Abstract
Human subjects maintain long-term immunologic memory against infective organisms but the mechanism is unclear. CD4+ T-helper memory (Thmem) cells are pivotal in controlling humoral and cellular responses, therefore their longevity and response to vaccination are critical for maintenance of protective immunity. To probe the dynamics of the Thmem-cell response to antigenic challenge, we investigated subjects following a booster injection with tetanus toxoid (TT). Expansion of TT-specific Thmem cells and cytokine production showed complex kinetics. Strikingly, parallel expansion and cytokine production occurred in pre-existing Thmem cells specific for 2 other common antigens: purified protein derivative of tuberculin and Candida albicans. Bystander expansion occurred in Thmem but not in Thnaive cells. Antibody production against TT peaked approximately 2 weeks after vaccination and gradually declined. However, pre-existing antibody against the other antigens did not change. It appears that although all Thmem cells are readily stimulated to expand, antibody responses are controlled by antigen availability. These findings relate to the maintenance of memory and have consequences for assessments of specific T-cell responses to vaccination.
Introduction
Immunologic memory, the key to long-term protection against pathogens, is mediated by antigen-specific memory T and B cells together with serum antibody. Although observations on resistance to infection have indicated longevity of memory,1 the mechanism of maintenance has been unclear. A periodic re-exposure to a pathogen is an obvious and effective way to maintain high levels of immunity. Recent investigations of human subjects vaccinated against smallpox have nonetheless revealed that although no exposure to smallpox (vaccinia) virus can have occurred for many years, survival of specific CD4+ and CD8+ T cells is evident decades after vaccination.2-4 Similarly, plateau levels of functional memory B cells persist for longer than 50 years.2 Since vaccinia virus is believed to neither persist nor become latent after the acute phase of infection, those studies support the idea that other antigen-independent mechanisms may contribute to the maintenance of immunologic memory.
Investigations of memory T (Tmem) cells have revealed heterogeneity in phenotype and function. In both mice5 and human subjects6 they display a higher rate of division in vivo than naive T cells. Human CD45RO+ Tmem cells have been subdivided into effector memory (TEM) cells, which migrate to sites of inflammation and have immediate effector function, and central memory cells (TCM), which home to secondary lymphoid tissue. Among blood CD4+ T cells, TCM cells predominate.7 Markers of TCM cells include CCR7 and CD62L, of importance in chemokine-mediated migration and extravasation through high endothelial venules, respectively.7 However, analysis of responses to persistent viruses have revealed further phenotypic complexity in CD4+ Tmem cells, which could be influenced by the nature and level of presented antigen.8
A major question is how an effective Tmem population can be maintained over the lifetime of the host in the apparent absence of antigen. One suggested route is via nonspecific stimulation by cytokines, and this has been shown to occur in human T-helper memory (Thmem) cells in vitro.7,9,10 In terms of the functional subsets, TEM cells proliferate in vitro when exposed to IL-7 and IL-15, whereas TCM cells are less responsive and naive T cells fail to respond.11 However, responsiveness is influenced by the presence of dendritic cells and associated cytokines, raising the question of the outcome in vivo.
One way to study antigen-independent bystander activation of memory T cells in vivo in human subjects is to evaluate the impact of infection or vaccination on resident memory cells with antigen specificities unrelated to the infectious agent or to the vaccine. Although there are few published studies, there have been some intriguing observations that point to bystander effects. In one study of vaccination with tetanus toxoid (TT), limiting dilution analysis of proliferating cells revealed the expected increase in the frequency of TT-specific cells, but this was observed to be accompanied by a modest but significant increase in the frequency of herpes simplex virus–specific cells.12 Similarly, after a TT boost, peripheral-blood mononuclear cells (PBMNCs) from 3 healthy donors showed an increased proliferative response to purified protein derivative of tuberculin (PPD) compared with preboost responses.13
In terms of B cells, maintenance of antibody levels can be partially explained by new estimates of the survival of plasma cells, which in mice can persist throughout life.14 Nondividing plasma cells are thought to occupy protective niches in bone marrow and spleen.15 However, in long-lived human subjects, it is unlikely that these can be the only source of persisting antibody, and continuous maturation of memory B cells may be required. Memory B cells can certainly persist without antigen stimulation16 and could be stimulated to differentiate via cross-reacting antigen or possibly via an antigen-independent pathway.17
In the present study, we have systematically investigated the effect of injecting human subjects with TT on both the specific T-cell response and on the surrounding Thmem cells specific for 2 other antigens. Bystander Thmem-cell stimulation with similar kinetics to the specific response was clearly evident from both proliferative assays and cytokine production. Interestingly, B-cell responses were constrained, with antibody production confined to the immunizing antigen.
Patients, materials, and methods
Vaccination and sample collection
After obtaining ethical approval for the study from the Southampton and Southwest Hampshire local research ethics committees (Southampton, United Kingdom) and informed consent in accordance with the Declaration of Helsinki, 12 healthy adults (8 males, 4 females; 31-44 years of age) received a single dose of TT vaccine intramuscularly (adsorbed tetanus vaccine BP; Aventis Pasteur MSD, Swiftwater, PA). All subjects had been vaccinated against TT but had not received a boost during the previous 5 years. All subjects had also received conventional vaccination with bacillus Calmette-Guérin (BCG). Blood samples were taken before vaccination (week 0) and then at weeks 1, according to availability, and 2. Subsequent samples were taken at 4-week intervals up to week 20. Serum was stored at –20°C. PBMNCs were isolated by centrifugation of heparinized blood on a Lymphoprep density gradient; frozen in 50% human AB serum (Sigma, Poole, United Kingdom), 40% RPMI, and 10% DMSO; and stored in liquid N2. The overall viability of defrosted cells was 90.2% ± 2.6% and the effect of freezing and thawing on the functional assays used was checked and found to be negligible (data not shown).
Antigens
Antigens were TT (not adsorbed), Candida albicans whole extract cytoplasmic protein (C albicans; National Institute for Biological Standards and Control [NIBSC], Potters Bar, United Kingdom); PPD prepared from human strains of Mycobacterium tuberculosis (Evans Vaccines, Mersey-side, United Kingdom); and normal human immunoglobulin for intravenous administration (Vigam Liquid 5g; Bio Products Laboratory, Elstree, United Kingdom) for control 1 (Ctrl1) and an IgA paraprotein purified from the serum of a patient with multiple myeloma for control 2 (Ctrl 2). These endotoxin-free proteins were selected as controls with no pre-existing immunity. In cellular assays TT was used at 1 μg/mL and all the remaining antigens at 10 μg/mL.
Proliferative responses
Cryopreserved PBMNCs were thawed and cultured in triplicates (2 × 105/well) in round-bottomed 96-well plates in RPMI-1640, 10% human AB serum (Sigma), l-glutamine, penicillin, streptomycin, and sodium pyruvate (complete medium) with either TT, PPD, C albicans, Ctrl1, or Ctrl2. PBMNCs cultured without antigen were used as controls. At day 5, cells were pulsed with 1 μCi/well (0.037 MBq/well) of [methyl-3H] thymidine (Amersham Biosciences, Little Chalfont, United Kingdom) and proliferative activity was measured after 18 hours using a scintillation counter. The response is reported as stimulation index (SI): mean counts per minute (cpm) of antigen-stimulated cultures/mean cpm of control. An SI of 3 or higher was taken as a positive response. Comparisons between postvaccine and prevaccine antigen-specific responses were made from the fold increase (FI) in the SI (postvaccine SI/prevaccine SI) and SDs of the 2 compared triplicates. The symbol “–” indicates no response and was given when FI less than 1; the symbol “=” indicates stable response and was given when FI equal to 1 or FI less than 1 SD from 1; finally, the symbols “+,” “++,” and “+++” indicate different degrees of response and were given when FI is between 1 and 2, at least 2 but less than 4, and 4 or more, respectively.
Generation of antigen-specific T-cell lines
PBMNCs were isolated from 2 healthy donors (D1 and D2) who, in a preliminary screening, showed strong T-cell responses (as measured by proliferative assays and IFNγ enzyme-linked immunospot [ELISPOT]) against TT and PPD, respectively (data not shown). Cells were cultured in complete medium in the presence of TT and PPD (10 μg/mL), respectively, at 2 × 105/well in round-bottomed 96-well plates. Human recombinant IL-2 (20 U/mL) was added at days 7 and 9 and medium replaced if necessary. At day 14 cells were harvested, pooled, and counted. Viable cells were then restimulated with antigen plus irradiated (40 Gy) autologous PBMNCs as antigen-presenting cells at a 1:3 ratio in 12- or 24-well plates. IL-2 was added at day 5. Lines were maintained by repeating this 14-day cycle of restimulation with antigen followed by culture in IL-2.
Antigen specificity of T-cell lines and identification of restricting HLA molecules
The antigen specificity of the T-cell lines was checked by a 3-day proliferative assay, performed as described in “Proliferative responses,” using 2.5 × 104 T cells plus irradiated (40 Gy) autologous PBMNCs as antigen-presenting cells at a 1:3 ratio in round-bottomed 96-well plates. Antigen (10 μg/mL) was added in each well. PHA (10 μg/mL) was used as positive control.
To identify the restricting HLA isotype of the T-cell lines, 10 μg/mL of monoclonal antibody (mAb) directed against HLA-DR (L243), HLA-DP (B7/21.2), or HLA class I (W6/32; all from Cancer Research UK) was added to the cell suspension 1 hour prior to addition of the antigen and kept in the medium for the duration of the proliferative assay.
Cytokine measurement by ELISPOT
ELISPOT plates (MAIPS4510; Millipore, Bedford, MA) were precoated overnight at 4°C with 10 μg/mL of mAb against human IFNγ, IL-13 (1-D1K and IL-13-I, respectively; Mabtech, Stockholm, Sweden), or IL-2 (NA/LE; BD Biosciences, San Jose, CA). After washing and blocking for 1 hour with complete medium, 2 × 105 PBMNCs were added in triplicates with either antigens or medium alone. Cytokine production was detected after 40 hours using the appropriate biotinylated mAb as recommended by the manufacturer, followed by streptavidin-alkaline phosphatase (Mabtech; 40 ng/well). Spots were developed after the addition of substrate (BCIP/NBT substrate kit; Zymed Laboratories, South San Francisco, CA) and counted using a computer-assisted video image analyzer (Autoimmun Diagnostika GmbH, Strassberg, Germany). Antigen (Ag)–specific responses are reported as the number of spots per 2 × 105 PBMNCs in Ag-stimulated cultures minus the number of spots in the corresponding control. The average number of spots in triplicate control wells was always less than 5. Responses were considered positive if (mean number of spots in triplicates of antigen-stimulated cultures) minus (mean number of spots in the control) was at least 10 per well and greater than the mean number of spots in the control + 2SD. Comparisons between postvaccine and prevaccine responses were done applying criteria used to evaluate proliferative responses (see “Proliferative responses”).
Flow cytometry analysis
White blood cell counts were done using an automated cell counter and analyzer at the local hospital. For the phenotype analysis, cryopreserved PBMNCs were thawed, washed with PBS, and stained with the following antibodies: anti–human CD3-PerCP (SK7), CD4-APC (RPA-T4), CD8-APC (RPA-T8), CD14-APC (M5E2), CD19-APC (HIB19), CD25-PE (M-A251), CD56-APC (B159), CD80-FITC (L307.4), CD86-PE (2331, FUN-1), and HLA-DR–PE (G46-6, L243; all BD Biosciences) or with their respective mouse immunoglobulin isotype controls. Samples were analyzed on a FACSCalibur flow cytometer using CELLQUEST software (both from BD Biosciences). Based on forward and side scatter characteristics, an initial gating was used to exclude dead cells and debris. A minimum of 20 000 viable cells was acquired.
Antibody measurement by enzyme-linked immunosorbent assay (ELISA)
ImmunoSorp plates (Nunc, Roskilde, Denmark) were coated overnight at 4°C with either 2.5 μg/mL TT, 10 μg/mL PPD, or 10 μg/mL C albicans. After washing and blocking, serial dilutions of serum samples or appropriate standards in at least 4 replicate wells were added. Antibody standards were as follows: TT, tetanus antitoxin human immunoglobulin (NIBSC); PPD, serum from a healthy individual showing relatively high anti-PPD antibody titers; C albicans, serum from a known antibody-positive subject (kindly provided by Dr R. Hobson, Mycology Reference Centre, University of Leeds, United Kingdom). An HRP-conjugated monoclonal anti–human IgG (Fc; The Binding Site, Birmingham, United Kingdom) was used for detection. For TT, results are expressed in IU/mL. For PPD and C albicans, results are expressed in arbitrary units (AU)/mL. Comparisons between postvaccine and prevaccine antigen-specific responses were done using fold increase in titers (postvaccine response/prevaccine response) and SDs of the 2 compared replicates. Symbols indicating no response (–), stable (=), or different degrees of positive response (+ to +++) were assigned using the same criteria as for the cellular responses.
Results
Vaccine-specific and bystander proliferative responses of Tmem cells following booster vaccination with TT
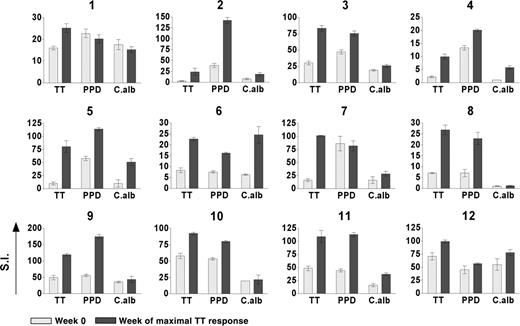
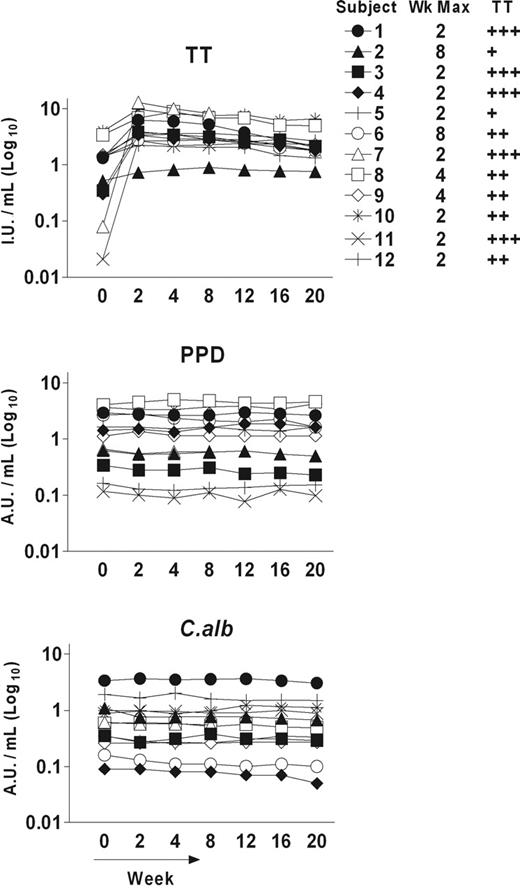
The kinetics of the TT-specific proliferative responses over 16 weeks following booster vaccination with TT are shown for 6 representative cases (Figure 1). Importantly, results were reproducible using samples thawed from stocks on 2 different occasions (data not shown). Pre-existing immunity, as indicated by a positive response at week 0, was evident in all subjects but at different levels. Increased responses were detected after vaccination but the time to peak responses varied from 2 to 12 weeks and the dynamic complexity of the responses was striking. Surprisingly, parallel kinetics were observed for proliferative responses against 2 unrelated antigens (PPD and C albicans) for which pre-existing immunity was also evident (Figure 1). Parallel kinetics were seen in individuals with early (cases 5, 3, 10) or late (cases 6, 8, 9) responses to TT. In patient 8, who lacked pre-existing immunity against C albicans, bystander proliferative responses did not develop, arguing that Tmem cells are required. In support of that, no responses against control antigens (Ctrl 1 and Ctrl 2) without pre-existing immunity were detected. It appears therefore that vaccination expands both specific and nonspecific Tmem cells coordinately and that contractions also occur in parallel. These contractions were observed in various individuals (eg, case 5 at wk 4, case 3 at wk 8, case 10 at wk 8, etc; Figure 1) and were not due to low viability of thawed cells, which was always approximately 90%. They were also observed when the assay was done using fresh PBMNCs (viability > 95%), excluding any other differential loss of functionality during cryopreservation (data not shown). Finally they were still observed when higher (superoptimal) doses of antigen were used (data not shown), arguing against a drop in assay sensitivity at those particular time points. Overall these contractions seem to represent a genuine phenomenon that, at least in some individuals, can been seen as the natural decline of the immune response following the peak.
Kinetics of vaccine-specific and bystander proliferative responses of Tmem cells following booster vaccination with TT. Six representative examples are shown. Vaccination (week 0) induces early (subjects 5, 3, and 10) and late (subjects 6, 8, and 9) TT-specific responses. Parallel kinetics were observed in the responses against 2 unrelated antigens (PPD and C albicans [C alb]) for which pre-existing immunity was evident, but no responses were detected against control antigens (Ctrl 1 and Ctrl 2) with no evident pre-existing immunity. Proliferative activity was measured by 3H-thymidine incorporation after 6 days of in vitro culture and reported for each time point as the mean value of stimulation index (SI) in triplicate cultures ± SD.
Kinetics of vaccine-specific and bystander proliferative responses of Tmem cells following booster vaccination with TT. Six representative examples are shown. Vaccination (week 0) induces early (subjects 5, 3, and 10) and late (subjects 6, 8, and 9) TT-specific responses. Parallel kinetics were observed in the responses against 2 unrelated antigens (PPD and C albicans [C alb]) for which pre-existing immunity was evident, but no responses were detected against control antigens (Ctrl 1 and Ctrl 2) with no evident pre-existing immunity. Proliferative activity was measured by 3H-thymidine incorporation after 6 days of in vitro culture and reported for each time point as the mean value of stimulation index (SI) in triplicate cultures ± SD.
Proliferative responses of the 12 subjects to the 3 antigens TT, PPD, and C albicans are shown in Figure 2 and Table 1. Due to the variable kinetics, the time of maximum response against TT varied from 1 to 12 weeks, and, in order to condense the data, responses against the other antigens are shown at this time. Responses have been summarized and graded (+ to +++) in terms of fold increases over pre-existing levels, as defined in “Patients, materials, and methods” (Table 1). Although this grading documents the statistically significant changes occurring, it minimizes the apparent responses of subjects, such as case 10, with a high initial level (SI ± SD = 57.9 ± 4) that clearly increased to an SI equal to 92.2 ± 1.8 (Figure 2). In contrast, it maximizes the apparent responses of subjects, such as case 4, with a very low pre-existing response (SI equal to 2.2 ± 0.3) but with a clear increase (SI = 9.9 ± 1.0). Overall, 12 of 12 subjects showed significantly increased responses to the TT vaccine, and the majority (11/12) had increased responses to at least one of the other nonvaccine antigens. For example, cases 2, 5, and 11, with strong responses to TT and pre-existing immunity to PPD and C albicans, showed high bystander effects. The single subject (case 1) who failed to expand the pre-existing response to PPD or C albicans had responded relatively weakly to the TT vaccine (Figure 2; Table 1). Seven of 12 subjects responded to all 3 antigens.
Summary of proliferative responses against PPD and C albicans at the week of maximal TT response
Subject . | Wk . | TT . | PPD . | C albicans . |
|---|---|---|---|---|
| 1 | 8 | + | = | = |
| 2 | 1 | +++ | ++ | ++ |
| 3 | 2 | ++ | + | + |
| 4 | 2 | +++ | + | +++ |
| 5 | 2 | +++ | ++ | +++ |
| 6 | 8 | ++ | ++ | ++ |
| 7 | 2 | +++ | = | + |
| 8 | 12 | ++ | ++ | - |
| 9 | 8 | ++ | ++ | = |
| 10 | 4 | + | + | = |
| 11 | 8 | ++ | ++ | ++ |
| 12 | 1 | + | + | + |
Subject . | Wk . | TT . | PPD . | C albicans . |
|---|---|---|---|---|
| 1 | 8 | + | = | = |
| 2 | 1 | +++ | ++ | ++ |
| 3 | 2 | ++ | + | + |
| 4 | 2 | +++ | + | +++ |
| 5 | 2 | +++ | ++ | +++ |
| 6 | 8 | ++ | ++ | ++ |
| 7 | 2 | +++ | = | + |
| 8 | 12 | ++ | ++ | - |
| 9 | 8 | ++ | ++ | = |
| 10 | 4 | + | + | = |
| 11 | 8 | ++ | ++ | ++ |
| 12 | 1 | + | + | + |
The week of maximal TT response is reported for each individual. Symbols indicating no response (-), stable (=), or different degrees (+ to +++) of a positive response were given according to the criteria stated in “Proliferative responses.”
Lack of cross-reactivity among the antigens
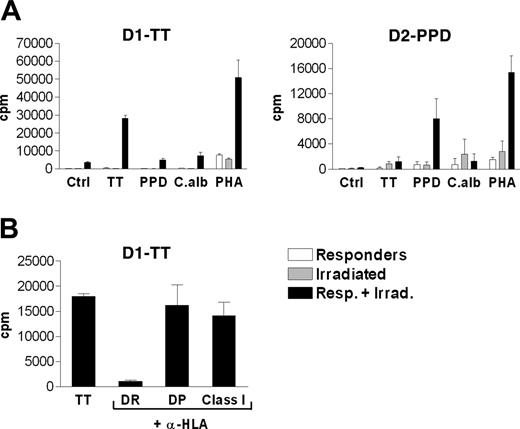
To exclude the possibility that the observed proliferative responses against the unrelated antigens PPD and C albicans were due simply to cross-reactivity, 2 antigen-specific T-cell lines, D1-TT and D2-PPD, were generated by repeated stimulation in vitro of PBMNCs with TT and PPD, respectively. Antigen specificity was then tested in a short-term proliferative assay. As shown in Figure 3A, the D1-TT cell line specifically responded to TT but not to PPD or C albicans, whereas the D2-PPD cell line specifically responded to PPD but not to TT or C albicans. Both T-cell lines strongly proliferated in the presence of PHA used as positive control. Taken together these data indicate lack of cross-reactivity among the antigens used in the present study.
Vaccine-specific and bystander proliferative responses of Tmem cells in 12 individuals following booster vaccination with TT. Responses are shown at the week of maximal TT response and are compared with the prevaccine (week 0) response. Proliferative activity was measured by 3H-thymidine incorporation after 6 days of in vitro culture and is reported as the mean value of stimulation index (SI) in triplicate cultures ± SD.
Vaccine-specific and bystander proliferative responses of Tmem cells in 12 individuals following booster vaccination with TT. Responses are shown at the week of maximal TT response and are compared with the prevaccine (week 0) response. Proliferative activity was measured by 3H-thymidine incorporation after 6 days of in vitro culture and is reported as the mean value of stimulation index (SI) in triplicate cultures ± SD.
Nature of the responding T cells
To identify the restricting HLA element and the phenotype of the responding cells, the proliferation assay was repeated for D1-TT in the presence of monoclonal antibodies (mAbs) directed against human major histocompatibility complex (MHC) antigens (Figure 3B). L243 and B7/21.2 recognize class II molecules HLA-DR and HLA-DP, respectively. W6/32 recognizes HLA class I molecules. The proliferative response to TT was strongly inhibited (> 95%) only in the presence of L243. This identifies HLA-DR as the restricting class II element involved in the presentation of antigen and CD4+ helper T cells as the population of lymphocytes responding in vitro to TT.
Vaccine-specific and bystander cytokine production by Thmem cells following booster vaccination with TT
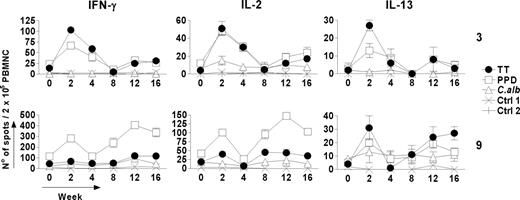
The effect of vaccination with TT on the ability of the T-cell population to produce cytokines in response to antigen in vitro also revealed complex dynamics. Kinetic data from 2 representative subjects (cases 3 and 9) showed parallel induction of IFNγ, IL-2, and IL-13 (Figure 4). IFNγ and IL-2 are generally considered to indicate Th1 responses, whereas IL-13 mainly reflects Th2 activation.18 In preliminary experiments, we confirmed that production of IL-13 correlated closely with that of IL-5, a classic Th2-associated cytokine (data not shown). However, the ELISPOT for IL-13 showed higher sensitivity and was therefore used. Production of all 3 cytokines in response to TT is consistent with a mixed Th1 and Th2 response (Figure 4). Cytokine production generally mirrored proliferative responses (Figure 1), with early and late peaks. However, in case 9, there was more evidence of cytokine-producing T cells at week 2, without a significantly increased proliferative response above the relatively high pre-existing levels.
Specificity of T-cell lines established against TT or PPD. (A) Lack of cross-reactivity among TT, PPD, and C albicans antigens. To exclude cross-reactivity among the antigens used, 2 different T-cell lines (D1-TT and D2-PPD) were generated from PBMNCs of 2 healthy donors by repeated cycles of in vitro stimulation with TT and PPD, respectively, as described in “Generation of antigen-specific T-cell lines.” Their antigen specificity was then tested in a 3-day in vitro proliferative assay. Proliferative activity was measured by 3H-thymidine incorporation and is reported as counts per minute (cpm) in triplicate cultures ± SD. (B) Identification of HLA-DR as the restricting MHC molecule of the T-cell lines. To identify the restricting HLA isotype of the T-cell lines, antigen presentation by autologous antigen-presenting cells was assayed by repeating the proliferative assay after addition of mAbs against HLA-DR (L243), HLA-DP (B7/21.2), or HLA class I (W6/32) at the beginning of the culture time. The proliferative response of the D1-TT to TT was strongly inhibited only when the HLA-DR–specific mAb L243 was added to the culture.
Specificity of T-cell lines established against TT or PPD. (A) Lack of cross-reactivity among TT, PPD, and C albicans antigens. To exclude cross-reactivity among the antigens used, 2 different T-cell lines (D1-TT and D2-PPD) were generated from PBMNCs of 2 healthy donors by repeated cycles of in vitro stimulation with TT and PPD, respectively, as described in “Generation of antigen-specific T-cell lines.” Their antigen specificity was then tested in a 3-day in vitro proliferative assay. Proliferative activity was measured by 3H-thymidine incorporation and is reported as counts per minute (cpm) in triplicate cultures ± SD. (B) Identification of HLA-DR as the restricting MHC molecule of the T-cell lines. To identify the restricting HLA isotype of the T-cell lines, antigen presentation by autologous antigen-presenting cells was assayed by repeating the proliferative assay after addition of mAbs against HLA-DR (L243), HLA-DP (B7/21.2), or HLA class I (W6/32) at the beginning of the culture time. The proliferative response of the D1-TT to TT was strongly inhibited only when the HLA-DR–specific mAb L243 was added to the culture.
Kinetics of vaccine-specific and bystander cytokine production by Thmem cells following booster vaccination (week 0) with TT. Two representative examples are shown. Characteristic Th1 (IFNγ and IL-2) and Th2 (IL-13) cytokines are produced following in vitro restimulation with TT and detected by ELISPOT assay. Parallel changes in the number of cytokine-producing cells are seen against PPD and (to a lesser extent) against C albicans but not against Ctrl1 or Ctrl2. Data for each time point represent the mean number of spots ± SD of triplicate wells of antigen-stimulated cultures after subtracting the mean number of spots in the corresponding negative control. The mean number of spots in the controls was always less than 5 spots per 2 × 105 PBMNCs.
Kinetics of vaccine-specific and bystander cytokine production by Thmem cells following booster vaccination (week 0) with TT. Two representative examples are shown. Characteristic Th1 (IFNγ and IL-2) and Th2 (IL-13) cytokines are produced following in vitro restimulation with TT and detected by ELISPOT assay. Parallel changes in the number of cytokine-producing cells are seen against PPD and (to a lesser extent) against C albicans but not against Ctrl1 or Ctrl2. Data for each time point represent the mean number of spots ± SD of triplicate wells of antigen-stimulated cultures after subtracting the mean number of spots in the corresponding negative control. The mean number of spots in the controls was always less than 5 spots per 2 × 105 PBMNCs.
For both cases, bystander cytokine production against PPD was also observed, with a pattern similar to the proliferative responses (Figures 1, 2 and 4; Table 1). PPD induced high IFNγ levels, as reported previously,19 but also IL-13, indicating a mixed Th1/Th2 response. Cytokine production against C albicans in these subjects showed less consistent changes, reflecting the more modest proliferative responses (Figure 1). However, a significantly increased IL-2 response was detected in case 3 at 2 weeks after vaccination, the time of maximum response to TT (Figures 1, 2 and 4; Table 1). In case 9, a significant IFNγ response was observed against C albicans at week 12, again in parallel with the high responses against TT and PPD (Figure 4).
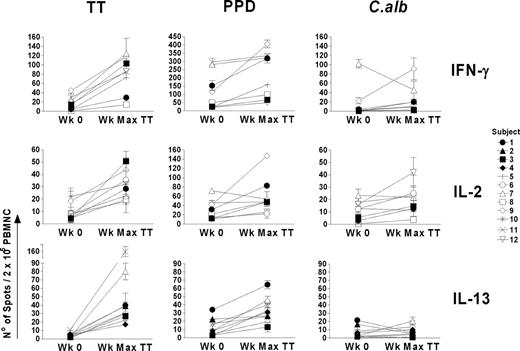
Cytokine profiles against PPD and C albicans for all subjects showing a response to TT were measured at the point of maximum response to TT, which generally matched that of the proliferative response. Data are shown in Figure 5 as the number of spots/2 × 105 cells. In Table 2 responses have been summarized and graded (+ to +++) in terms of fold increases over prevaccine levels, following the same criteria applied for the evaluation of proliferative responses, and the week of maximum response to TT is reported for each cytokine. The pattern of cytokine production against PPD tended to mirror that against TT, with production of IFNγ, IL-2, and IL-13 (Figure 5; Table 2). Cytokine responses against C albicans were less common, probably due to a lower degree of pre-existing immunity. For example, subject 8, with no apparent pre-existing or post-TT vaccination proliferative response to C albicans (Figure 1), failed to produce IL-2, whereas this was readily produced in response to TT and PPD (Figure 5; Table 2). The largest increase in the IFNγ response against C albicans was in subject 9 at week 12 (Figures 4, 5; Table 2), which paralleled responses to TT and PPD. However, in contrast to TT and PPD, there was no detectable increase in IL-2– and IL-13–producing T cells against C albicans at this time point (Figure 5; Table 2).
Summary of cytokine responses against PPD and C albicans at the week of maximal TT response
Subject and cytokine . | Wk . | TT . | PPD . | C albicans . |
|---|---|---|---|---|
| 1 | ||||
| IFNγ | 2 | +++ | ++ | +++ |
| IL-2 | 2 | +++ | ++ | +++ |
| IL-13 | 2 | +++ | ++ | - |
| 2 | ||||
| IFNγ | NA | = | NA | NA |
| IL-2 | NA | - | NA | NA |
| IL-13 | 2 | +++ | = | - |
| 3 | ||||
| IFNγ | 2 | +++ | ++ | - |
| IL-2 | 2 | +++ | +++ | ++ |
| IL-13 | 2 | +++ | +++ | - |
| 4 | ||||
| IFNγ | NA | - | NA | NA |
| IL-2 | NA | - | NA | NA |
| IL-13 | 2 | ++ | ++ | = |
| 5 | ||||
| IFNγ | 2 | +++ | +++ | - |
| IL-2 | NA | ND | ND | ND |
| IL-13 | 2 | +++ | +++ | - |
| 6 | ||||
| IFNγ | NA | - | NA | NA |
| IL-2 | 8 | +++ | = | ++ |
| IL-13 | NA | - | NA | NA |
| 7 | ||||
| IFNγ | 9 | +++ | + | - |
| IL-2 | 2 | ++ | - | = |
| IL-13 | 4 | +++ | +++ | +++ |
| 8 | ||||
| IFNγ | 1 | + | + | - |
| IL-2 | 12 | +++ | ++ | - |
| IL-13 | NA | - | NA | NA |
| 9 | ||||
| IFNγ | 12 | ++ | ++ | +++ |
| IL-2 | 12 | ++ | ++ | = |
| IL-13 | 2 | +++ | +++ | = |
| 10 | ||||
| IFNγ | NA | - | NA | NA |
| IL-2 | 2 | + | = | = |
| IL-13 | NA | - | NA | NA |
| 11 | ||||
| IFNγ | 1 | ++ | + | +++ |
| IL-2 | NA | ND | ND | ND |
| IL-13 | 1 | +++ | = | - |
| 12 | ||||
| IFNγ | 1 | +++ | ++ | - |
| IL-2 | NA | = | NA | NA |
| IL-13 | 1 | +++ | ++ | - |
Subject and cytokine . | Wk . | TT . | PPD . | C albicans . |
|---|---|---|---|---|
| 1 | ||||
| IFNγ | 2 | +++ | ++ | +++ |
| IL-2 | 2 | +++ | ++ | +++ |
| IL-13 | 2 | +++ | ++ | - |
| 2 | ||||
| IFNγ | NA | = | NA | NA |
| IL-2 | NA | - | NA | NA |
| IL-13 | 2 | +++ | = | - |
| 3 | ||||
| IFNγ | 2 | +++ | ++ | - |
| IL-2 | 2 | +++ | +++ | ++ |
| IL-13 | 2 | +++ | +++ | - |
| 4 | ||||
| IFNγ | NA | - | NA | NA |
| IL-2 | NA | - | NA | NA |
| IL-13 | 2 | ++ | ++ | = |
| 5 | ||||
| IFNγ | 2 | +++ | +++ | - |
| IL-2 | NA | ND | ND | ND |
| IL-13 | 2 | +++ | +++ | - |
| 6 | ||||
| IFNγ | NA | - | NA | NA |
| IL-2 | 8 | +++ | = | ++ |
| IL-13 | NA | - | NA | NA |
| 7 | ||||
| IFNγ | 9 | +++ | + | - |
| IL-2 | 2 | ++ | - | = |
| IL-13 | 4 | +++ | +++ | +++ |
| 8 | ||||
| IFNγ | 1 | + | + | - |
| IL-2 | 12 | +++ | ++ | - |
| IL-13 | NA | - | NA | NA |
| 9 | ||||
| IFNγ | 12 | ++ | ++ | +++ |
| IL-2 | 12 | ++ | ++ | = |
| IL-13 | 2 | +++ | +++ | = |
| 10 | ||||
| IFNγ | NA | - | NA | NA |
| IL-2 | 2 | + | = | = |
| IL-13 | NA | - | NA | NA |
| 11 | ||||
| IFNγ | 1 | ++ | + | +++ |
| IL-2 | NA | ND | ND | ND |
| IL-13 | 1 | +++ | = | - |
| 12 | ||||
| IFNγ | 1 | +++ | ++ | - |
| IL-2 | NA | = | NA | NA |
| IL-13 | 1 | +++ | ++ | - |
The week of maximal TT response is reported for each individual and for each cytokine. Symbols indicating no response (-), stable (=), or different degrees (+ to +++) of a positive response were given according to the criteria stated in “Proliferative responses.”
NA, indicates not available. ND, not done.
Vaccine-specific and bystander cytokine production in 12 individuals following booster vaccination with TT. Responses are shown at the week of maximal TT (Wk Max TT) response and are compared with the prevaccine (Wk 0) response. Cytokine responses were measured by ELISPOT assay after 40 hours of in vitro culture and are reported as the mean number of spots ± SD of triplicate wells of antigen-stimulated cultures after subtracting the corresponding negative control. The mean numbers of spots in the controls were always less than 5 spots per 2 × 105 PBMNCs.
Vaccine-specific and bystander cytokine production in 12 individuals following booster vaccination with TT. Responses are shown at the week of maximal TT (Wk Max TT) response and are compared with the prevaccine (Wk 0) response. Cytokine responses were measured by ELISPOT assay after 40 hours of in vitro culture and are reported as the mean number of spots ± SD of triplicate wells of antigen-stimulated cultures after subtracting the corresponding negative control. The mean numbers of spots in the controls were always less than 5 spots per 2 × 105 PBMNCs.
One question is whether the increased number of cytokine-producing cells observed in postvaccine samples after 40-hour incubation in vitro with PPD or C albicans was by T cells expanded in vivo. An alternative explanation might be that Thmem cells against bystander antigens might not have divided in vivo but could have been rendered more sensitive to specific antigenic proliferative signals in vitro. To investigate this, we used CFSE labeling20 to track cell division occurring in vitro in CD3+CD4+ T cells from the blood of case 3 at day 0 and 2 weeks after TT injection (the time of maximum response against TT; Figures 4, 5; Table 2). We found that cell division did not occur during incubation with antigen for 40 hours in vitro, up to the point where cytokine production was measured (data not shown). The number of cytokine-producing cells, as measured by ELISPOT, therefore reflects cells already existing in vivo. Booster vaccination has apparently increased functional antigen-specific and bystander Tmem cells in vivo, able to respond in vitro to TT or to PPD/C albicans, respectively, by proliferation and cytokine production.
To investigate any relationship between the observed kinetic pattern in the antigen-specific responses and possible changes in the number and/or phenotype of immunologic relevant cell subsets in the peripheral blood, a detailed phenotypic analysis was carried out at each time point in one individual (case 12) and analyzed in parallel with IFNγ, IL-2, and IL-13 antigen-specific responses. Results are shown (Supplemental Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article) up to week 4 (the remaining time points are not shown for clarity). While a clear expansion in the number of TT-specific and bystander Tmem cells measured by ELISPOT is visible at week 1, followed by a down-regulation to prevaccine levels at week 2 (Figure S1A), a parallel remarkable stability was found in the number and phenotype of all the analyzed cell subsets (Figure S1B). This analysis points to no gross changes in relative proportions, composition, or activation status of cell populations during vaccination. An analysis of phenotypic changes in activation markers within selected populations of TCM,TEM, and effector cells using multi-color flow cytometry might be more revealing and is now planned on a new cohort of vaccinees.
Antibody responses
Measurement of the antibody responses against TT showed the expected boost after vaccination, with a maximum at or near week 2. Levels were variable but all subjects showed a significant increase to a plateau followed by a very slow decline over 20 weeks (Figure 6), confirming other studies.12,17 The most dramatic change was observed in 2 individuals (7 and 11) showing prevaccination TT-specific antibody titers below the level of 0.1 IU/mL, known to be required for full protection (fold increase at wk 2 = 162 and 135, respectively). Interestingly, the same individuals had shown the strongest IL-13 responses in the ELISPOT assay (Figure 5; Table 2). In contrast to TT, there was no significant change in antibody levels against PPD or C albicans, which remained remarkably stable. These findings indicate a dependence of antibody formation by B cells on specific antigen and argue for restriction of a functional bystander effect to T cells.
Discussion
The control of memory T cells has obvious implications for natural and vaccine-induced immunity. We have focused on Thmem cells known to be the population responding to TT and to PPD in vitro.21,22 Our data here support the view that human Thmem cells are susceptible in vivo to stimulation from surrounding Thmem cells that are responding to a specific antigen. This bystander response has parallel kinetics to the specific response and is likely to be mediated by cytokines. In this respect the immune system uses Thmem cells to add an innate “infectious” element to the specific response. Although there is a tempting parallel with infectious tolerance,23 this cannot be simply drawn. Infectious tolerance has been studied mainly at the point of priming and appears to require presentation of both specific and bystander antigens by the same dendritic cells (DCs).24 For Thmem-cell stimulation the bystander antigens apparently need not be present.
Kinetics of antigen-specific antibody responses as measured by ELISA in 12 individuals following booster vaccination (week 0) with TT. A boost in the levels of TT-specific serum IgG was evident in all the vaccinated subjects, with a maximum at the indicated week, but no significant changes were detected in either PPD- or C albicans–specific antibody responses. Data for each time point represent the mean value of at least 4 replicate wells. SD bars are not shown for clarity. Positive symbols indicate different degrees of positive responses analyzed at the week of maximal TT response (indicated) compared with the prevaccine (week 0) response and were given according to the criteria stated in “Proliferative responses.”
Kinetics of antigen-specific antibody responses as measured by ELISA in 12 individuals following booster vaccination (week 0) with TT. A boost in the levels of TT-specific serum IgG was evident in all the vaccinated subjects, with a maximum at the indicated week, but no significant changes were detected in either PPD- or C albicans–specific antibody responses. Data for each time point represent the mean value of at least 4 replicate wells. SD bars are not shown for clarity. Positive symbols indicate different degrees of positive responses analyzed at the week of maximal TT response (indicated) compared with the prevaccine (week 0) response and were given according to the criteria stated in “Proliferative responses.”
In vitro proliferative responses against TT and PPD were detected at various degrees in all subjects prior to vaccination with TT, indicating pre-existing immunity probably generated by the prophylactic TT and BCG vaccinations that all the individuals had received. Pre-existing immunity against C albicans is more difficult to assess as it would depend on natural exposure, and initial proliferative responses were more heterogeneous (Figure 2; Table 1).
In terms of immune protection, the ability of the wider bystander population to secrete proinflammatory cytokines at the site of infection would enhance the effectiveness of the specific response. Clearly it is now necessary to dissect the CM and EM components of these Thmem-cell populations in human subjects and model it in mice to allow more definitive analysis.
The ability of human CD4+ Tmem cells to respond directly to cytokines has been suspected for some time from in vitro data.9 However, the focus in mouse models has been mainly on CD8+ Tmem cells. The latter are clearly susceptible to cytokine stimulation, with the main mediators being IL-15 and IL-7.25,26 In fact, “bystander” activation of human CD8+ T cells has been suggested in a recent study of responses to 2 different latent herpes viruses where contemporaneous cofluctuations of virus-specific CD8+ T cells were observed without perturbation of the total number or phenotype of CD3+ T cells.27 This stability in the CD3+ T-cell population seen during spontaneous activation of immunity against latent herpes viruses was also evident in our study of the effects of vaccination (Figure S1).
In contrast to CD8+ T cells, it has been proposed that CD4+ Tmem cells do not need cytokine signals for survival or expansion.28 However, CD4+ Tmem cells do express IL-7 receptors, and it has been reported that they are regulated by signaling via IL-7, with an overlapping influence of T-cell–receptor (TCR) signals.29 Dependence on IL-7 for generation and survival of transgenic CD4+ Tmem cells has also been observed,30 with evidence for involvement in the transition from effector to memory T cells.31
A close parallel to our observations in human subjects was described recently in a mouse model where infection with Leishmania donovani led to expansion of adoptively transferred transgenic ovalbumin (OVA)–specific memory CD4+ T cells.32 In this case, OVA antigen was required, possibly to maintain the transferred cells, whereas in our case it is unlikely, but not impossible, that PPD or C albicans antigens or cross-reactive antigens persist in vivo. While definitive data on generation and survival of Tmem cells can be obtained from transgenic and knockout mice, it is difficult to mimic the situation in human subjects who have a normal Tmem-cell repertoire under continuous exposure to environmental antigens. Perhaps in this case, data gleaned from human subjects, although more difficult to manipulate, can be illuminating.
Our data on the behavior of human Thmem cells tie together several previous observations on T-cell responses following vaccination with TT,33 including the fact that transient expansion of TT-specific T cells occurred during a flulike illness.21 It presents a problem for evaluation of specific memory responses to vaccines, since cells could be expanding simply as bystanders. It will be necessary next to determine if there are phenotypic or functional features that distinguish the T-cell population responding to specific antigen from the bystander population by analyzing isolated subpopulations. One particular question will be the balance between TEM and TCM cells in the 2 responses.
While the bystander Thmem cells can secrete cytokines and could potentially influence B cells, no increase in bystander antibody was evident. This would appear to argue against the proposal, again derived from subjects vaccinated with TT, that antigen-independent serologic memory can be maintained by bystander activation of memory B cells.17 Our data are more consistent with a study on the effects of a booster injection of diphtheria vaccine on diphtheria- and tetanus toxoid–specific B-cell responses, which showed a rise in the antibody response only against the immunizing antigen.34
However, it remains possible that activation of B cells is occurring, which could contribute to survival, but that no significant antibody results. Our data indicate that B cells are dependent on engagement of antigen by the B-cell receptor before they can receive the help apparently on offer from stimulated Thmem cells. Without this control, the antibody response would perhaps be too easily induced, with possible autoimmune consequences.
Prepublished online as Blood First Edition Paper, December 8, 2005; DOI 10.1182/blood-2005-08-3255.
Supported by the Leukaemia Research Fund UK and Tenovus UK.
G.D.G. designed and performed research, analyzed data, and cowrote the paper; J.R. and F.M. performed research; and F.K.S. designed research, analyzed data, and cowrote the paper.
The online version of this article contains a data supplement The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 1. Kinetics of vaccine-specific and bystander proliferative responses of Tmem cells following booster vaccination with TT. Six representative examples are shown. Vaccination (week 0) induces early (subjects 5, 3, and 10) and late (subjects 6, 8, and 9) TT-specific responses. Parallel kinetics were observed in the responses against 2 unrelated antigens (PPD and C albicans [C alb]) for which pre-existing immunity was evident, but no responses were detected against control antigens (Ctrl 1 and Ctrl 2) with no evident pre-existing immunity. Proliferative activity was measured by 3H-thymidine incorporation after 6 days of in vitro culture and reported for each time point as the mean value of stimulation index (SI) in triplicate cultures ± SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/7/10.1182_blood-2005-08-3255/5/m_zh80070693450001.jpeg?Expires=1767770351&Signature=TWaSLSKZ3IPR8AoLEFJVtjMDPJ34GGoI55vLU0crfFAYdKydTzmCcVkzVplnenoipTdt0oR8KWuqAmrgcujfDQJ51GE4uy1s6oRGtPfj8QqoKUPGqnVhkRxXSp29ySEX1tgtncMxgMMcpJKpoeDDzi5d7swC1Trtw-8Cdj8cdAIN9z89T2zw0lmfunN18za9cjZgQWvtHpdhKcnWfyG8IQ~i~cs0jEN3SqsMdMFKpedvZXQ8lLlAYGr5tUcBNhHxcy99O~8lBk7z~09RzxwdZETs7WtOj8exGexSRfDq8iF8og9KH2oGJy1UYsv3erk8BwYBfVOgUbD4DHcVVpgNtg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal