Abstract
It is a longstanding question which bone marrow–derived cell seeds the thymus and to what level this cell is committed to the T-cell lineage. We sought to elucidate this issue by examining gene expression, lineage potential, and self-renewal capacity of the 2 most immature subsets in the human thymus, namely CD34+CD1a– and CD34+CD1a+ thymocytes. DNA microarrays revealed the presence of several myeloid and erythroid transcripts in CD34+CD1a– thymocytes but not in CD34+CD1a+ thymocytes. Lineage potential of both subpopulations was assessed using in vitro colony assays, bone marrow stroma cultures, and in vivo transplantation into nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice. The CD34+CD1a– subset contained progenitors with lymphoid (both T and B), myeloid, and erythroid lineage potential. Remarkably, development of CD34+CD1a– thymocytes toward the T-cell lineage, as shown by T-cell receptor δ gene rearrangements, could be reversed into a myeloid-cell fate. In contrast, the CD34+CD1a+ cells yielded only T-cell progenitors, demonstrating their irreversible commitment to the T-cell lineage. Both CD34+CD1a– and CD34+CD1a+ thymocytes failed to repopulate NOD/SCID mice. We conclude that the human thymus is seeded by multipotent progenitors with a much broader lineage potential than previously assumed. These cells resemble hematopoietic stem cells but, by analogy with murine thymocytes, apparently lack sufficient self-renewal capacity.
Introduction
The thymic microenvironment is exceptional in its ability to sustain production of T cells.1 However, hematopoietic stem cells (HSCs) that will eventually give rise to T cells are derived from the bone marrow (BM). The nature of the thymus-seeding cell and its relation to several BM progenitors has remained elusive, despite being the subject of intense investigation. Furthermore, it is controversial whether cells commit to the T-cell lineage prethymically or intrathymically.2,3
Aided by modern cell-sorting techniques, many studies in the mouse have recently readdressed these issues. Adult murine BM has been demonstrated to contain precursors with a restricted T-/B-lymphoid potential, so-called common lymphoid progenitors (CLPs).2,4 However, the earliest thymic immigrants were shown to differ from CLPs in several aspects.5 In peripheral blood, T-lineage potential appeared to be restricted to Lin–Sca-1+c-Kit+ (LSK) progenitor populations, rather than to CLPs.6 Recently, two detailed analyses of the earliest subpopulations in the murine thymus showed variable lineage potential of different subsets.7,8 Together, these studies point toward a model in which a range of BM-derived progenitors colonize the thymus, probably including multipotent progenitors and more lineage-restricted precursor cells.9 For the human system, comparable experimental data are lacking. Determining lineage potential of early thymocytes will help to unveil the identity of the thymus-seeding cell in humans.
In both humans and mice, the most immature cells in the thymus do not express CD4 and CD8 and therefore are called double negative (DN). In humans, the DN stage can be further subdivided by staining for CD34 and CD1.10,11 CD34 is a marker for hematopoietic stem cells and progenitors in all hematopoietic organs. Also in the thymus, the most immature cells are highly positive for CD34, and levels decline as the cells mature.10,11 Concomitant with the decrease of CD34, thymocytes acquire expression of CD1.12 This is usually detected by staining for CD1a, but CD1b, CD1c, CD1d, and CD1e are also expressed. This order of developmental steps is supported by the rearrangement status of the T-cell receptor (TCR) loci in the consecutive stages. The CD34+CD1a– cells start to rearrange their TCRD genes, but mostly have their TCRG and TCRB loci still in germ line position, whereas expression of CD1a is accompanied by rearrangements of TCRD (V to DJ), TCRG, and TCRB (D to J) loci.13 The immature single-positive (ISP) cells have lost CD34 expression and contain mature TCRB (V to DJ) rearrangements.13 The CD34+CD1a– and CD34+CD1a+ subsets comprise about 0.4% and 0.6% of all thymocytes, respectively.11
Because of their TCR gene rearrangement status, CD34+CD1a+ thymocytes are thought to be irreversibly committed to the T-cell lineage. Nevertheless, their potential to differentiate into other lineages has not been studied in detail. Lineage potential of CD34+CD1a– is generally thought to be less restricted, but the range of progeny they can generate is under debate. The capability of human CD34+ thymocytes to develop into natural killer (NK) cells and dendritic cells (DCs) has been demonstrated by several studies, in both fetal14,15 and adult15,16 thymus. B-cell precursor potential of CD34+CD1a– thymocytes has, to our knowledge, never been reported. However, we and others have shown the presence of significant numbers of B cells in the human thymus.11,17,18 Furthermore, in the thymus we could detect the presence of all BM B-cell progenitor stages, indicating that B cells develop in the thymus, albeit at much lower frequencies than in the BM.11
Myeloid potential of CD34+ thymocytes was investigated by a number of studies several years ago, yielding contradictory results. Although one study could generate myeloid colonies from CD34+ as well as from CD34– thymocytes,19 others could not confirm any myeloid potential in CD34+ thymocytes.20 De Yebenes et al21 demonstrated that myeloid DC precursors can be generated from CD34+ thymocytes in cultures containing M-CSF.
In this study, we investigated the potential of CD34+CD1a– and CD34+CD1a+ thymocytes to develop into all hematopoietic lineages, as well as their ability to reconstitute nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice.
Materials and methods
Thymus and umbilical cord blood material
Human thymus, umbilical cord blood (UCB), and BM material were obtained according to the informed consent guidelines of the Medical Ethical Committee of Erasmus MC, Rotterdam and approved by its institutional review board; consent was obtained in accordance with the Declaration of Helsinki. Thymus material was obtained from children requiring surgery for congenital heart disease. All children were between 8 days and 2 years of age and did not have hematologic or immunologic diseases. Thymocytes were isolated by disrupting the thymus on an iron filter and frozen until further use. After thawing, viable thymocytes were isolated using Ficoll density centrifugation.
BM material was obtained from remaining graft cells of BM transplantation procedures. UCB and BM mononuclear cells were isolated using Ficoll density centrifugation and frozen until further use. Greater than 90% of UCB mononuclear cells were viable after thawing.
Cell purification
For each sorting experiment, frozen thymocytes or UCB mononuclear cells from 3 to 5 different human donors were used. CD34+ progenitor cells were prepurified by AutoMACS (magnetic cell sorter; (Miltenyi Biotec, Bergisch Gladbach, Germany) before fluorescence-activated cell sorting (FACS). Cells were stained with CD1a-RD1 (Beckman Coulter, Fullerton, CA) and/or CD34-APC (BD Pharmingen, San Diego, CA) for 30 minutes on ice, washed, and sorted on a FACS Digital Vantage (DiVa) cell sorter (BD Biosciences, Mountain View, CA) (Figure 1).
BM mononuclear cells were not MACS prepurified but immediately stained with CD3-FITC, CD20-FITC, CD34-APC (all from BD Pharmingen), CD13-RD1, and CD33-RD1 (Beckman Coulter) and sorted on a DiVa cell sorter.
Microarray analysis
RNA was isolated from purified UCB and thymocyte subsets and processed to cDNA and cRNA according to standard methods for microarray analysis. Gene-expression profiles were generated using Affymetrix U133A microarrays (Affymetrix, Santa Clara, CA) and have been described previously.13
Flow cytometric validation of microarray results
For all flow cytometric analyses, CD34+ cells were purified using AutoMACS (Miltenyi Biotec). For surface markers, cells were stained with CD34-APC (BD Pharmingen), CD1a-RD1 (Beckman Coulter), CXCR4-FITC (R&D Systems, Minneapolis, MN), or CD13-RD1 and CD33-RD1 (Beckman Coulter) for 10 minutes at room temperature. For intracellular staining of MPO, cells were stained first with surface markers, then fixed and permeabilized using IntraPrep reagent (Beckman Coulter) and stained with MPO-FITC (DakoCytomation, Carpinteria, CA). Cells were analyzed using a FACSCalibur flow cytometer (BD Biosciences) and Cell Quest Pro software.
In vitro colony assays
Purified thymocyte and UCB subsets and chimeric mouse BM samples were assayed for the presence of human granulocyte-macrophage colony-forming units (CFU-GMs) and erythroid burst-forming units (BFU-Es) by in vitro colony formation in viscous methylcellulose culture medium as described previously.22 After 14 days of culture, the number of colonies was counted under an Olympus CKX41 microscope equipped with a 10 × ocular and a 10 ×/22 objective lens (Olympus, Tokyo, Japan). Images were captured using an Olympus C-5050 digital camera and Jpegger sofware version 4.73 (Vallen Systeme, Icking, Germany). Images were transferred to Adobe Photoshop 7.0 (Adobe Systems, San José, CA).
Real-time quantitative–polymerase chain reaction (RQ-PCR) of TCR gene rearrangements
DNA was isolated from cell samples using a GenElute mammalian genomic miniprep kit (Sigma, St Louis, MO). Gene rearrangements in the TCRD locus were quantitatively determined by using TaqMan-based RQ-PCR as described previously.13 Albumin DNA was determined in each sample to normalize for cDNA input. Nalm16 DNA was used as clonal control and reference sample in each experiment. For validation of microarray experiments, RQ-PCR was done for CD34, Flt3, CD1a, pTα, RAG1, BTK, and NF-E2.
Stroma cultures
OP9 cells (BM stromal-cell line; obtained from ATCC, Manassas, VA) that expressed either human Delta-like 1 (DL1) in combination with GFP (hereafter called OP9-DL1) or GFP alone (OP9-GFP) were generated by retroviral transduction. OP9-GFP and OP9-DL1 were grown in confluent layers in 96-well plates in αMEM (Cambrex, Walkersville, MD) containing 20% fetal calf serum. CD34+CD1a– and CD34+CD1a+ cells were sorted onto the OP9 layers using a FACS Digital Vantage (DiVa) cell sorter (BD Biosciences). CD34+CD1a– thymocytes were seeded on OP9-GFP at 1000, 300, 100, 30, 10, and 3 cells per well (48 wells per condition per experiment) and on OP9-DL1 at 1000, 300, 100, and 30 cells per well (12 wells per condition) or 100, 30, 10, and 3 cells per well (48 wells per condition) or 1 cell per well (192 wells). CD34+CD1a+ cells were seeded on OP9-GFP and OP9-DL1 at 1000 cells per well (12 wells). Cells were cultured for 14 days in the presence of 5 ng/mL human IL-7 and 25 ng/mL human SCF (both R&D Systems) and in 1 experiment with 80 ng/mL human GM-CSF (PeproTech, Rocky Hill, NJ).
Transplantations performed upon NOD/SCID mice
Ten- to 15-week-old NOD/LtSz-scid/scid (NOD/SCID) mice received a sublethal dose of 3.5 Gy total body irradiation. Mice were intravenously injected with 105 FACS-sorted CD34+ UCB cells or 106 (first experiment) or 5 × 106 (second experiment) sorted CD34+CD1a– or CD34+CD1a+ thymocytes in 250 μL PBS. In the first experiment, 5 mice per group were used. In the second experiment, 2 mice per thymocyte group were used, because of the difficulty of obtaining sufficient cell numbers. To prevent infections, mice received 100 mg/L ciprofloxacin (Bayer, Berkeley, CA) in their drinking water.
Thirty-five days after transplantation, the mice were killed, and femurs, spleen, and thymus were isolated. In each organ, total cell numbers were determined, and the percentage of human cells and their subset distribution was assayed by flow cytometry using the following antibodies: CD8-PE (Sanquin, Amsterdam, the Netherlands) and CD4-FITC, CD14-FITC, CD19-PE, CD3-PerCP, CD45-APC (all from BD Pharmingen). Cell samples of mice not receiving a transplant were stained as negative controls.
Results
CD34+CD1a– but not CD34+CD1a+ thymocytes express genes of the myeloid and erythroid/megakaryocyte lineages
Previously, gene-expression profiles have been generated of 8 thymocyte subsets corresponding to consecutive differentiation stages.13 We here studied the expression profiles of 2 populations in greater detail: the most immature CD34+CD1a– subset that at least retains NK and DC potential10 and the CD34+CD1a+ thymocytes that are presumed to be T-cell committed (Figure 1).
As expected, CD34+CD1a– cells expressed high levels of progenitor marker transcripts such as CD34 and CD133 (Figure 2A), reflecting their immature phenotype. In the CD34+CD1a+ population up-regulation of T-cell–specific transcripts (eg, CD3, PCTRA, and LAT) and genes involved in TCR rearrangements (eg, RAG, DNTT, sterile TCRB transcripts) occurred (Figure 2B). To our surprise, we found that concurrently transcription of many myeloid-associated genes, including myeloperoxidase (MPO), CD13, and CD33, was down-regulated during development from CD34+CD1a– to CD34+CD1a+ cells (Figure 2C). In addition, the gene for the erythrocyte/megakaryocyte-specific transcription factor NF-E2 (nuclear factor erythroid-derived 2) was transcribed at significantly lower levels in the CD34+CD1a+ cells (Figure 2C). Low-level expression of these lineage-specific genes presumably represents “promiscuous” expression that is associated with multipotency.23
Our microarray data were validated using quantitative reverse transcriptase (RQ)–PCR and flow cytometry. Differential expression of CD34, Flt3, CD1a, pTα, RAG1, BTK, and NF-E2 was confirmed (Figure 2D) at the mRNA level. At the protein level, the percentage of cells positive for CD133, MPO, and CD13/33 was higher in the CD34+CD1a– population than in the CD34+CD1a+ cells (Figure 2E).
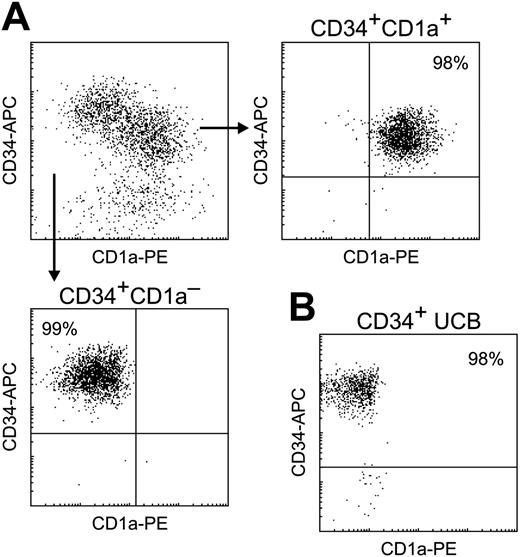
Purification of cell subsets. (A) Total CD34+ cells were isolated from human thymus using AutoMACS and subsequently FACS sorted into CD34+CD1a– (bottom) and CD34+CD1a+ (right) fractions. (B) CD34+ cells were isolated from human UCB using AutoMACS and FACS sorting. All populations were greater than 98% pure.
Purification of cell subsets. (A) Total CD34+ cells were isolated from human thymus using AutoMACS and subsequently FACS sorted into CD34+CD1a– (bottom) and CD34+CD1a+ (right) fractions. (B) CD34+ cells were isolated from human UCB using AutoMACS and FACS sorting. All populations were greater than 98% pure.
Gene expression of CD34+CD1a– and CD34+CD1a+ thymocytes. Expression levels (in arbitrary fluorescence units) of several genes were extracted from previously performed Affymetrix microarrays.13 (A) “Stem cell–like” genes, (B) T-cell–specific genes, (C) “non-T lineage” genes. (D) RQ-PCR validation: fold increase or decrease in expression between CD34+CD1a– and CD34+CD1a+ subsets. (E) Expression patterns of CD133, MPO, and CD13/CD33 were validated at the protein level using flow cytometry. CD34+ cells from thymus and UCB were purified using AutoMACS and gated for the indicated populations. Expression of CD13/33 in mature granulocytes was at least 10-fold higher than in CD34+ UCB cells.
Gene expression of CD34+CD1a– and CD34+CD1a+ thymocytes. Expression levels (in arbitrary fluorescence units) of several genes were extracted from previously performed Affymetrix microarrays.13 (A) “Stem cell–like” genes, (B) T-cell–specific genes, (C) “non-T lineage” genes. (D) RQ-PCR validation: fold increase or decrease in expression between CD34+CD1a– and CD34+CD1a+ subsets. (E) Expression patterns of CD133, MPO, and CD13/CD33 were validated at the protein level using flow cytometry. CD34+ cells from thymus and UCB were purified using AutoMACS and gated for the indicated populations. Expression of CD13/33 in mature granulocytes was at least 10-fold higher than in CD34+ UCB cells.
These data suggest that CD34+CD1a– thymocytes not only are immature and not yet committed to the T-cell lineage but may in addition still possess myeloid and erythroid potential.
CD34+CD1a– but not CD34+CD1a+ thymocytes have myeloid and erythroid potential in vitro
To study the myeloid and erythroid lineage potential of the 2 CD34+ thymocyte populations, cells were sorted and cultured in in vitro assays that allow outgrowth of either CFU-GMs or BFU-Es. As a positive control, CD34+ cells from human UCB were used. The CD34+CD1a+ thymocytes did not give rise to any colonies in our assays, but the CD34+CD1a– thymocytes yielded clearly detectable colonies, both CFU-GM and BFU-E (Table 1; Figure 3A-B). The efficiency of CD34+CD1a– thymocytes to yield myeloid colonies was about 30 times lower than that of CD34+ UCB cells, whereas erythroid potential was about 100 times less efficient (Table 1).
In vitro colony assays
Numbers of myeloid (CFU-GM) and erythroid (BFU-E) colonies calculated per 105 input cells. Means and standard errors of triplicate wells. Results are representative of three independent experiments.
In vitro colony assays. Sorted CD34+CD1a– and CD34+CD1a+ thymocytes and CD34+ UCB cells were plated in semisolid cultures containing appropriate cytokines to generate myeloid (CFU-GM) or erythroid (BFU-E) colonies. (A) Typical myeloid (left) and erythroid (right) colonies generated from CD34+CD1a– thymocytes. (B) Overview of BFU-E dishes in which 105 CD34+CD1a– or CD34+CD1a+ thymocytes or 103 CD34+ UCB cells were plated. No colonies were detected in dishes with CD34+CD1a+ thymocytes. (C) RQ-PCR analysis of immature TCRD gene rearrangements (Dδ2-Dδ3) in original CD34+CD1a– thymocytes, myeloid and erythroid colonies generated from CD34+CD1a– thymocytes, myeloid colonies generated from CD34+ UCB cells, and CD34+ cells from children's BM sorted into either CD13/33– (mostly B-cell progenitors) and CD13/33+ (myeloid progenitors) fractions. Percentages of rearranged alleles in each sample are shown.
In vitro colony assays. Sorted CD34+CD1a– and CD34+CD1a+ thymocytes and CD34+ UCB cells were plated in semisolid cultures containing appropriate cytokines to generate myeloid (CFU-GM) or erythroid (BFU-E) colonies. (A) Typical myeloid (left) and erythroid (right) colonies generated from CD34+CD1a– thymocytes. (B) Overview of BFU-E dishes in which 105 CD34+CD1a– or CD34+CD1a+ thymocytes or 103 CD34+ UCB cells were plated. No colonies were detected in dishes with CD34+CD1a+ thymocytes. (C) RQ-PCR analysis of immature TCRD gene rearrangements (Dδ2-Dδ3) in original CD34+CD1a– thymocytes, myeloid and erythroid colonies generated from CD34+CD1a– thymocytes, myeloid colonies generated from CD34+ UCB cells, and CD34+ cells from children's BM sorted into either CD13/33– (mostly B-cell progenitors) and CD13/33+ (myeloid progenitors) fractions. Percentages of rearranged alleles in each sample are shown.
Rearrangements of the TCRD locus were quantitatively determined in the acquired colonies. Although colonies derived from CD34+ UCB cells did not contain any rearranged TCRD genes, the most immature TCRD rearrangements (Dδ2-Dδ3) were present in about 10% of the alleles in the CD34+CD1a– thymocyte-derived colonies, both CFU-GM and BFU-E (Figure 3C). The proportion of Dδ2-Dδ3 found in the original CD34+CD1a– thymocytes was approximately 60%,13 whereas CD34+CD13/33+ myeloid progenitor cells from children's BM did not contain any TCRD rearrangements (Figure 3C). CD34+CD13/33– BM cells, which represent mainly B-cell precursors, did have (very low) levels of TCRD rearrangements (Figure 3C), consistent with earlier reports.24 We confirmed these data performing single-colony RQ-PCR on sorted CD34+lin– UCB and CD34+CD1a– thymocytes, forced to differentiate into myeloid colonies in CFU-GM. Because these colonies arise from single cells, we can draw conclusions about the fate of individual cells in the absence of a true single-cell assay. We detected TCRD rearrangements in 10% of the colonies (Table 2), in line with the percentages found by pooling all the colonies in bulk assays, indicating that at least some CD34+CD1a– thymocytes that had initiated TCRD rearrangement and therefore initiation of T-lineage commitment reversed their developmental decision to become myeloid and erythroid cells.
Dδ2-Dδ3 rearrangements in individual colonies
. | No. of colonies analyzed . | Dδ2-Dδ3+ colonies . | . | |
|---|---|---|---|---|
. | . | No. . | % . | |
| CFU-GMs from CD34+CD1a- thymocytes | 40 | 4 | 10 | |
| CFU-GMs from CD34+ UCB | 40 | 0 | 0 | |
. | No. of colonies analyzed . | Dδ2-Dδ3+ colonies . | . | |
|---|---|---|---|---|
. | . | No. . | % . | |
| CFU-GMs from CD34+CD1a- thymocytes | 40 | 4 | 10 | |
| CFU-GMs from CD34+ UCB | 40 | 0 | 0 | |
Individual colonies from CFU-GMs were picked, DNA was extracted, and RQ-PCR was done for albumin (to check presence and quality of DNA) and the Dδ2-Dδ3 rearrangements. In all colonies albumin PCR gave appositive signal, the Dδ2-Dδ3 rearrangements only in 4 of 40 colonies from CD34+CD1a- thymocytes.
Lineage capacity and frequency of CD34+CD1a– and CD34+CD1a+ thymocytes
To assess the ability of CD34+CD1a– and CD34+CD1a+ thymocytes to develop into different hematopoietic lineages in more detail, we performed cultures on OP9 BM stromal cells expressing either GFP alone (OP9-GFP) or Notch ligand Delta-like 1 (DL1) in combination with GFP (OP9-DL1). Culturing of CD34+ UCB cells on OP9-GFP has been previously shown to induce myeloid and B-lymphoid development, whereas OP9-DL1 allows the development of T cells.25
Both CD34+CD1a– and CD34+CD1a+ thymocytes efficiently developed into CD4+CD8+ double-positive cells when cultured for 14 days on OP9-DL1 (Figure 4A), although CD34+CD1a– cells yielded 4-fold higher cell numbers (Figure 4B), probably because they have higher proliferative capacity.
As was expected from the colony assays (Table 1), there were large differences between CD34+CD1a– and CD34+CD1a+ thymocytes when cultured on OP9-GFP. The CD34+CD1a– cells were able to proliferate on OP9 cells lacking DL1, although they yielded significantly lower cell numbers than when cultured with DL1 (Figure 4B). In contrast, CD34+CD1a+ cells did not grow on OP9-GFP (Figure 4B), illustrating their irreversible commitment to the T-cell lineage and thereby need for continuous Notch signaling.26
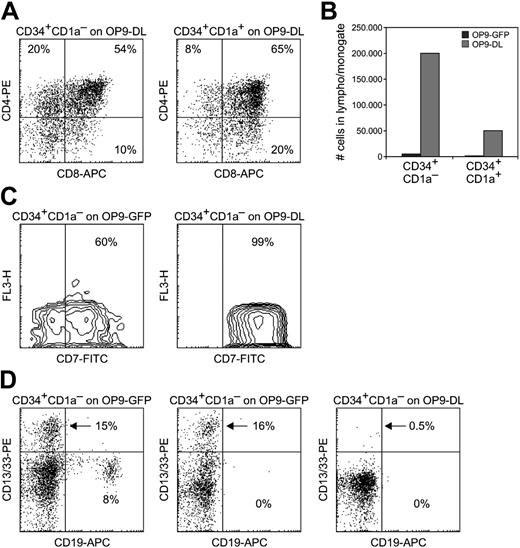
OP9 cocultures. (A) Development of CD4+CD8+ double-positive cells from CD34+CD1a– (left) and CD34+CD1a+ (right) thymocytes cultured for 14 days on OP9-DL1; 1000 cells per well were seeded. (B) Cell numbers per well after 14 days of culturing 1000 CD34+CD1a– or CD34+CD1a+ thymocytes on OP9-GFP or OP9-DL1. (C) CD7 staining of 300 CD34+CD1a– thymocytes cultured on OP9-GFP (left) or OP9-DL1 (right) for 14 days. (D) Presence of CD19+ B cells and/or CD13/33+ myeloid cells in 14-day cocultures of 300 CD34+CD1a– thymocytes on OP9-GFP (left and middle) or on OP9-DL1 (right).
OP9 cocultures. (A) Development of CD4+CD8+ double-positive cells from CD34+CD1a– (left) and CD34+CD1a+ (right) thymocytes cultured for 14 days on OP9-DL1; 1000 cells per well were seeded. (B) Cell numbers per well after 14 days of culturing 1000 CD34+CD1a– or CD34+CD1a+ thymocytes on OP9-GFP or OP9-DL1. (C) CD7 staining of 300 CD34+CD1a– thymocytes cultured on OP9-GFP (left) or OP9-DL1 (right) for 14 days. (D) Presence of CD19+ B cells and/or CD13/33+ myeloid cells in 14-day cocultures of 300 CD34+CD1a– thymocytes on OP9-GFP (left and middle) or on OP9-DL1 (right).
After culturing CD34+CD1a– thymocytes on OP9-GFP for 14 days, a subset of cells was found to be negative for CD7 (Figure 4C) and had developed into myeloid and B cells (Figure 4D). In some cultures both myeloid and B-lymphoid cells were detected, whereas in others cells from a single lineage were observed (Figure 4D). On the contrary when cultured on OP9-DL1, all CD34+CD1a– thymocytes developed into CD7+ cells (Figure 4C), and only minute percentages (< 1%) of cells representing non–T-cell lineages were detected (Figure 4D, right).
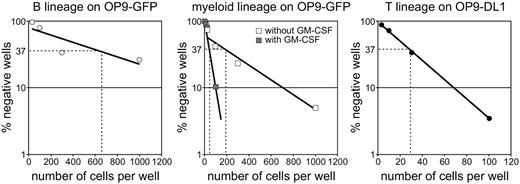
To determine the frequencies by which CD34+CD1a– thymocytes can develop into the different lineages, we performed limiting dilution cultures on OP9-GFP. The frequency of progenitors with B-lineage potential was approximately 1 in 640 and with myeloid potential approximately 1 in 160 (Figure 5). In the presence of GM-CSF, the frequency of myeloid progenitors was increased to 1 in 48 CD34+CD1a– thymocytes (Figure 5).
As a control, the progenitor frequency of CD34+CD1a– thymocytes that can give rise to T cells was determined by culturing cells on OP9-DL1 for 14 days and staining for CD4 and CD8. The T-cell progenitor frequency was found to be only 1 in 27 CD34+CD1a– thymocytes (Figure 5, right). A similar frequency was found when single cells were seeded on OP9-DL1: after 14 days, growing cells were found in 7 of 192 wells. Because it can be presumed that in an in vivo thymus environment the vast majority of CD34+CD1a– cells would develop into T cells, the OP9 system appears to be rather inefficient for culturing human cells.
CD34+CD1a– and CD34+CD1a+ thymocytes lack in vivo–repopulating capacity inherent to HSCs
The finding that CD34+CD1a– thymocytes have T-/B-lymphoid, myeloid, and erythroid developmental capacity, together with the previously demonstrated NK-cell and DC potential, raises the possibility that these cells behave either as very immature multilineage progenitors or as true HSCs. A hallmark of HSCs is self-renewal capacity, of which NOD/SCID-repopulating ability is the most commonly accepted read-out for the human system.27
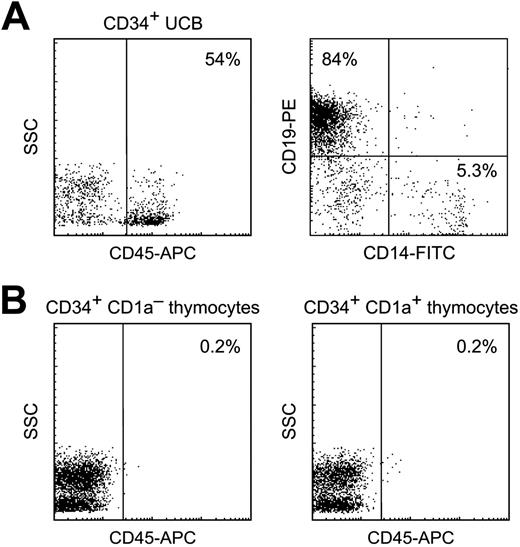
Consequently, we performed transplantations of CD34+CD1a– and CD34+CD1a+ thymocytes into sublethally irradiated immunodeficient NOD/SCID mice. As a control we transplanted 105 sorted CD34+ UCB cells and showed that these efficiently repopulated BM and spleen (but not the thymus) of all mice tested (4%-54% of BM cells positive for human CD45; mean, 26%) and gave rise to B-cell and myeloid lineages (Figure 6A). In contrast, transplantation with CD34+CD1a– or CD34+CD1a+ thymocytes did not yield any cells positive for human CD45 above background levels (mice not receiving a transplant) in any organ, even when administered in a very high dose (5 × 106 CD34+ thymocytes per mouse) (Figure 6B). These results show that CD34+ thymocytes do not have NOD/SCID-repopulating ability.
Frequencies of myeloid, B-, and T-cell lineages. Precursor frequencies within the CD34+CD1a– thymocyte population were determined by limiting dilution assays and calculated using the method of maximum likelihood.43 (Left) CD19+ B cells on OP9-GFP; frequency 1 in 644 (720 and 270 in subsequent experiments). (Middle) CD13/33+ myeloid cells on OP9-GFP; frequency 1 in 193 (126 in a second experiment) in the absence of GM-CSF (□) and 1 in 48 in the presence of GM-CSF (▦). (Right) CD8+ T cells on OP9-DL1; frequency 1 in 29.
Frequencies of myeloid, B-, and T-cell lineages. Precursor frequencies within the CD34+CD1a– thymocyte population were determined by limiting dilution assays and calculated using the method of maximum likelihood.43 (Left) CD19+ B cells on OP9-GFP; frequency 1 in 644 (720 and 270 in subsequent experiments). (Middle) CD13/33+ myeloid cells on OP9-GFP; frequency 1 in 193 (126 in a second experiment) in the absence of GM-CSF (□) and 1 in 48 in the presence of GM-CSF (▦). (Right) CD8+ T cells on OP9-DL1; frequency 1 in 29.
Repopulation of NOD/SCID mice. (A) Human CD34+ UCB cells efficiently repopulate BM of NOD/SCID mice. (Left) Expression of human CD45 within the lympho/monogate. (Right) Expression of CD19 and CD14 within the CD45 gate. (B) No human CD45+ cells were detected in BM of mice that received a transplant with CD34+CD1a– thymocytes (left) or with CD34+CD1a+ thymocytes (right).
Repopulation of NOD/SCID mice. (A) Human CD34+ UCB cells efficiently repopulate BM of NOD/SCID mice. (Left) Expression of human CD45 within the lympho/monogate. (Right) Expression of CD19 and CD14 within the CD45 gate. (B) No human CD45+ cells were detected in BM of mice that received a transplant with CD34+CD1a– thymocytes (left) or with CD34+CD1a+ thymocytes (right).
Discussion
Our study demonstrates that CD34+CD1a– thymocytes have the potential to generate T, B, myeloid, and erythroid cells in vitro. Megakaryocytic potential was suggested by the expression of NF-E2 in CD34+CD1a– thymocytes and was confirmed by culturing thymocytes in suspension in the presence of TPO and SCF. Although CD34+CD1a+ thymocytes did not survive, CD34+CD1a– thymocytes could be cultured in this condition for at least 14 days and developed into large cells resembling megakaryocytes, although definitive flow cytometric characterization (eg, by staining for CD41) was hindered by high autofluorescence (data not shown). As previous studies have already provided evidence for the development into NK cells and DCs from CD34+CD1a– thymocytes,14-16 precursors for the full range of hematopoietic cells appear to be present in the human thymus. Although we did not formally prove that all these lineages can be attributed to a single progenitor cell, our data are evocative of the existence of an immature, stem cell–like progenitor in the human thymus. Also studies in the murine thymus have shown that the DN1 subset (the earliest DN subset in the mouse) contains multilineage progenitors.7,8,28-30 These included precursors for T, B, and NK cells and DCs and macrophages, but erythrocyte development has not been shown before.
Frequencies of myeloid and erythroid potential, as determined by in vitro colony assays, were markedly lower in the CD34+CD1a– thymocytes than in CD34+ UCB cells, probably because UCB contains higher numbers of more mature progenitors. This is visible, for instance, in the higher expression of CD13/33 and MPO on CD34+ cells from UCB. Also in the OP9 cocultures we found low frequencies of myeloid and B-lineage progenitors in the CD34+CD1a– subset. As frequencies of the different lineages did not overlap, we could not provide definitive evidence for a thymic pluripotent progenitor. Instead, the CD34+CD1a– population may contain committed precursors for alternative (non-T) lineages. However, we think it highly unlikely that, for instance, a committed erythroid progenitor would enter the thymus. More efficient single-cell assays will be needed to definitely solve the question of pluripotent progenitors versus mixtures of lineage-committed progenitors. In our hands, single-cell cultures were technically difficult and inconclusive, probably because the OP9 system is not optimal for human cells. La Motte-Mohs et al25 have demonstrated that the OP9-DL1 culture system allows T-cell development from human CD34+CD38– UCB cells, but in this study large numbers of cells (> 10 000 per well) were seeded and no limiting dilutions were performed.
Although seeding efficiencies on OP9-GFP were too low to draw definitive conclusions, we did find wells containing both B and myeloid cells, also among wells seeded with limited numbers of cells (eg, 10 or 30). Furthermore, the frequency of myeloid progenitors, when cultured on OP9-GFP in the presence of GM-CSF, was only 2 times lower than the frequency of T-cell progenitors. Together these data point toward the existence of multilineage progenitors in the human thymus.
The remarkable finding that normal human thymocytes that have undergone immature TCRD rearrangements can still be induced to develop into myeloid and erythroid cells, whereas these rearrangements are absent in CD13/33+ myeloid progenitors in BM, demonstrates that the distinction between lymphoid, myeloid, and erythroid development is not as rigid as often assumed. A close lineage relation between myeloid cells and T cells has also been suggested by experiments in the fetal mouse, by Kawamoto et al.31
The range of lineage potentials of early thymocytes may give clues about the identity of the BM progenitor that seeds the thymus. In the mouse, several candidate thymus-colonizing progenitors have been proposed. These include lymphoid-restricted common lymphoid progenitors (CLPs),2 early lymphoid progenitors (ELPs) that have limited myeloid potential,32 multipotent Lin–Sca-1+c-Kit+ (LSK) cells,6 and bipotent T/myeloid progenitors.33 Experimental data in the mouse argue against the CLP as a likely candidate. Although CLPs were detected in murine BM,2 cells with the same surface markers could not be detected in the thymus.5,6 Furthermore, BM contains other progenitors upstream of CLPs (eg, LSK cells) that can efficiently generate T cells.6,34 Our finding that the earliest subset in the human thymus (as a population) has a very broad lineage potential also suggests that the human thymus is seeded not by lymphoid-restricted CLPs, but probably by more stem cell–like progenitors. This view is strengthened by our observation in OP9-GFP cocultures that the frequency of myeloid potential within CD34+CD1a– thymocytes was higher than that of B cells, as is the case for normal human HSCs. Adolfsson et al35 recently identified a population in the LSK compartment of murine BM that has lymphoid and myeloid, but not erythroid and megakaryocytic, potential. The fact that we find cells with erythroid potential within the CD34+CD1a– population suggests that at least part of the thymus-seeding cells are multipotent progenitors that resemble HSCs.
The hypothesis that the human thymus is seeded by a stem cell–like cell is supported by the fact that HSCs circulate in adult peripheral blood, in both mice36 and humans37 and can repopulate the thymus of irradiated mice.6 We used the entire CD34+CD1a– population, the homolog of murine DN1 population, so as to certainly include the thymus-seeding cells, even though they may represent a tiny fraction of this subset. Because of the large cell numbers in the human thymus and the easy purification of CD34+ cells, we were able to culture large numbers of cells in colony assays. This may have been the reason why we could detect the very low erythroid and megakaryocytic potential of these very immature thymocytes, which was never found in the mouse. However, this suggestion is contradicted by our finding that CD34+CD1a– thymocytes could not repopulate NOD/SCID mice. In the mouse it has been shown that proliferation of a precursor that enters the thymus is limited to several weeks and that no permanent endogenous stem cell exists.38 This makes it likely that, similar to the corresponding cells in the mouse, CD34+CD1a– cells have very limited self-renewal. Thus, it is possible that the thymus is seeded by a HSC that rapidly loses self-renewal capacity and reduces non–T-cell potential after entering the thymus and interacting with molecules on the thymic epithelium, for instance, Notch ligands. Another possibility is that only a minute fraction of the cells that seed the thymus retains sufficient self-renewal and that this population is too rare to be detected with currently used assays. In line with this, it may be that a similar situation exists in the mouse, but that very stringent lineage depletion or insufficient cell numbers used in colony assays have excluded detection of, for instance, erythroid potential.
An alternative explanation for the fact that CD34+CD1a– thymocytes could not repopulate NOD/SCID mice is that they lack BM homing capability. One of the adhesion molecules necessary for BM homing is CXCR4.39,40 However, using flow cytometry we could not detect significant differences in CXCR4 expression between CD34+ UCB cells, which efficiently repopulate NOD/SCID BM, and CD34+ thymocytes (data not shown). This suggested that the BM homing capacity through CXCR4 is not affected, although absence of other homing signals in CD34+ thymocytes cannot be excluded. Poor homing capability can be considered as an additional argument against the existence of a thymic HSC, because murine HSCs were shown to have high BM-seeding efficiency.41
In contrast to the immature characteristics of the CD34+CD1a– cells, the CD34+CD1a+ thymocytes showed a T-cell–specific gene-expression pattern and did not yield any non–T-lineage cells in in vitro cultures. We did not test NK and DC potential, but previous studies have shown a very limited NK and no DC precursor activity of CD34+CD1a+ cells.10 Together these findings suggest that human CD34+CD1a+ thymocytes are the equivalents of murine DN3 cells, in which final commitment to the T-cell lineage occurs.42 This confirms our recently proposed scheme in which the stages of human T-cell development are highly similar to those in the mouse.13
In summary, we here characterized the CD34+CD1a– and CD34+CD1a+ thymic populations by gene-expression profiling and functional studies with respect to lineage potential and self-renewal capacity. We show that lineage potential of the CD34+CD1a– thymocytes is much broader than previously thought and includes erythrocytic and B-lymphocytic capacity. Although clonal assays with a higher seeding efficiency than those used here are necessary to prove whether all lineages can be attributed to a single progenitor, our data strongly suggest that the human thymus is seeded by multipotent progenitors. These progenitors are distinct from CLPs and may be pluripotent HSCs, which immediately lose self-renewal capacity on contact with the thymic epithelium.
Prepublished online as Blood First Edition Paper, December 29, 2005; DOI 10.1182/blood-2005-08-3412.
Supported by the Trustfund of the Erasmus University Rotterdam (F.W.), the 5th and 6th framework for gene therapy (grants QLK3-CT-2001-00427 and LSHB-CT-2004-005242) (F.J.T.S. and G.W.), a gene therapy grant from the Netherlands Organization for Scientific Research (NWO) (40-40300-98-04016; F.J.T.S. and G.W.), and VIRGO (grant 1754065; F.J.T.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank M. Comans-Bitter for preparing the figures, Dr M. Versteeg for help with colony assay experiments, Prof A. Bogers for providing thymus material, and Dr M. van der Burg for bone marrow material.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal