+) along with 200 000 recipient-type (CD45.2+) whole bone marrow cells. The dose of CD150+ or CD150– donor cells was based on the number of cells from each population contained in 200 000 old bone marrow, mobilized spleen, or reconstituted bone marrow cells as done in previous studies of marker expression on HSCs.3,10,13,19 Mice were considered long-term multilineage reconstituted if donor-type myeloid, B, and T cells were present for at least 16 weeks after transplantation.
SLAM family members improve the purity of HSCs from different contexts
These results suggest that by further selecting the CD150+CD48- subset of Sca-1+Lineage-c-kit+ cells that it might be possible to significantly increase the purity of HSCs isolated from old, reconstituted, and mobilized mice. To test this possibility, we transplanted 3 CD150+CD48-Sca-1+Lineage-c-kit+ cells from old bone marrow, reconstituted bone marrow, or day-7 cyclophosphamide/G-CSF-mobilized spleen into lethally irradiated mice along with a radioprotective dose of recipient-type bone marrow cells. This donor population engrafted and gave long-term multilineage reconstitution much more efficiently than observed from ThylowSca-1+Lineage-c-kit+ cells (Table 3). Previously, we reported that only around 1 in every 150 ThylowSca-1+Lineage-c-kit+ cells from day-7 cyclophosphamide/G-CSF-mobilized spleen engrafted and gave long-term multilineage reconstitution after intravenous transplantation into irradiated mice.13 In this study, we found that 1 in 74.5 ThylowSca-1+Lineage-c-kit+ cells from mobilized spleen engrafted and gave long-term multilineage reconstitution (Table 3). In contrast, 1 in 9.0 CD48-Thy-1lowSca-1+Lineage-c-kit+ cells (Table 3) or 1 in 3.6 CD150+CD48-Sca-1+Lineage-c-kit+ cells from mobilized spleen engrafted and gave long-term multilineage reconstitution (Table 5), suggesting a dramatic increase in HSC purity.
CD150+CD48-Sca-1+Lineage-c-kit+ cells from old, reconstituted, and cyclophosphamide/G-CSF-mobilized mice are highly enriched for long-term self-renewing, multipotent HSCs
Donor/experiment . | No. mice that engrafted/no. mice total . | No. mice with long-term multilineage reconstitution/no. mice total . | Frequency of cells that long-term multilineage reconstituted . |
|---|---|---|---|
| Young | |||
| 1 | 8/8 | 7/8 | 1 in 2.0 |
| Old | |||
| 1 | 4/7 | 3/7 | 1 in 5.9 |
| 2 | 6/12 | 5/12 | 1 in 6.1 |
| 3 | 5/9 | 3/9 | 1 in 7.9 |
| 4 | 4/9 | 3/9 | 1 in 7.9 |
| Reconstituted | |||
| 1 | 3/6 | 3/6 | 1 in 4.8 |
| 2 | 12/13 | 10/13 | 1 in 2.6 |
| 3 | 7/9 | 3/9 | 1 in 7.9 |
| Mobilized | |||
| 1 | 5/8 | 5/8 | 1 in 3.6 |
Donor/experiment . | No. mice that engrafted/no. mice total . | No. mice with long-term multilineage reconstitution/no. mice total . | Frequency of cells that long-term multilineage reconstituted . |
|---|---|---|---|
| Young | |||
| 1 | 8/8 | 7/8 | 1 in 2.0 |
| Old | |||
| 1 | 4/7 | 3/7 | 1 in 5.9 |
| 2 | 6/12 | 5/12 | 1 in 6.1 |
| 3 | 5/9 | 3/9 | 1 in 7.9 |
| 4 | 4/9 | 3/9 | 1 in 7.9 |
| Reconstituted | |||
| 1 | 3/6 | 3/6 | 1 in 4.8 |
| 2 | 12/13 | 10/13 | 1 in 2.6 |
| 3 | 7/9 | 3/9 | 1 in 7.9 |
| Mobilized | |||
| 1 | 5/8 | 5/8 | 1 in 3.6 |
Three (CD45.1+) CD50+CD48–Sca-1+lin–c-kit+ cells from old, reconstituted, or day-7 cyclophosphamide/G-CSF–mobilized splenocytes were transplanted intravenously into lethally irradiated recipients (CD45.2+) along with 300 000 CD150– recipient-type cells or 200 000 whole bone marrow cells for radioprotection. There was no apparent difference in the frequency of donor cells that long-term multilineage reconstituted based on the nature of cells used for radioprotection. Recipients were considered long-term multilineage reconstituted if donor-type myeloid, B, and T cells were present for at least 16 weeks after transplantation.
Previously, we reported that only 1 of every 185 ThylowSca-1+Lineage-c-kit+ cells from reconstituted mice gave long-term multilineage reconstitution after intravenous transplantation into irradiated mice.12 In this study, we found that 1 in 70.0 to 1 in 105 ThylowSca-1+Lineage-c-kit+ cells from reconstituted bone marrow engrafted and gave long-term multilineage reconstitution (Table 3). In contrast, 1 in 20.4 CD48-Thy-1lowSca-1+Lineage-c-kit+ cells (Table 3) or an average of 1 in 5.1 CD150+CD48-Sca-1+Lineage-c-kit+ cells from reconstituted bone marrow engrafted and gave long-term multilineage reconstitution in several independent experiments (Table 5). This again suggests a dramatic increase in HSC purity in this population from reconstituted bone marrow.
Previously, we found that only 1 of every 78 intravenously injected ThylowSca-1+Lineage-c-kit+ cells from old mice was able to engraft and give long-term multilineage reconstitution.11 By gating more stringently on Lineage markers in this experiment, we found that an average of 1 in 12.2 ThylowSca-1+Lineage-c-kit+ cells from old mice engrafted and gave long-term multilineage reconstitution (Table 3). On average, 1 in 6.9 ± 1.1 CD150+ CD48-Sca-1+Lineage-c-kit+ cells from old bone marrow engrafted and gave long-term multilineage reconstitution (Table 5). While the reconstitution assays using old ThylowSca-1+Lineage-c-kit+ cells were not repeated enough to determine whether this difference is statistically significant, the data demonstrating the presence of contaminating CD48+ and CD150- cells within the old ThylowSca-1+Lineage-c-kit+ population suggest that the selection of CD150+CD48- cells does contribute to increased purity in this population.
Discussion
Although a number of recent studies have identified new markers or strategies for improving HSC purification from young adult bone marrow,4,5,19,22-24 little attention has been paid to other contexts in which HSCs must be studied. This is despite the fact that standard HSC markers, such as ThylowSca-1+Lineage-c-kit+, do a much poorer job of enriching long-term multilineage-reconstituting cells from old mice, reconstituted mice, or cytokine-mobilized mice.11-16 It is possible that newly identified HSC markers might enhance the enrichment of old, reconstituted, or mobilized HSCs. However, the fact that HSC markers change their expression under a variety of circumstances, including after 5-fluorouracil treatment,25 at different stages of development,10,26,27 and after cytokine mobilization,16 makes it necessary to confirm that HSC markers identified in one circumstance are also expressed under other circumstances.
In this study, we have demonstrated that SLAM family receptors exhibit similar expression patterns on HSCs isolated from old, reconstituted, and mobilized mice compared with their expression on HSCs from young adult bone marrow. We recently showed that SLAM family receptors including CD150, CD48, and CD244 are differentially expressed among stem and progenitor cells at different stages of the hematopoiesis hierarchy in a way that correlates with primitiveness.3 The reconstituting potential of primitive hematopoietic progenitors can be predicted based upon the combination of SLAM family members they express (SLAM codes). CD150 is expressed by HSCs in young adult bone marrow, while CD244 is expressed by non-self-renewing multipotent progenitors, and CD48 is expressed by restricted progenitors. These surface receptors are so precisely differentially expressed that HSCs can be isolated from young adult bone marrow as CD150+CD48- cells, with a similar degree of purity as in the ThylowSca-1+Lineage-c-kit+ population. Our observation that HSCs from old, reconstituted, and mobilized mice are CD150+CD48-, just like HSCs from young adult bone marrow, further emphasizes the robustness of these HSC markers.
A recent study reported that CD48 was up-regulated on enriched HSCs isolated from 5-fluorouracil-treated bone marrow cells17 and proposed that CD48 is part of the molecular signature of activated HSCs. However, the reconstituting potential of CD48+ cells was not tested in this study, and the functional purity of the cell populations used for gene expression profiling was not indicated. We observed that long-term multilineage-reconstituting cells from young adult bone marrow, old adult bone marrow, reconstituted bone marrow, and mobilized spleen (a context in which HSCs are activated13 ) are always CD48- despite the consistent presence of contaminating CD48+ cells that lack multilineage-reconstituting activity. Indeed, we previously found that CD48 was preferentially expressed by restricted colony-forming progenitors but not HSCs or transiently reconstituting multipotent progenitors in young adult bone marrow.3 This suggests that the CD48+ cells from 5-fluorouracil-treated bone marrow may also be non-HSC-contaminating cells, though the reconstituting potential of these cells must be tested. These observations emphasize the importance of functionally confirming markers identified by gene expression profiling to ensure that they do not reflect changes in contaminating cells.
The use of SLAM family markers has improved the purity with which HSCs can be isolated from old, reconstituted, or mobilized mice. ThylowSca-1+Lineage-c-kit+ cells from each of these contexts were heterogeneous for their expression of CD48 (Figure 1) and CD150 (Figure 2), with most cells in these populations being CD48+ and/or CD150-. Yet, these CD48+ cells or CD150- cells had no long-term multilineage-reconstituting activity upon transplantation into irradiated mice (Tables 1, 2, 3, 4). Indeed, the CD48+ cells from within the Flk2-Sca-1+Lineage-c-kit+ population had no detectable reconstituting activity of any type (Table 2). This demonstrates that the ThylowSca-1+Lineage-c-kit+ population from old, reconstituted, and cytokine-mobilized mice contains mainly contaminating cells that lack HSC activity. When these cells were eliminated by selecting the CD150+CD48- subset of Sca-1+Lineage-c-kit+ cells, the frequency of cells that gave long-term multilineage reconstitution increased, sometimes dramatically.
The dramatic increase in purity afforded by the SLAM family markers should greatly increase the precision with which HSCs can be studied in old, reconstituted, and mobilized mice. The low rate of reconstitution from ThylowSca-1+Lineage-c-kit+ cells isolated from these contexts had previously raised the possibility that these cells were grossly defective in their ability to home to bone marrow and engraft following intravenous transplantation into irradiated mice (4- to 20-fold less than young adult cells).11-16 The increased reconstituting efficiency that was obtained with the addition of SLAM family markers rules out the possibility of dramatic (> 4-fold) homing defects in these cells.
Nonetheless, the data in this paper remain consistent with the possibility of smaller engraftment defects. CD150+CD48-Sca-1+Lineage-c-kit+ cells from young mice gave long-term multilineage reconstitution approximately 3-fold more efficiently than CD150+CD48-Sca-1+Lineage-c-kit+ cells from old or reconstituted mice (Table 5). This is consistent with the engraftment defect that was previously inferred based on reconstitution experiments with enriched HSCs from old mice.11 These data are also consistent with the observed 3-fold reduction in the engraftment efficiency of old compared with young competitive repopulating units.14 While our data leave open the possibility that CD150+CD48-Sca-1+Lineage-c-kit+ cells from old, reconstituted, and mobilized mice may still contain more contaminating non-HSCs than the same population from young mice, the functional analysis of engraftment efficiency performed by Liang et al14 strongly suggests that purified HSCs from old mice exhibit a somewhat attenuated ability to engraft after transplantation. If so, the CD150+CD48-Sca-1+Lineage-c-kit+ population from old mice may contain a similar degree of HSC purity as observed in the same population from normal young adult mice.3
We and others had reported previously that HSCs from old mice and cyclophosphamide/G-CSF-mobilized mice are increased in frequency and mitotic activity relative to young adult bone marrow HSCs.11,13,28,29 Examination of the more highly purified HSC populations using SLAM family markers continues to support these conclusions. For example, we find that even using the more highly purified HSCs described in this paper, we continue to observe a 3-fold increase in HSC frequency and a 3-fold increase in the percentage of HSCs in S/G2/M phases of the cell cycle in C57BL mice older than 22 months of age (data not shown).
The use of SLAM family markers to enhance the purification of HSCs from diverse contexts should continue to refine our understanding of the biology of these cells by increasing the purity with which the HSCs can be isolated.
Prepublished online as Blood First Edition Paper, October 11, 2005; DOI 10.1182/blood-2005-05-2140.
Supported by the Howard Hughes Medical Institute and the US Army Research Laboratory/Office under grant no. DAAD19-03-1-0168. Flow cytometry was partially supported by the UM-Comprehensive Cancer NIH CA46592, and the UM-Multipurpose Arthritis Center NIH AR20557. Antibody production was partially supported by the Rheumatic Core Disease Center (1 P30 AR48310). Ö.H.Y. was supported by a predoctoral fellowship from the University of Michigan (UM) Institute of Gerontology and M.J.K. was supported by a Medical Scientist Training Program Fellowship.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal