Hematopoiesis is maintained by specific interactions between both hematopoietic and nonhematopoietic cells. Whereas hematopoietic stem cells (HSCs) have been extensively studied both in vitro and in vivo, little is known about the in vivo characteristics of stem cells of the nonhematopoietic component, known as mesenchymal stem cells (MSCs). Here we have visualized and characterized human MSCs in vivo following intramedullary transplantation of enhanced green fluorescent protein-marked human MSCs (eGFP-MSCs) into the bone marrow (BM) of nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice. Between 4 to 10 weeks after transplantation, eGFP-MSCs that engrafted in murine BM integrated into the hematopoietic microenvironment (HME) of the host mouse. They differentiated into pericytes, myofibroblasts, BM stromal cells, osteocytes in bone, bone-lining osteoblasts, and endothelial cells, which constituted the functional components of the BM HME. The presence of human MSCs in murine BM resulted in an increase in functionally and phenotypically primitive human hematopoietic cells. Human MSC-derived cells that reconstituted the HME appeared to contribute to the maintenance of human hematopoiesis by actively interacting with primitive human hematopoietic cells.
Introduction
Mesenchymal stem cells (MSCs) present in bone marrow (BM) are thought to give rise to cells that constitute the hematopoietic microenvironment (HME).1 MSCs have been isolated from BM and various tissues from humans and many other species, expanded in culture, and shown to differentiate into osteocytes, chondrocytes, adipocytes, and myoblasts under defined conditions in vitro.2 In culture, MSCs produce a number of cytokines and extracellular matrix proteins and express cell adhesion molecules, all of which are involved in the regulation of hematopoiesis.3,4 They also support the development of hematopoietic colonies in vitro.4 However, in contrast to hematopoietic stem cells (HSCs) that have been prospectively isolated and extensively studied at the single-cell level both in vitro and in vivo, MSCs have only been defined and isolated by physical and functional properties in vitro. Consequently, little is known about their phenotypic and functional characteristics in vivo.
Systemic administration of MSCs for facilitation of bone marrow transplantation has been proposed based on the in vitro characteristics of MSCs.5 In recent studies, cotransplantation of human MSCs and HSCs resulted in increased chimerism or accelerated hematopoietic recovery (or both) in animal models and in humans,6-9 suggesting a role for MSCs in the engraftment and repopulation of HSCs. Although the existence of donor MSCs has been documented in the BM of recipient animals following MSC infusion,9,10 the methods used to detect engraftment, such as polymerase chain reaction (PCR) or staining of cytospin samples, could not unambiguously distinguish engraftment from cell survival or nonspecific lodgment on the vascular bed. In addition, Awaya et al examined stromal cells of patients who received BM transplants and confirmed that all donor signals were, in fact, derived from macrophages.11 To our knowledge, there is no physical evidence that transplanted human MSCs have indeed engrafted in the BM of adult animals and directly participated in the enhanced engraftment of HSCs.
To assess the engraftment, spatial distribution, and lineage commitment of MSCs as well as their roles in hematopoiesis in vivo, we transplanted enhanced green fluorescent protein (eGFP)-marked human MSCs into the tibiae of nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice by intra-bone marrow transplantation (IBMT), a method previously shown to improve the engraftment of both hematopoietic and nonhematopoietic cells in mice.12-14 We used a dual-color genetic marking strategy15 along with immunofluorescent staining to distinguish and investigate transplanted cells in situ. We show that transplanted human MSCs integrated into the functional components of the HME and that these MSC-derived cells appeared to be actively involved in the maintenance of human hematopoiesis in murine BM.
Materials and methods
Isolation of human cord blood CD34+ cells
Human umbilical cord blood (CB) samples were obtained from full-term deliveries with informed consent of the mother and used in accordance with the institutional guidelines approved by the Tokai University Committee on Clinical Investigation. CD34+ cells were selected using the CD34 Progenitor Cell Isolation Kit (Miltenyi Biotec, Sunnyvale, CA) according to the manufacturer's instructions as described previously.12 The purity of selected cord blood CD34+ (CBCD34) cells was always greater than 95%, and they were cryopreserved in liquid nitrogen until use. In some experiments, CBCD34 cells were further fractionated into CD34+CD38+ and CD34+CD38- populations at the day of transplantation.
Human MSCs
Human MSCs were purchased from Cambrex BioScience Walkersville (Walkersville, MD) and cultured according to the directions supplied by the company. The ability to differentiate into adipocytes, chondrocytes, and osteoblasts was confirmed in vitro before they were used for the experiments.2
Antibodies
The following antibodies were used for tissue immunostaining: anti-CD15 (80H5, 1:75; Coulter/Immunotech, Marseille, France), anti-CD31 (1:100; TECNE, Minneapolis, MN), anti-CD34 (My10, 1:20; BD Biosciences, San Jose, CA), anti-CD45 (2D1, 1:75; BD Biosciences), anti-glycophorin A (JC159, 1:400; Dako, Glostrup, Denmark), anti-N-cadherin (1:20; IBL, Gunma, Japan), antiosteocalcin (1:25; Biogenesis, Poole, United Kingdom), anti-smooth muscle (SM) actin (1A4, 1:800; Sigma-Aldrich, St Louis, MO), anti-alkaline phosphatase (B4-78, 1:30; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), antivimentin (1:400; Progen Biotechnik, Heidelberg, Germany), anti-GFP (1:500; MBL, Nagoya, Japan), antiosteopontin (10A16, 1:100; IBL), and anti-SDF-1 (1:200, Santa Cruz Biotechnology, Santa Cruz, CA). The following monoclonal antibodies (mAbs) were used for flow cytometry: fluorescein isothiocyanate-conjugated anti-CD19 (SJ25C1; BD Biosciences), phycoerythrin-conjugated anti-CD33 (WM53) and anti-CD34 (581), and allophycocyanin-conjugated anti-CD45 (J.33; all from Coulter/Immunotech).
Experimental animals, lentiviral gene transduction, and cell transplantation
Eight- to 10-week old male NOD/Shi-scid (NOD/SCID) mice were purchased from Clea Japan (Tokyo, Japan) and housed in sterile microisolator cages in the animal facility of Tokai University School of Medicine. Mice were given autoclaved food and water. Twenty-four hours before transplantation, mice were irradiated with 300 cGy from an x-ray irradiator (HW-300, Hitex, Osaka, Japan) and thereafter fed acidified water. All procedures were approved by the Animal Care Committee of Tokai University. Prior to transplantation into mice, MSCs and CBCD34 were genetically marked with eGFP or its yellow variant enhanced yellow fluorescent protein (eYFP). Transduction of CBCD34 and MSCs was carried out as described previously.15,16 For in situ examination of transplanted cells, 1 × 106 eGFP-marked MSCs and 2 × 105 eYFP-marked CBCD34, 2 to 4 × 105 CD34+CD38+ cells, or 1.5 to 4 × 104 CD34+CD38- cells were suspended in 10 μL PBS and transplanted directly into the right tibia of NOD/SCID mice using a Hamilton syringe equipped with a 31-gauge needle.12 In some experiments, gene-marked MSCs or CBCD34 cells were separately transplanted by IBMT or by the intravenous route.
Analysis of human cell homing
Staining of CBCD34 cells with PKH26 dye (Sigma-Aldrich) and analysis of cells that homed into BM were conducted as described previously.12 Each mouse received 1 × 106 MSCs into the right tibia and 10 μL PBS into the left tibia by IBMT, followed by administration of 1 × 106 PKH26-labeled CBCD34 cells into the retro-orbital plexus. Twenty hours after transplantation, mice were humanely killed, and BM cells were collected separately from the tibiae that had been injected with MSC and the PBS.
Analysis of human cell engraftment
A total of 1 × 106 MSCs was injected into the right tibiae of irradiated NOD/SCID mice, and then 5 × 104 CBCD34 cells were injected into the retro-orbital plexus of the mice. Control groups received the same amount of PBS in the right tibia. At 6 weeks after transplantation, mice were humanely killed, and BM cells were collected separately from each tibia. Aliquots of cells were used to examine the percentages of CD45-, CD19-, CD33-, and CD34-expressing cells in the respective tibia. Two-color flow cytometric analysis was conducted using FACSCalibur. Quadrants were set to include at least 97% of the isotype-negative cells. The proportion of each lineage was calculated from 20 000 events acquired using CellQuest software (Becton Dickinson, San Jose, CA). Remaining cells were saved for the clonogenic cell assay and secondary transplantation.
Clonogenic cell assay
Human hematopoietic cells were isolated from BM cells of mice given transplants using CD45 MicroBeads (Miltenyi Biotec) according to the manufacturer's protocol. The purity of selected cells was 36% to 96% (mean, 73%). CD45-enriched populations containing 10 000 CD45+ cells were plated in MethoCult GFH4434V (StemCell Technologies, Vancouver, BC, Canada). The number and morphology of colonies formed during the 14-day culture period were determined under inverted microscope. Morphologic designation of colony type by light microscopy was confirmed by Wright-Giemsa staining of cytospin preparations. The specificity of the assay was confirmed by PCR on individual colonies using primers specific for the human chromosome 17-α satellite sequence12 and the expression of CD45 by flow cytometric analysis.
Secondary transplantation
BM cells obtained separately from each tibia of primary mice were intravenously transplanted into irradiated secondary recipient mice. Because the number of cells recovered from one tibia was small (2.4-3.5 × 106/tibia), we used NOD/SCID IL-2Rγnull mice, which have been shown to be a better recipient of human cells than NOD/SCID mice,17,18 as secondary recipients. Six weeks after transplantation, BM cells were obtained from tibiae and femurs of each secondary recipient, and the presence of human cells was analyzed by flow cytometry.
Tissue processing and immunofluorescent staining
Anesthetized mice were perfused with 4% paraformaldehyde in PBS. The tibiae were excised, decalcified, infiltrated with sucrose, embedded in OCT compound (Sakura, Tokyo, Japan), and frozen in liquid nitrogen. Frozen sections of decalcified bone were obtained using a cryostat microtome (CM3050, Leica, Germany) and stored at -80°C until staining. Immunofluorescent staining and enzyme immunohistochemistry were performed as described previously.16 For immunofluorescent analysis, slides were examined and images were captured using an LSM510 META confocal microscope and a 63 ×/1.2 numeric aperture (NA) c-Apochromat objective lens (Carl Zeiss, Jena, Germany). Images were transferred to Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA).
Quantitative microscopic examination
Spatial distribution of human hematopoietic cells. The location of cells was designated as either endosteal (within 12 cells of the endosteum) or central (>12 cells of the endosteum) as described previously.19 To accurately assess the proportion of endosteal area, the diameter of the diaphyseal shaft was evaluated by counting the number of cells from one side of the endosteum to the other along the line perpendicular to the longitudinal axis of the bone. CD34-, CD15-, and glycophorin A-reactive human hematopoietic cells present in the diaphyseal shaft were counted under light microscopy with assistance of a Zeiss KS400.
Interaction between human MSCs and human HSCs. Entire fields of longitudinal sections cut through the center of bones were examined for cell counting. All eGFP-expressing cells and immunophenotyped human hematopoietic cells present in BM were counted under fluorescent microscopy. When cells were in physical contact, they were categorized as interacting.
Statistical analysis
All values are presented as the mean plus or minus standard deviation (SD). The 2-sided P value was determined, testing the null hypothesis that the 2 population medians are equal. P below .05 was considered significant.
Results
Spatial distribution of human hematopoietic cells in the murine BM compartment
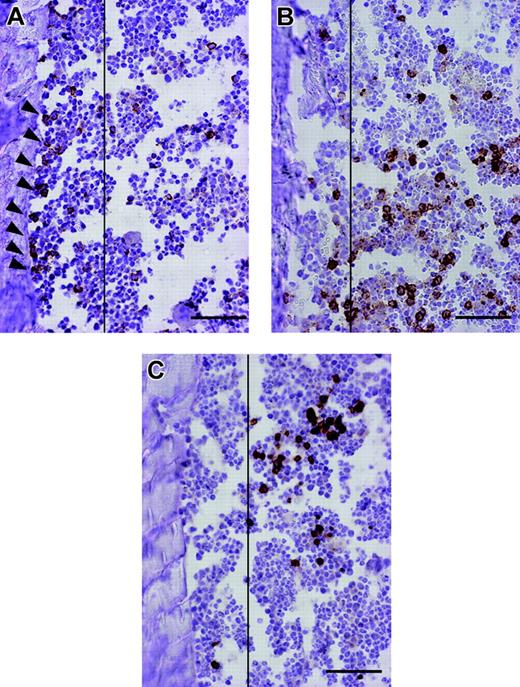
The potential of human HSCs in vivo can be assessed by using the SCID-repopulating cell (SRC) assay based on the ability to reconstitute hematopoiesis in the host following transplantation into NOD/SCID mice.20 However, analysis of SRCs by flow cytometry does not allow identification of individual SRCs in situ. Consequently, the behavior of transplanted SRCs during repopulation has not been elucidated. To address this issue, we prepared mice that were highly chimeric with human hematopoietic cells by directly injecting CBCD34 cells into the tibia12 and investigated the spatial organization of individual engrafted cells in murine BM at 10 weeks after transplantation. Close examination of the bone specimens revealed that cells stained positive for human CD34, a marker of human HSCs and progenitor cells, were specifically localized to an area near the endosteum of the bone (the endosteal region) (Figure 1A). In contrast, cells positively stained for human CD15, a marker of committed myelocytes, or glycophorin A (GlyA), a marker of the erythrocyte lineage, were distributed distant to the endosteum (Figure 1B-C). These results reflect previous morphologic studies of BM that found that hematopoietic stem and progenitor cells are concentrated in the endosteal region rather than the central marrow area, which mostly is composed of mature cells.19,21,22 To quantitatively confirm these observations, the numbers of CD34+, CD15+, and GlyA+ cells present in the endosteal region or in the central BM cavity of the diaphyseal shaft were counted. Consistent with the microscopic observations, a surprisingly higher proportion of CD34+ cells was located in the endosteal region compared to the lineage-committed cells (Table 1). Because the endosteal region consisted of only 21.4% ± 3.8% of the BM cavity examined for this assay, there were approximately 10-fold more CD34+ cells in the endosteal area. By visualizing the fate of transplanted SRCs in situ and confirming their physiologic organization in BM, these results validate the SRC assay for human cell analysis. Hence, we used this xenogeneic transplantation system for the phenotypic and functional characterization of human MSCs in vivo.
Spatial distribution of human hematopoietic cells in the murine BM compartment at 10 weeks after IBMT of CBCD34
. | Total no. cells counted . | Total no. cells in the endosteal region . | Cells in the endosteal region/slide, % . |
|---|---|---|---|
| CD34 | 1469 | 1086 | 71.4 ± 12.5* |
| CD15 | 2940 | 451 | 14.9 ± 6.2 |
| GlyA | 1514 | 171 | 10.7 ± 2.2 |
. | Total no. cells counted . | Total no. cells in the endosteal region . | Cells in the endosteal region/slide, % . |
|---|---|---|---|
| CD34 | 1469 | 1086 | 71.4 ± 12.5* |
| CD15 | 2940 | 451 | 14.9 ± 6.2 |
| GlyA | 1514 | 171 | 10.7 ± 2.2 |
Fourteen slides from at least 8 different mice were examined to count each cell type. Slides containing 115 ± 19 cells in the diaphyseal shaft were chosen for this analysis. Because the endosteal region was arbitrarily decided within 12 cells of both endosteum, the endosteal area comprised approximately 21.4% ± 3.8% of the BM cavity in this study. The proportion of cells located in the endosteal area was calculated for each slide and expressed as the means ± SD.
P < .001 relative to the CD15 and GlyA groups
Visualization of human MSCs and human CBCD34 engraftment in the murine BM compartment
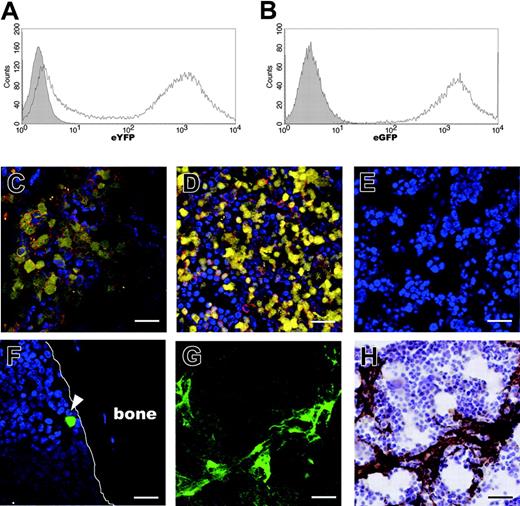
The route of administration is an important factor for effective delivery of transplanted cells into the target tissue. We compared the degree of cell engraftment in BM at 4 and 10 weeks after IBMT or intravenous transplantation of eYFP-marked CBCD34 cells (eYFP-CBCD34; Figure 2A) or eGFP-marked MSCs (eGFP-MSCs; Figure 2B). When excess numbers of eYFP-CBCD34 cells (2 × 105) were transplanted intravenously or by IBMT, similar amounts of eYFP+ human hematopoietic cells (eYFP-cells) that expressed human CD45, a pan-leukocyte marker, were observed in BM at both time points examined (Figure 2C-D and data not shown). In marked contrast to the sufficient engraftment of human hematopoietic cells, intravenous administration of 1 × 106 eGFP-MSCs resulted in virtually no visible MSCs in BM at either 4 (Figure 2F) or 10 weeks after transplantation. At 10 weeks after transplantation, analysis of 250 bone sections prepared from 5 mice yielded a total of only 10 eGFP-MSCs in BM. However, when the same number of MSCs was administered by IBMT, eGFP-MSCs were clearly identified in BM at the both time points. At 4 weeks after transplantation, eGFP-MSCs were broadly distributed in BM (Figure 2G-H). At 10 weeks after transplantation, eGFP-MSCs were detected in all sections examined (> 1000 sections), although the number of eGFP-MSCs per section varied considerably (range, 1-57; median, 13; mean, 14.8; from 91 sections) depending on the plane of sectioning. Interestingly, unlike eYFP-cells, which migrated into the contralateral tibia,12 no eGFP-MSCs were detected in the BM of the tibia that had not received injections (data not shown), suggesting no or minimal migration of transplanted MSCs in this system.
Spatial distribution of human hematopoietic cells in the murine BM compartment. Representative pictures of bone specimens from NOD/SCID mice at 10 weeks after IBMT of 2 × 105 CBCD34 cells are shown. Slides were stained with either anti-CD34, anti-CD15, or anti-glycophorin A antibody, and cells expressing the respective antigens were distinguished by brown reactive products of DAB on immunohistochemistry. CD34+ cells are localized to the endosteal region (A; black arrowheads), whereas CD15+ (B) and GlyA+ cells (C) are distributed away from the endosteum. Vertical lines demarcate 12 cells of the endosteum. Bars represent 100 μm. Images were obtained using an Olympus AX80 microscope and a 20 ×/0.7 NA UPlanApo objective lens. Images were captured using a DP50 digital camera fitted with Viewfinder Lite (all from Olympus, Tokyo, Japan).
Spatial distribution of human hematopoietic cells in the murine BM compartment. Representative pictures of bone specimens from NOD/SCID mice at 10 weeks after IBMT of 2 × 105 CBCD34 cells are shown. Slides were stained with either anti-CD34, anti-CD15, or anti-glycophorin A antibody, and cells expressing the respective antigens were distinguished by brown reactive products of DAB on immunohistochemistry. CD34+ cells are localized to the endosteal region (A; black arrowheads), whereas CD15+ (B) and GlyA+ cells (C) are distributed away from the endosteum. Vertical lines demarcate 12 cells of the endosteum. Bars represent 100 μm. Images were obtained using an Olympus AX80 microscope and a 20 ×/0.7 NA UPlanApo objective lens. Images were captured using a DP50 digital camera fitted with Viewfinder Lite (all from Olympus, Tokyo, Japan).
Integration of human MSCs into the murine microenvironment
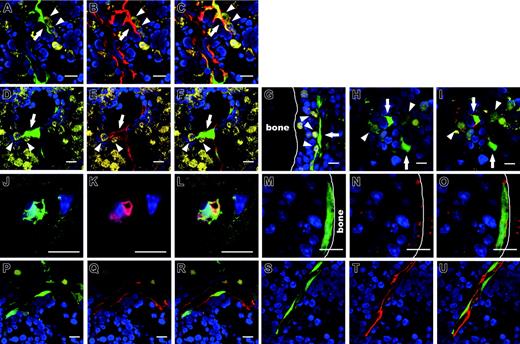
Having confirmed the effectiveness of delivering MSCs into BM, IBMT was used for all subsequent experiments. The phenotypes of transplanted MSCs and their progeny at 10 weeks after transplantation were investigated in detail. In contrast to the bone specimens prepared at 4 weeks after transplantation, in which eGFP-MSCs were located throughout the marrow cavity (Figure 2G-H), eGFP-MSCs at 10 weeks after transplantation were preferentially localized to the endosteal region (73.8% ± 18.4%, n = 251 eGFP-MSCs), frequently within 5 cells from the surface of the bone. When human MSC-derived eGFP-expressing cells (eGFP-cells) were found away from the endosteum, they were often associated with the vasculature (Figure 3A,D). Those vasculature-associated eGFP-cells expressed α-SM actin (Figure 3B-C,E-F), the expression of which has been documented in pericytes, SM cells of the vascular wall as well as myofibroblasts in BM.23,24 A total of 59.9% ± 21.6% of eGFP-cells (n =251) found in BM were positive for α-SM actin.
Two other types of eGFP-cells that were negative for α-SM actin were also present in BM: flattened cells located in the hematopoietic cords but not specifically associated with the vasculature (Figure 3G) and cells characterized by long cytoplasmic extensions, so-called reticular cells (Figure 3H) that are considered to be the predominant cells of the HME.25,26 BM was often interspersed with a fine network of cell processes that expressed alkaline phosphatase (ALP; Figure 3I), an enzyme that distinguishes reticular cells from the stromal component of acid phosphatase- or nonspecific esterase-expressing macrophages.11,25 A total of 28.2% ± 11.2% of eGFP-cells (n = 242) in BM were ALP+.
In addition, eGFP-cells were found within or on the surface of the bone (Figure 3J-O). Cells in the bone stained positive for osteocalcin, a specific marker of mature osteoblasts and osteocytes (Figure 3K-L), indicating an active participation in skeletal remodeling.27,28 Interestingly, eGFP-cells on the bone surface resembled spindle-shaped osteoblasts (Figure 3M; see also Figure 7), a key component of the stem cell niche in murine hematopoiesis.29-32 These cells expressed osteopontin (data not shown) and N-cadherin (Figure 3M-O), both of which are involved in regulating murine HSCs.29,33,34 In a small number of mice examined (4 of 39), eGFP-cells associated with the vasculature stained positive for CD31 (Figure 3P-R) and CD34 (Figure 3S-U), markers of endothelial cells. MSCs expanded ex vivo prior to transplantation did not react with any of these markers either by the immunohistochemical or flow cytometric method, but they did stain positive for vimentin, a marker of fibroblasts (data not shown). These results indicate that within 10 weeks after transplantation, human MSCs differentiated into pericytes, myofibroblasts, reticular cells, osteocytes in bone, bone-lining osteoblasts, and endothelial cells, which constitute the 3-dimensional structure of hematopoietic parenchyma and provide the milieu of hematopoiesis.26 Therefore, we designated these cells as human MSC-derived hematopoietic microenvironment reconstituting cells (HMRCs).
Engraftment of human hematopoietic cells
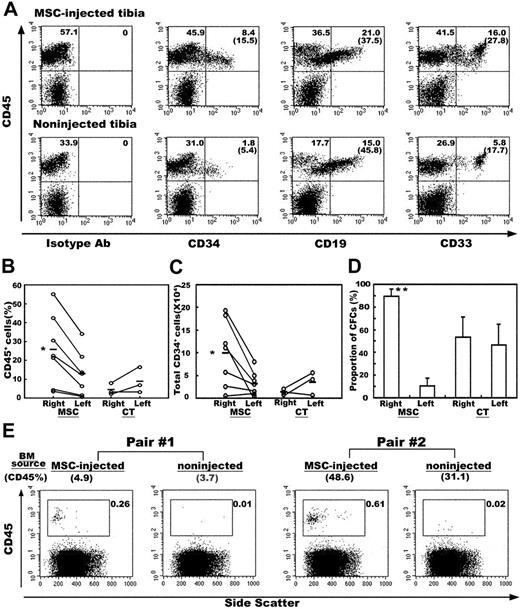
During analysis of the engraftment and differentiation of transplanted MSCs, we frequently observed physical contact between HMRCs and hematopoietic eYFP-cells (Figure 3A-I). In particular, eYFP-cells were intimately associated with ALP+ eGFP-reticular cells, where eYFP-cells were almost surrounded by the thin delicate cytoplasm of the eGFP-reticular cells (Figure 3H-I). To address whether the presence of HMRCs in the murine HME contributes to the development of human hematopoietic cells, we analyzed the engraftment and differentiation of CBCD34 cells after transplantation with or without human MSCs. MSCs were injected into the right tibiae of irradiated NOD/SCID mice by IBMT, and then CBCD34 cells were administered intravenously into each mouse. Consistent with our histologic findings, more human CD45+ cells were found in the tibiae into which MSCs had been injected than the tibiae that had not received injections. In addition, the presence of MSCs increased the percentage of CD34+ cells without affecting the proportion of CD19+ B lymphocytes or CD33+ myeloid cells (Figure 4A). There were 2-fold more human CD45+ cells in the tibiae into which MSCs had been injected compared with the tibiae that had not received injections (25.7% ± 17.6% versus 12.8% ± 11.1%, P = .002, Figure 4B), and the absolute number of CD34+ cells present in the tibiae into which MSCs had been injected was 3-fold higher than that in the tibiae that had not received injections (99 296 ± 68 189 versus 31 525 ± 25 224; P = .007, Figure 4C). Control experiments, in which the same volume of PBS was injected into the right tibia, were conducted to exclude the possibility that this effect was due to injury from the injection. There were no significant differences in either the percentage of CD45+ cells or the number of CD34+ cells between the tibiae into which PBS had been injected and the contralateral tibiae (Figure 4B-C). We then examined the colony-forming ability of BM cells recovered from the mice that had received injections of MSCs and control mice given PBS. The cumulative numbers of colony-forming cells (CFCs) recovered from both tibiae of experimental animals were 2663 for the group that had received injections of MSCs and 1578 for the control group (n = 3 for each group). The proportion of colony types formed from BM cells obtained from both groups was similar (data not shown). Because the numbers of CFCs recovered from each experimental animal varied considerably, we compared the proportion of CFCs present in the tibiae into which MSCs had been injected with the tibiae that had not received injections over the total number of CFCs in each animal. In mice given MSCs, the tibiae into which MSCs had been injected contained the majority of CFCs (89.4% ± 6.7% versus 10.6% ± 6.7%, P = .007), whereas CFCs were evenly distributed in the right and left tibiae (53.3% ± 18.1% and 46.7% ± 18.1%, respectively) in control mice (Figure 4D). These results confirmed that the presence of human MSCs positively affected the engraftment of human HSCs.
In situ visualization of human MSCs and hematopoietic cells in the murine BM compartment at 4 weeks after IBMT or intravenous transplantation. Transduction efficiencies of eYFP into CBCD34 cells and eGFP into MSCs, determined by flow cytometric analysis at the day of transplantation, were approximately 65% (A) and 99% (B), respectively. The shaded histograms indicate nontransduced cells. (C-E) The presence of human hematopoietic cells was determined by eYFP fluorescence and immunofluorescence staining with an anti-human CD45 antibody followed by Alexa-fluor 594 goat anti-mouse immunoglobulin secondary antibody. Cell nuclei were visualized by staining with TOTO3. Similar amounts of eYFP-cells expressing human CD45 (red) are present in BM after intravenous transplantation (C) or IBMT (D). (E) No eYFP- or CD45-reactive cells are present in the BM of noninjected mouse. (F) eGFP-MSCs are rarely seen in BM after intravenous transplantation (white arrowhead). After IBMT, eGFP-MSCs are easily identified in the BM cavity by eGFP fluorescence (G) and immunohistochemistry (H). (G-H) The same section was stained with an anti-GFP antibody after examining for eGFP fluorescence. All bars represent 10 μm. Images in panels C-G were obtained using an LSM510 META confocal microscope; image in panel H was obtained using an Olympus AX80 microscope and a 20 ×/0.7 NA UPlanApo objective lens.
In situ visualization of human MSCs and hematopoietic cells in the murine BM compartment at 4 weeks after IBMT or intravenous transplantation. Transduction efficiencies of eYFP into CBCD34 cells and eGFP into MSCs, determined by flow cytometric analysis at the day of transplantation, were approximately 65% (A) and 99% (B), respectively. The shaded histograms indicate nontransduced cells. (C-E) The presence of human hematopoietic cells was determined by eYFP fluorescence and immunofluorescence staining with an anti-human CD45 antibody followed by Alexa-fluor 594 goat anti-mouse immunoglobulin secondary antibody. Cell nuclei were visualized by staining with TOTO3. Similar amounts of eYFP-cells expressing human CD45 (red) are present in BM after intravenous transplantation (C) or IBMT (D). (E) No eYFP- or CD45-reactive cells are present in the BM of noninjected mouse. (F) eGFP-MSCs are rarely seen in BM after intravenous transplantation (white arrowhead). After IBMT, eGFP-MSCs are easily identified in the BM cavity by eGFP fluorescence (G) and immunohistochemistry (H). (G-H) The same section was stained with an anti-GFP antibody after examining for eGFP fluorescence. All bars represent 10 μm. Images in panels C-G were obtained using an LSM510 META confocal microscope; image in panel H was obtained using an Olympus AX80 microscope and a 20 ×/0.7 NA UPlanApo objective lens.
Site-specific differentiation of human eGFP-MSCs in the murine BM compartment. The engraftment and differentiation of transplanted eGFP-MSCs were determined by eGFP fluorescence and immunofluorescence staining for lineage-specific antigens. eGFP-cells were located on the abluminal side of endothelial cells (A) or lined the sinus wall (D). Those vasculature-associated eGFP-cells express α-SM actin (red in panels B and E, and merged in panels C and F). (A-F) eYFP-cells reside next to eGFP-cells. (G) An elongated cell in the endosteal hematopoietic cord interacts with eYFP-cells. eGFP-reticular cells (H) with ALP+ (red) cytoplasmic extensions (I), which haphazardly radiate into the hematopoietic parenchyma, interact with eYFP-cells. An eGFP-cell in the bone exhibits the morphology of authentic osteocytes with filopodial processes surrounded by bone matrix and extending into the canaliculi (J), and expresses osteocalcin (red, K-L). A bone-lining eGFP-cell (M) expresses N-cadherin (red, N-O). N-cadherin is localized to the cell surface. Vasculature-associated eGFP-cells (P,S) express CD31 (red, Q-R) and CD34 (red, T-U). Interactions between eGFP-cells (arrows) and eYFP-cells (arrowheads in A-I) are seen in the specimens from mice in which eGFP-MSCs and eYFP-CBCD34 were cotransplanted. All bars represent 10 μm.
Site-specific differentiation of human eGFP-MSCs in the murine BM compartment. The engraftment and differentiation of transplanted eGFP-MSCs were determined by eGFP fluorescence and immunofluorescence staining for lineage-specific antigens. eGFP-cells were located on the abluminal side of endothelial cells (A) or lined the sinus wall (D). Those vasculature-associated eGFP-cells express α-SM actin (red in panels B and E, and merged in panels C and F). (A-F) eYFP-cells reside next to eGFP-cells. (G) An elongated cell in the endosteal hematopoietic cord interacts with eYFP-cells. eGFP-reticular cells (H) with ALP+ (red) cytoplasmic extensions (I), which haphazardly radiate into the hematopoietic parenchyma, interact with eYFP-cells. An eGFP-cell in the bone exhibits the morphology of authentic osteocytes with filopodial processes surrounded by bone matrix and extending into the canaliculi (J), and expresses osteocalcin (red, K-L). A bone-lining eGFP-cell (M) expresses N-cadherin (red, N-O). N-cadherin is localized to the cell surface. Vasculature-associated eGFP-cells (P,S) express CD31 (red, Q-R) and CD34 (red, T-U). Interactions between eGFP-cells (arrows) and eYFP-cells (arrowheads in A-I) are seen in the specimens from mice in which eGFP-MSCs and eYFP-CBCD34 were cotransplanted. All bars represent 10 μm.
To assess if there were any functional differences between human cells engrafted in the tibiae into which MSCs had been injected and tibiae that had not received injections, we transplanted BM cells obtained from each tibia of primary mice to separate secondary hosts. Two of 3 secondary host pairs showed human cell engraftment. A pair of recipients, which received cells from the primary host with minimal engraftment (CD45% content; 1.44% for tibiae into which MSCs had been injected and 0.52% for tibiae that had not received injections), failed to show engraftment. As expected, secondary hosts that received BM cells of the tibiae into which MSCs had been injected demonstrated a markedly higher level of engraftment than the hosts that received cells of tibiae that had not received injections (Figure 4E). Taken together, the presence of HMRCs in murine BM augmented not only the proportion of human cells but also the function of human cells engrafted in mouse BM.
Effect of human MSCs on the engraftment of human hematopoietic cells. (A) BM cells obtained from the MSC-injected or noninjected tibia from the same mouse were examined by flow cytometry at 6 weeks after transplantation. Representative flow cytometric profiles are shown. The relative frequencies of each population are shown at the corner of the respective quadrants. The numbers in parentheses indicate the proportion of CD45+ cells positive for each marker. (B) Percentages of human CD45+ cells in the right (injected) and the left (noninjected) tibia of the MSC-injected (MSC) and control mice (CT). (C) Absolute numbers of CD34+ cells in the right and the left tibia of the MSC-injected and control mice. Each white circle represents one mouse. Values obtained from the same mouse are connected with lines. (D) Distribution of clonogenic progenitors into the right and the left tibia of the MSC-injected and control mice. The proportions of clonogenic cells present in the respective tibia over the total number of clonogenic cells recovered from both tibiae of each mouse are shown (n = 3). *P < .05 and **P < .01 relative to the noninjected (left) tibia. (E) Secondary transplantation. BM cells obtained separately from the MSC-injected or noninjected tibia from the same mouse were transplanted into separate secondary hosts. Human cell engraftment was analyzed by CD45 expression at 6 weeks after transplantation. Results of 2 pairs of secondary host are shown. Numbers in parentheses above each flow cytometric profile show the percentage of CD45 cells in the BM cells of primary host. The relative frequencies of CD45 cells in secondary hosts are shown in each profile.
Effect of human MSCs on the engraftment of human hematopoietic cells. (A) BM cells obtained from the MSC-injected or noninjected tibia from the same mouse were examined by flow cytometry at 6 weeks after transplantation. Representative flow cytometric profiles are shown. The relative frequencies of each population are shown at the corner of the respective quadrants. The numbers in parentheses indicate the proportion of CD45+ cells positive for each marker. (B) Percentages of human CD45+ cells in the right (injected) and the left (noninjected) tibia of the MSC-injected (MSC) and control mice (CT). (C) Absolute numbers of CD34+ cells in the right and the left tibia of the MSC-injected and control mice. Each white circle represents one mouse. Values obtained from the same mouse are connected with lines. (D) Distribution of clonogenic progenitors into the right and the left tibia of the MSC-injected and control mice. The proportions of clonogenic cells present in the respective tibia over the total number of clonogenic cells recovered from both tibiae of each mouse are shown (n = 3). *P < .05 and **P < .01 relative to the noninjected (left) tibia. (E) Secondary transplantation. BM cells obtained separately from the MSC-injected or noninjected tibia from the same mouse were transplanted into separate secondary hosts. Human cell engraftment was analyzed by CD45 expression at 6 weeks after transplantation. Results of 2 pairs of secondary host are shown. Numbers in parentheses above each flow cytometric profile show the percentage of CD45 cells in the BM cells of primary host. The relative frequencies of CD45 cells in secondary hosts are shown in each profile.
Interaction between human MSC-derived cells and human hematopoietic cells
Having shown the effect of MSCs on human hematopoietic cell engraftment, we examined the underlying basis of increased engraftment in phenotypically and functionally primitive cells. First, we asked whether MSCs play a role in the migration and homing of HSCs to BM, critical steps in the engraftment and initiation of hematopoiesis after transplantation. Prior to intravenous administration of CBCD34, each mouse was given injections of MSCs into the right tibia and PBS into the left tibia. The percentages of CBCD34 cells homed to the tibiae into which MSCs and PBS had been injected were not different (0.05% ± 0.02% and 0.04% ± 0.01%, respectively; n = 3). Thus, MSCs did not seem to function in the initial homing of CBCD34 cells.
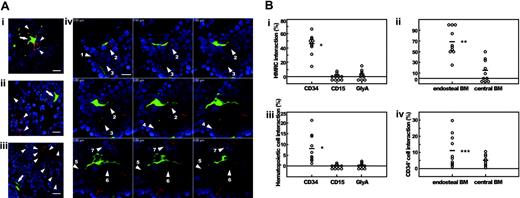
We then asked whether MSCs participate in the repopulation process of human hematopoietic cells in the murine HME. At 10 weeks after transplantation, human MSC-derived HMRCs and CBCD34-derived hematopoietic cells frequently interacted (Figure 3), suggesting the role of HMRCs in the maintenance of human hematopoiesis. When those hematopoietic cells were immunophenotyped, CD34+ cells were often in contact or within close proximity to HMRCs (Figure 5Ai), contrasting to lineage-committed CD15+ and GlyA+ cells that were usually found away from HMRCs (Figure 5Aii-iii). Interestingly, one HMRC interacted with 7 CD34+ cells (Figure 5Aiv). These results suggest that HMRCs interact specifically with primitive human hematopoietic cells; therefore, we quantitated HMRCs that were in physical contact with CD34+, CD15+, or GlyA+ cells (Table 2; Figure 5B). Whereas approximately half of all HMRCs in BM were in contact with CD34+ cells, less than 4% of HMRCs were in contact with the lineage-committed cells (Figure 5Bi). Furthermore, the vast majority of HMRCs located in the endosteal region interacted with CD34+ cells (Figure 5Bii). We then examined the human hematopoietic cells that were in contact with HMRCs. A significantly higher proportion of CD34+ cells interacted with HMRCs compared to the lineage-committed cells (Figure 5Biii). The proportion of CD34+ cells interacting with HMRCs in the endosteal region was 2-fold higher than that of CD34+ cells in the central marrow (Figure 5Biv). The result suggests that CD34+ cells in the endosteal region and the central marrow represent different populations of CD34+ cells.
Interactions between HMRCs and human hematopoietic cells at 10 weeks after IBMT of eGFP-MSCs plus CBCD34
. | HMRC interaction with hematopoietic cells . | . | . | Hematopoietic cell interaction with HMRC . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | HMRCs interacting . | . | . | Hematopoietic cells interacting . | . | ||||
. | No. cells counted . | Total no. . | Frequency, % . | No. cells counted . | Total no. . | Frequency, % . | ||||
| CD34 | ||||||||||
| Total | 150 | 66 | 45.9 ± 13.5* | 1609 | 76 | 8.0 ± 6.2* | ||||
| Endosteal | 81 | 49 | 68.0 ± 24.8† | 1012 | 59 | 11.0 ± 9.4‡ | ||||
| Central | 59 | 17 | 14.6 ± 17.9 | 597 | 17 | 5.0 ± 3.1 | ||||
| CD15 | 100 | 3 | 1.2 ± 2.4 | 2603 | 3 | 0.14 ± 0.28 | ||||
| GlyA | 154 | 5 | 3.2 ± 4.5 | 1624 | 5 | 0.36 ± 0.49 | ||||
. | HMRC interaction with hematopoietic cells . | . | . | Hematopoietic cell interaction with HMRC . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | HMRCs interacting . | . | . | Hematopoietic cells interacting . | . | ||||
. | No. cells counted . | Total no. . | Frequency, % . | No. cells counted . | Total no. . | Frequency, % . | ||||
| CD34 | ||||||||||
| Total | 150 | 66 | 45.9 ± 13.5* | 1609 | 76 | 8.0 ± 6.2* | ||||
| Endosteal | 81 | 49 | 68.0 ± 24.8† | 1012 | 59 | 11.0 ± 9.4‡ | ||||
| Central | 59 | 17 | 14.6 ± 17.9 | 597 | 17 | 5.0 ± 3.1 | ||||
| CD15 | 100 | 3 | 1.2 ± 2.4 | 2603 | 3 | 0.14 ± 0.28 | ||||
| GlyA | 154 | 5 | 3.2 ± 4.5 | 1624 | 5 | 0.36 ± 0.49 | ||||
BM sections stained with either anti-CD34, anti-CD15, or anti–glycophorin A (GlyA) antibody were examined under fluorescent microscope (10 slides from at least 5 different mice for each cell type). The total numbers of HMRCs and immunophenotyped hematopoietic cells present in the sections were counted, and individual cells were examined for physical contact between HMRCs and hematopoietic cells. Proportions of interacting cells/slide were calculated for each cell type and expressed as the means ± SD. Interactions in CD34+ cell group were further categorized into the endosteal and the central BM groups based on the histoanatomic location of cells.
P < .005 relative to the CD15 and GlyA groups
P < .005 relative to the central group
P < .05 relative to the central group
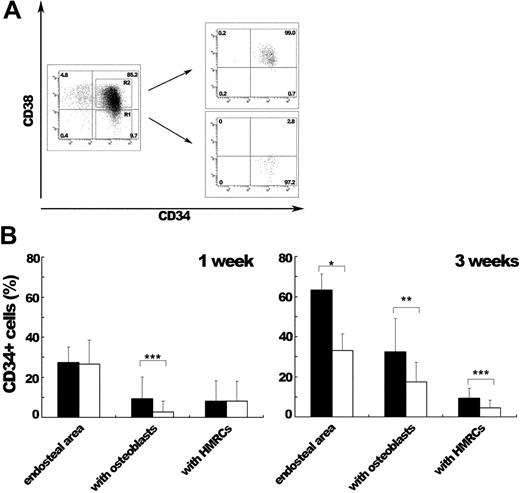
Because the heterogeneity among CD34+ stem/progenitor cells is known,35-39 we further examined the interaction between CD34+ cells and HMRCs by transplanting SRC-enriched CD34+CD38- cells or more mature CD34+CD38+ progenitor cells together with MSCs (Figure 6). At 1 week after transplantation, there were no differences in the proportions of CD34+ cells localized to the endosteal region (27.4% ± 7.8% for the CD34+CD38- group and 26.5% ± 12.1% for the CD34+CD38+ group) or CD34+ cells interacting with HMRCs (8.1% ± 10.2% and 8.2% ± 9.7%, respectively) between the 2 experimental groups. At 3 weeks after transplantation, the proportion of CD34+ cells in the endosteal area was 2-fold higher in the group that had received transplants of SRC-enriched CD34+CD38- cells (63.3% ± 7.9% versus 33.1% ± 8.3%; P < .001). To our interest, a significantly higher proportion of CD34+ cells were in contact with HMRCs in the group that had received transplants of CD34+CD38- cells (9.4% ± 4.8% versus 4.4% ± 4.0%; P < .05). In the group that had received CD34+CD38+ transplants, CD34+ cells interacting with HMRCs became progressively rarer (8.2% ± 9.7% at 1 week and 4.4% ± 4.0% at 3 weeks) and were rarely observed at 6 weeks after transplantation (1.1% ± 1.0%). At both time points examined, the group that had received CD34+CD38- transplants contained higher proportions of CD34+ cells interacting with bone-lining osteoblasts (9.3% ± 10.9% versus 2.7% ± 5.5% at 1 week and 32.4% ± 16.8% versus 17.4% ± 9.9% at 3 weeks). This tendency of CD34+CD38- cells, the preferential localization of CD34+ cells in the endosteal area and their interaction with bone-lining osteoblasts, may reflect their high repopulation potential. In another words, SRC-enriched CD34+CD38- cells localize to the endosteal area and interact with bone-lining osteoblasts as well as HMRCs, and progressively become distant to the endosteal area as they differentiate into progenitor cells and finally to mature cells. These results confirmed our earlier observation that HMRCs interacted with the primitive population of CD34+ cells, which resulted in the enhanced engraftment of human hematopoietic cells.
Interaction between HMRCs and CD34+, CD15+, or GlyA+ cells. (A) Bone slides were stained with either anti-CD34, anti-CD15, or anti-glycophorin A antibody followed by Alexa-fluor 594 goat anti-mouse immunoglobulin secondary antibody, and examined under fluorescent microscope. (Ai) CD34+ cells are in physical contact with the cell body and cytoplasmic extensions of a HMRC. CD15+ (Aii) or GlyA+ cells (Aiii) are not found close to HMRCs. HMRCs and immunophenotyped cells are indicated by arrows and arrowheads, respectively. (Aiv) One HMRC in the hematopoietic parenchyma interacts with 7 CD34+ cells (numbered arrowheads). Numerical letters at the left corner of each panel indicate the position of z-axis in the analytical planes. All bars represent 10 μm. (B) Quantification of the interaction between HMRCs and CD34+, CD15+, or GlyA+ cells. (Bi) Frequencies of HMRCs interacting with CD34+, CD15+, or GlyA+ cells. (Bii) The vast majority of HMRCs located in the endosteal region interact with CD34+ cells. (Biii) Frequencies of CD34+, CD15+, or GlyA+ cells interacting with HMRCs. (Biv) In the endosteal region, 2-fold more CD34+ cells interact with HMRCs than in the central marrow. Each white circle represents a value obtained by counting. *P < .005 relative to the CD15 and GlyA groups; **P < .005 and ***P < .05 relative to the central group.
Interaction between HMRCs and CD34+, CD15+, or GlyA+ cells. (A) Bone slides were stained with either anti-CD34, anti-CD15, or anti-glycophorin A antibody followed by Alexa-fluor 594 goat anti-mouse immunoglobulin secondary antibody, and examined under fluorescent microscope. (Ai) CD34+ cells are in physical contact with the cell body and cytoplasmic extensions of a HMRC. CD15+ (Aii) or GlyA+ cells (Aiii) are not found close to HMRCs. HMRCs and immunophenotyped cells are indicated by arrows and arrowheads, respectively. (Aiv) One HMRC in the hematopoietic parenchyma interacts with 7 CD34+ cells (numbered arrowheads). Numerical letters at the left corner of each panel indicate the position of z-axis in the analytical planes. All bars represent 10 μm. (B) Quantification of the interaction between HMRCs and CD34+, CD15+, or GlyA+ cells. (Bi) Frequencies of HMRCs interacting with CD34+, CD15+, or GlyA+ cells. (Bii) The vast majority of HMRCs located in the endosteal region interact with CD34+ cells. (Biii) Frequencies of CD34+, CD15+, or GlyA+ cells interacting with HMRCs. (Biv) In the endosteal region, 2-fold more CD34+ cells interact with HMRCs than in the central marrow. Each white circle represents a value obtained by counting. *P < .005 relative to the CD15 and GlyA groups; **P < .005 and ***P < .05 relative to the central group.
Molecular interaction of HMRCs and CD34+ cells
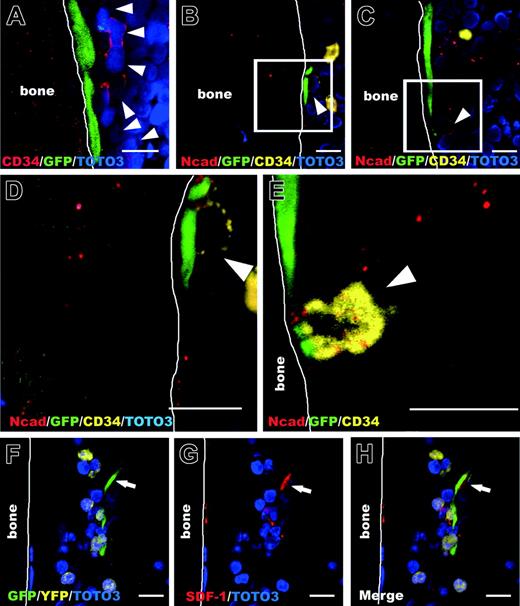
To determine how HMRCs participated in human hematopoietic cell repopulation in mice, we investigated the molecular interaction of HMRCs and CD34+ cells. We found that CD34+ cells adhered to HMRCs on the bone surface and appeared to proliferate along the endosteal surface, suggesting the existence of specific local signals between CD34+ cells and bone-lining HMRCs (Figure 7A). Double staining for N-cadherin and CD34 demonstrated that bone-lining HMRCs associated with CD34+ cells through the colocalization of N-cadherin (Figure 7B-E). In addition, an HMRC in the endosteal hematopoietic parenchyma expressed stromal cell-derived factor 1 (SDF-1) and interacted with a few eYFP-human hematopoietic cells (Figure 7F-H), although SDF-1 was not detected in ex vivo expanded MSCs by immunofluorescence analysis (data not shown). In BM, SDF-1 is constitutively expressed by osteoblasts, endothelial cells, and BM stromal cells.40 In addition to its well-established role in homing and retention of HSCs in BM,41 SDF-1 has been implicated in regulating the status of primitive HSCs both in vitro and in vivo.42-44 Therefore, HMRCs may contribute to the maintenance of primitive human HSCs through N-cadherin-mediated interactions and the production of SDF-1.
In vivo localization of CD34+ cells and their interaction with HMRCs after IBMT of CD34+CD38- or CD34+CD38+ populations together with human MSCs. (A) Sorting profiles of CD34+CD38+ and CD34+CD38- populations. The relative frequencies of each population are shown in the corner of the respective quadrants. (B) At 1 or 3 weeks after IBMT, bone sections were stained with an anti-CD34 antibody and examined for counting. Eighteen slides from 3 mice for each group were counted to obtain the proportion of CD34+ cells localized to the endosteum (endosteal area) at the both time points. CD34+ cells in the endosteal area were further categorized into cells attaching to bone-lining osteoblasts (with osteoblasts) and cells interacting with HMRCs (with HMRCs). The proportions of CD34+ cells in each category are shown. At 1 week after IBMT, the proportions of CD34+ cells both in the endosteal area and in contact with HMRCs were not different between the 2 groups. At 3 weeks after IBMT, the SRC-enriched CD34+CD38--transplanted group had the higher proportions of CD34+ cells in the endosteal area as well as those interacting with HMRCs. At the both time points examined, the proportion of CD34+ cells interacted with osteoblasts was higher in the SRC-enriched CD34+CD38--transplanted group. Bars represent the CD34+CD38--transplanted group (▪) and the CD34+CD38+-transplanted group (□). *P < .001, **P < .005, and ***P < .05 relative to the CD34+CD38+ group.
In vivo localization of CD34+ cells and their interaction with HMRCs after IBMT of CD34+CD38- or CD34+CD38+ populations together with human MSCs. (A) Sorting profiles of CD34+CD38+ and CD34+CD38- populations. The relative frequencies of each population are shown in the corner of the respective quadrants. (B) At 1 or 3 weeks after IBMT, bone sections were stained with an anti-CD34 antibody and examined for counting. Eighteen slides from 3 mice for each group were counted to obtain the proportion of CD34+ cells localized to the endosteum (endosteal area) at the both time points. CD34+ cells in the endosteal area were further categorized into cells attaching to bone-lining osteoblasts (with osteoblasts) and cells interacting with HMRCs (with HMRCs). The proportions of CD34+ cells in each category are shown. At 1 week after IBMT, the proportions of CD34+ cells both in the endosteal area and in contact with HMRCs were not different between the 2 groups. At 3 weeks after IBMT, the SRC-enriched CD34+CD38--transplanted group had the higher proportions of CD34+ cells in the endosteal area as well as those interacting with HMRCs. At the both time points examined, the proportion of CD34+ cells interacted with osteoblasts was higher in the SRC-enriched CD34+CD38--transplanted group. Bars represent the CD34+CD38--transplanted group (▪) and the CD34+CD38+-transplanted group (□). *P < .001, **P < .005, and ***P < .05 relative to the CD34+CD38+ group.
Discussion
Our study demonstrated that intramedullary transplanted human MSCs reconstituted the HME and provided direct evidence for a role of transplanted human MSCs in the enhancement of human hematopoietic cell repopulation in mice. The initial histologic analysis unveiled the integration of human hematopoietic cells into the specially and functionally compartmentalized HME of NOD/SCID mice. Based on this finding, we established a model system that enables the identification of the phenotype and function of human MSCs in vivo by directly injecting genetically marked human MSCs into the BM of NOD/SCID mice. Analogous to human HSCs, human MSCs persisted long-term in murine BM to at least 10 weeks after transplantation and were able to differentiate into the key components of the HME in the host. The presence of human MSCs in murine BM correlated with the increase in human hematopoietic cells that were phenotypically and functionally primitive. Engrafted human MSCs appeared to be involved in the maintenance of human hematopoiesis via secreted factors as well as by physically interacting with primitive hematopoietic cells.
The stem cell niche is a key determinant of stem cell development.45,46 We are beginning to understand the murine HSC niche and the molecular mechanisms that govern the fate of murine HSCs,47 but there exists a paucity of data on the cellular and molecular microenvironmental regulation of human hematopoiesis in vivo due largely to a lack of good experimental tools. Although the identification of SRCs has facilitated detailed characterization of human HSCs in vivo,20,48 the key niches that function in human cell repopulation have not been identified. Our study has demonstrated for the first time that CD34-expressing stem/progenitor cells localize to the endosteal surface and mobilize toward the central marrow as they differentiate in BM. In addition, SRC-enriched CD34+CD38- cells demonstrated a distinct trend to localize in the endosteal region and to interact with bone-lining osteoblasts, even at the early stage of hematopoietic reconstitution. This may be one of the reasons that CD34+CD38- cells have the high repopulation potential. We also found that human MSC-derived HMRCs locally created human HME in the murine environment. Visualization of human hematopoietic cells and human HMRCs in situ made it possible to elucidate the physical interaction between human hematopoietic cells and the human microenvironment. In this way, the SRC assay recapitulates human hematopoiesis in the murine environment both structurally and functionally and could serve as a good experimental system to study human hematopoiesis.
The presence of human MSCs in murine BM resulted in significantly more engraftment of phenotypically and functionally primitive human hematopoietic cells. With this newly established experimental system, we were able to present 3 lines of evidence that explain how human MSCs may facilitate human hematopoietic cell engraftment. First, the interaction of human MSC-derived HMRCs and human CD34+ cells was mostly observed in the endosteal region, at a significantly higher frequency than that of lineage-committed cells. When this interaction was examined further by using sorted populations of SRC-enriched CD34+CD38- cells and more mature CD34+CD38+ progenitor cells, a preferential interaction of HMRCs with a primitive population of CD34+ cells was evident. Whereas the proportion of CD34+ cells interacting with HMRCs rapidly decreased in the group that had received CD34+CD38+ progenitor cells, this frequency in the group that had received CD34+CD38- transplants did not change and was equivalent to the proportion of CD34+ cells interacting with HMRCs at 10 weeks after transplantation. Considering that the proportion of CD34+CD38- cells originally contained in the CD34+ cells (representing 5%-8% of CD34+ cells in this study) was similar to HMRC-interacting CD34+ cells (8.0% ± 6.2% at 10 weeks), transplanted human MSCs, which became an integral part of the functional HME, interacted with primitive cell populations, provided supportive environment of human hematopoiesis, and augmented human cell engraftment. Second, bone-lining HMRCs interacted with CD34+ cells through the asymmetric expression of N-cadherin, similar to the way bone-lining osteoblasts maintain primitive HSCs in mouse. This specific interaction of CD34+ cells with human MSC-derived bone-lining osteoblasts, a cellular component of the stem cell niche, indicates that similar regulatory mechanisms operate in human and murine hematopoiesis. Third, HMRCs in the endosteal hematopoietic parenchyma produced SDF-1 and interacted with human hematopoietic cells. This strengthens the previous observations that SDF-1 regulates the proliferation and survival of primitive HSCs and progenitor cells.12,42-44 Taken together, these results suggest that human MSC-derived HMRCs contribute not only to the proliferation and differentiation of human hematopoietic progenitor cells that results in the increased chimerism but also to the maintenance of primitive human HSCs.
Expression of N-cadherin and SDF-1 by HMRCs that interact with human hematopoietic cells. (A) CD34+ cells (arrowheads) appear to colonize near the bone-lining HMRCs. (B-C) Bone-lining HMRCs colocalize with CD34+ cells (arrowheads) through the asymmetrical expression of N-cadherin. (D-E) Higher magnifications of panels B and C. (F-H) An HMRC in the endosteal hematopoietic parenchyma (arrow) expresses SDF-1 and interacts with a few eYFP hematopoietic cells. All bars represent 10 μm.
Expression of N-cadherin and SDF-1 by HMRCs that interact with human hematopoietic cells. (A) CD34+ cells (arrowheads) appear to colonize near the bone-lining HMRCs. (B-C) Bone-lining HMRCs colocalize with CD34+ cells (arrowheads) through the asymmetrical expression of N-cadherin. (D-E) Higher magnifications of panels B and C. (F-H) An HMRC in the endosteal hematopoietic parenchyma (arrow) expresses SDF-1 and interacts with a few eYFP hematopoietic cells. All bars represent 10 μm.
Embryonic development is strictly regulated through sequential and concerted events that are orchestrated by interactions between tissue stem cells and the microenvironment. This developmental paradigm, including signaling molecules that regulate stem cell self-renewal,49 is conserved for generating and maintaining specific tissues in adult life, and dysregulation of this process leads to pathologic conditions such as cancer.50 Current empiric cancer studies have focused on identifying intrinsic genetic changes that lead to the aberrant proliferation of cells, and, as a result, therapeutic agents targeting the genetic mutations are emerging. However, our understanding of extrinsic, or microenvironmental, signals in the context of tumorigenesis has lagged behind. Because the microenvironment is responsible for homeostatic controls,45,51,52 is there a specific microenvironment that permits, initiates, or complements tumorigenesis and supports progression of tumors? Could the microenvironment be a new target for cancer therapy? The experimental system we described here, which allows the visualization and reconstitution of a human microenvironment, provides a unique tool to study the maintenance of tissue homeostasis, which may lead to the elucidation of the tumorigenic microenvironment. In addition, the functional persistence of transplanted MSCs in the host environment means that MSCs may be used to deliver therapeutic genes or agents to target tissues.
Prepublished online as Blood First Edition Paper, November 10, 2005; DOI 10.1182/blood-2005-06-2211.
Supported by a grant-in-aid for a Research Grant of Scientific Frontier Program and Scientific Research, and of Regenerative Medicine Project from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and Research Grant on Human Genome, Tissue Engineering (H17-014) from the Ministry of Health, Labor and Welfare of Japan. Y.M. and T.Y. participated in designing and performing the research; Y.M. wrote the paper; H.M., T.S., T.U., and J.I. analyzed the data; M.I. provided experimental animals; S.K., T.H., and K.A. analyzed and interpreted the data; and all authors checked the final version of the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The monoclonal antibody for alkaline phosphatase developed by Dr Katzmann was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. The authors thank Dr Hiroyuki Miyoshi, BioResource Center, RIKEN Tsukuba Institute, for providing lentivirus vectors, Hideyuki Matsuzawa and Tamaki Saso for technical assistance, members of Tokai Cord Blood Bank for providing cord blood samples, members of the animal facility of Tokai University for care of experimental animals, and all members of Research Center of Regenerative Medicine for their support.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal