Comment on Ting–De Ravin et al, page 3091
Dogs with severe combined immunodeficiency (SCID) were cured with intravenous neonatal injection of a retroviral vector expressing the common γ chain. This simple procedure for transducing hematopoietic stem cells in vivo might also treat other blood disorders.
Gene therapy could treat a variety of disorders that involve blood cells. Most hematopoietic stem cell (HSC)–directed gene therapy has involved an ex vivo approach in which HSCs obtained from blood, bone marrow (BM), or cord blood are transduced in culture, and modified cells are returned to the body after BM ablation. BM ablation is not necessary for diseases such as X-linked severe combined immunodeficiency (SCID) due to a deficiency of the common γ chain for cytokine receptors, where the modified cells have a selective advantage. Although ex vivo HSC-directed gene therapy has resulted in correction of hematopoietic disorders in animals and humans, there are several disadvantages: (1) obtaining HSCs from BM or blood requires a medical procedure; (2) the isolation of stem cells from blood or marrow and the in vitro culture have the potential for contamination with infectious agents; and (3) the in vitro culture with cytokines may select for insertions that promote immortalization1 or may alter the potential for cells to engraft.2 Identification of an in vivo gene therapy approach would make it easier to perform gene therapy outside of specialized medical centers and might reduce the risks of the procedure.FIG1
Longitudinal analysis of T-cell development and persistence of gene marking in different blood cell lineages. See the complete figure in the article beginning on page 3091.
Longitudinal analysis of T-cell development and persistence of gene marking in different blood cell lineages. See the complete figure in the article beginning on page 3091.
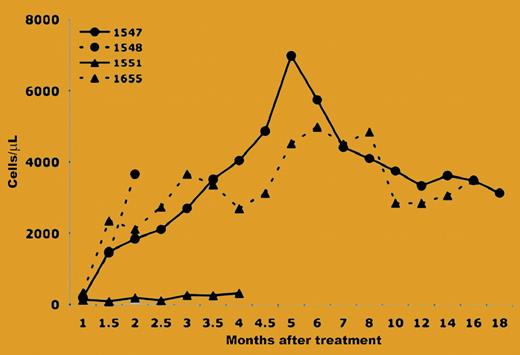
The paper from Ting–De Ravin and colleagues demonstrated that X-linked SCID could be corrected with a simple intravenous injection of a retroviral vector (RV) expressing the common γ chain within 3 days after birth. It was previously demonstrated that intravenous injection of an amphotropic RV shortly after birth resulted in transduction of approximately 1% blood cells, and transduced cells were maintained at stable levels for up to 4 years.3 This transduction of HSCs may be related to the fact that hematopoiesis occurs in the liver in newborn dogs, and the liver has direct contact with blood. The study by Ting–De Ravin et al took advantage of this phenomenon by injecting an RD114-pseudotyped RV that expressed both the common γ chain and the green fluorescence protein (GFP) into newborn dogs with X-linked SCID. They found that 3 of 4 dogs achieved normal levels (> 750 cells/mL) of T cells within 1.5 months after gene transfer (see the figure), and more than 90% of the T cells expressed GFP. The T cells were maintained at normal levels for up to 1.5 years and led to reconstitution of immune responses. Although this result is very exciting, it is unlikely that neonatal intravenous injection of an RV will be effective for disorders that require that more than 1% of cells be modified, unless the therapeutic gene confers a selective advantage. However, it is possible that an in vivo selection strategy could be applied transiently around the time of gene transfer to achieve a high level of transduction of HSCs, and that this level could be maintained over time.
Although X-linked SCID was the first disease for which gene therapy was clearly curative in humans,4 leukemias have developed at about 3 years after transduction in 3 patients; 2 of these leukemias involved integration near the LMO2 locus, which is a known T-cell oncogene.5 In contrast, leukemias did not develop in X-linked SCID mice that received gene therapy, which may be due in part to their shorter lifespan. These RV-treated dogs should represent a very important model in which to determine how often insertional mutagenesis causes an adverse event and to test vectors that have been modified to reduce this risk. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal