The pathophysiology of microthrombocytopenia in the Wiskott-Aldrich syndrome (WAS) and its milder form, X-linked thrombocytopenia (XLT), is unclear. Although quantitative defects are correctable by splenectomy, residual platelet abnormalities are suggestive of intrinsic disturbances of production. In contrast to human patients, murine models of WASp deficiency exhibit only mild thrombocytopenia, and platelets are of normal size. Here, we have identified a critical role for WASp during murine platelet biogenesis. By electron microscopy, WASp-deficient MKs appeared to have shed platelets ectopically within the bone marrow space. WASp-deficient megakaryocytes (MKs) also displayed defects in response to fibrillar collagen I (CI) in vitro, the major matrix component of bone. These included a loss of normal CI receptor (α2β1 integrin)-mediated inhibition of proplatelet formation, a marked abrogation of SDF-1-induced chemotactic migration of CD41+ MKs adherent to CI, and an almost complete lack of actin-rich podosomes, normally induced by interaction between CI and its receptors GPVI or α2β1 integrin. These findings highlight the central and highly specialized role of WASp in MKs during platelet biogenesis, and may provide a mechanism for the mild thrombocytopenia observed in WASp-deficient mice. In addition, they suggest a novel explanation for some of the platelet abnormalities characteristic of patients with WAS. (Blood. 2006;108:134-140)
Introduction
Megakaryocytes (MKs) shed platelets by forming cytoplasmic extensions called proplatelets.1 At the end of maturation, MKs migrate toward marrow sinusoids and either entirely transmigrate before forming platelets2 or protrude their proplatelets through the vascular endothelium.3 Ectopic localization of this process within the bone marrow environment would severely compromise efficient platelet production because platelets have low capacities for chemotaxis and transmigration, and therefore need to be directly released into the bloodstream. For this reason, efficient platelet production requires a synchronous process of cell migration and proplatelet formation. ECM4 and stromal cells5 appear to play an important role in the inhibition of platelet production by MKs within the marrow microenvironment, and the regulated loss of this specific interaction may precede MK migration from the bone marrow, and promote the onset of proplatelet formation.
The Wiskott-Aldrich syndrome protein (WASp), which is exclusively expressed in hematopoietic cells, has emerged as a key signaling molecule6 involved in cellular processes such as (1) reorganization of the actin cytoskeleton in response to specific signals,7 (2) formation of podosomes, specialized actin-rich structures identified in motile primary cells such as macrophages, dendritic cells (DCs), and osteoclasts,8 and (3) migration and cell trafficking of lymphocytes, DCs, and granulocytes.9 Accordingly, abnormalities of immune-cell adhesion and migration observed in WAS, the X-linked primary immunodeficiency caused by mutations in the gene encoding WASp, have been partly ascribed to the lack of podosomes and concomitant cell migration defects in human and mouse models. In contrast, the pathophysiology of microthrombocytopenia, which is characteristic of WAS and its attenuated forms, remains unclear, although it is considered that increased platelet peripheral destruction is the most important mechanism.6 Indeed, splenectomy of WAS patients usually results in substantial (though sometimes incomplete) correction of thrombocytopenia, but persistence of small platelet size.10 Isotope studies of platelet survival and release have also demonstrated that a component of the thrombocytopenia is related to inefficient platelet production.11 This is unlikely to be due to a direct role of WASp in the process of pseudopod extension, which leads to proplatelet formation because platelet production by human WAS MKs is normal in liquid culture in vitro.12 In this system, platelet shedding occurs directly in the absence of cell-cell and cell-ECM interactions, and it is therefore limited in its ability to evaluate the important influences of MK interaction with the microenvironment and MK endothelial transmigration. WASp, an important regulator of the actin cytoskeleton,7 may play an important role in these last 2 mechanisms. Mice generated by gene targeting have provided a powerful model to study the role of WASp in multiple cell lineages of the hematopoietic system. However, until now study of the mechanism of thrombocytopenia in WAS has been limited because, unlike human patients, mice have only a moderate decrease in their platelet count and no abnormalities in their platelet size.13
In spite of these species differences, we report here that proplatelet formation in WASp-deficient mice may occur ectopically within the bone marrow. We also show that WASp-deficient MKs have a defect in the negative regulation of proplatelet formation mediated by α2β1, one of the main receptors of fibrillar collagen I (CI), which is an important component of the bone marrow microenvironment.14 In addition, in the presence of CI, WASp-deficient MKs show a profound defect in chemotactic migration, which is consistent with marked cytoskeletal abnormalities, such as the lack of actin-rich podosome structures that were selectively induced by CI in wild-type MKs.
Taken together, we suggest that WASp is involved in the modulation of MK actin reorganization, and that this participates in the prevention of premature transmigration of immature MKs in response to chemotactic factors and early platelet biogenesis in the bone marrow microenvironment. These events may provide a novel explanation for the defective platelet production in WASp-deficient mice and human patients.
Materials and methods
Animals and reagents
C57Bl/6 and wild-type 129Sv/Ev mice were purchased from Iffa Credo (L'arbresle, France). WASp-deficient mice were provided by Drs Scott B. Snapper (Boston, MA) and Adrian Thrasher (London, United Kingdom). Mice were housed in animal facilities at the Institut Gustave Roussy (Villejuif, France) under specific pathogen-free conditions.
CI (Horm) was purchased from Nycomed (Munich, Germany). Poly-l-lysine (PLL), fibronectin, antivinculin mAb, and tetramethyl rhodamine isothiocyanate (TRITC)-conjugated phalloidin were from Sigma (St Quentin, France). Growth factor-reduced matrigel was obtained from BD Biosciences (San Jose, CA). Bovine fibrinogen was from Pentex (Kankakee, IL). Rabbit anti-von Willebrand factor (VWF) and anti-WASp (H-250) antibodies were obtained from Dakocytomation (Trappes, France) and Santa Cruz Biotechnology (Perray en Yvelines, France), respectively. Secondary antibodies were Alexa 488 goat anti-rabbit and Alexa 647 anti-mouse IgG (Molecular Probes, Eugene, OR). Convulxin (CVX) and GFOGER peptide were purified as described.15,16
Culture of MKs
Bone marrow (BM) nucleated cells from 129Sv, WASp-deficient mice, or C57Bl/6 mice were obtained from femurs and tibiae of 6- to 8-week-old adult mice. The lineage-negative (lin-) fraction was purified from BM nucleated cells and grown for 72 hours in serum-free medium containing 10 ng/mL murine recombinant thrombopoietin (TPO; R&D Systems, Oxon, United Kingdom) as previously described.17
Optical microscopy
Standard. Epon-embedded thick sections (1 μm) of wild-type and WASp-deficient mouse bone marrow were stained with toluidine blue and examined by optical microscopy.
Immunohistochemistry. Immunohistochemistry was performed on paraffin-embedded 4-μm tissue sections from the WASp and control femora previously fixed in 10% neutral-buffered formalin and embedded in paraffin. Standard indirect immunoperoxidase procedures were used. The primary antibody was a polyclonal rabbit antibody factor VIII anti-humanrelated antigen (dilution, 1:2; DAKO, Carpinteria, CA); an avidinbiotinylated peroxidase system was applied for the immunohistochemical stains. Hematoxylin was the nuclear counterstain. Negative controls were performed by replacing the primary antibody with buffer.
Electronic microscopy
Samples were fixed in 1.5% glutaraldehyde for 1 hour and washed 3 times in 0.1 M phosphate buffer, pH 7.4. For morphologic examination, samples were postfixed in 1% osmic acid, dehydrated in ethanol, and embedded in Epon by standard methods. Samples were counterstained and were observed on a Philips CM 10 electron microscope (Eindhoven, the Netherlands).
Chemotactic assay
Cell migration was quantified through 12-μm-pore filters (Transwell, 24-well cell clusters; VWR, Strasbourg, France). Serum-free medium (100 μL) containing 2 × 105 cells was placed in the upper chamber of the transwell precoated by matrigel or CI. In the lower chamber, 600 μL serum-free medium with (30, 100, or 300 ng/mL) or without SDF-1α (Abcys, Paris, France) was added. After 4 hours at 37°C in 5% CO2, the cells from the lower chambers and the initial population were recovered in phosphate-buffered saline (PBS) containing 2% BSA, and then incubated with a purified rat anti-mouse CD41 antibody (MWReg30) for 20 minutes at 4°C. Negative controls were performed using purified rat IgG1 antibody. The cells were washed and labeled with an allophycocyanin-conjugated goat antirat antibody for 20 minutes at 4°C, washed, recovered in equal volumes of PBS containing 7-AAD, and counted by flow cytometry. The percentage of migration was calculated according to the following formula: number of CD41+ cells in the lower chamber divided by the number of CD41+ cells in the initial population. The 7-AAD DNA dye was used to exclude dead cells. All antibodies were purchased from BD Biosciences, and flow cytometry analyses were performed on a FACSort instrument (BD Biosciences).
Immunofluorescence analysis
CI (50 μg/mL), CVX (15 μg/mL), fibronectin (20 μg/mL), or fibrinogen (20 μg/mL) was incubated on coverslips overnight at 4°C. GFOGER peptide or PLL (20 μg/mL) was incubated for 1 hour at room temperature (RT). Cells were seeded on precoated coverslips at 37°C for the indicated times and processed for labeling as described.17 Briefly, cells were fixed in 2% paraformaldehyde for 10 minutes, washed with PBS, and permeabilized using Triton X-100 (0.2%) for 3 minutes. They were then incubated sequentially with antivinculin (1/400), rabbit anti-VWF (1/1000), or anti-WASp (1/400) antibodies. After washing, coverslips were incubated with TRITC-phalloidin (1/1000) and Alexa 647 goat anti-mouse or 488 anti-rabbit IgG (1/200), washed, and mounted with Vectashield antifading mounting medium (Vector, Burlingame, CA).
Cells were examined under an epifluorescence microscope (Nikon Eclipse 600; Nikon, Tokyo, Japan) equipped with a 63 ×/1.4 NA objective lens and a Zeiss laser scanning microscope (LSM 510; Zeiss, Jena, Germany) equipped with a Planapo oil-immersion lens (63 × magnification). Images were processed using CLSM5 Zeiss Browse Image software.
At least 300 MKs showing VWF+ staining were counted in each experiment in 3 independent experiments to determine the percentage of MKs showing VWF+ proplatelets (defined as cells exhibiting one or more cytoplasmic processes with areas of constriction) or actin-rich podosome structures and/or stress fibers.
Statistical analysis
The results are presented as mean plus or minus SD. The data were analyzed using the 2-tailed Student t test.
Results
Platelet formation may occur inside the bone marrow of WASp-deficient mice
Microthrombocytopenia is a consistent feature of WAS and its mild form X-linked thrombocytopenia (XLT).6 However, in previous studies, we did not detect any obvious cytologic alterations in primary human WASp-deficient MKs grown in vitro.12 To investigate whether WASp might be involved in MK function under more physiologic conditions, we analyzed bone marrow femurs of WASp-deficient mice and their normal counterpart 129Sv mice by conventional histology, immunohistochemistry, and electron microscopy.
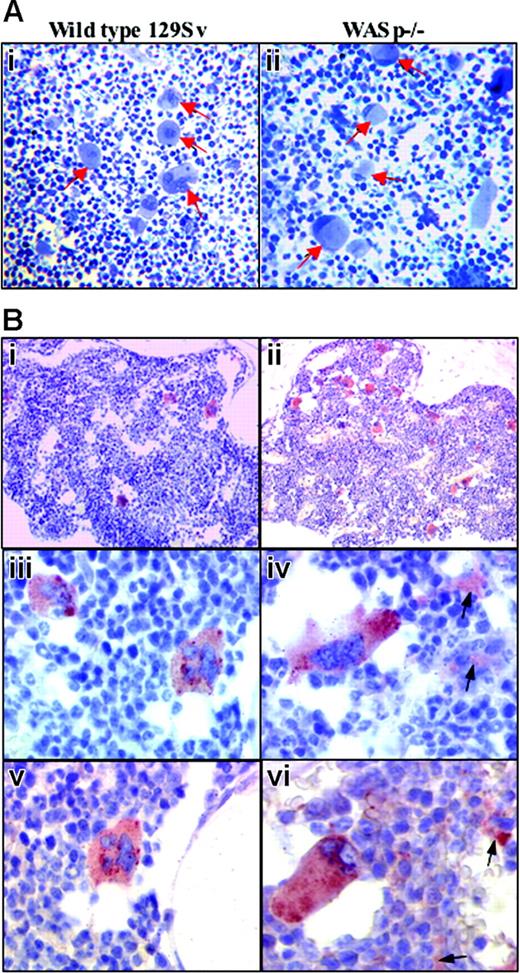
In situ cytologic alterations of MKs in WASp-deficient bone marrow.(A) Semithin toluidine blue staining sections of wild-type and WASp-deficient bone marrows. Epon-embedded sections of wild-type bone marrow MKs examined by light microscopy show centrally located nuclei (i, arrows), whereas MKs in WASp-deficient bone marrow (ii, arrows) display eccentric moon crescent-like nucleus attesting for the cell structure disorganization (original magnification, × 200). (B) Immunohistochemical staining of VWF in wild-type (i,iii,v) and WASp-deficient (ii,iv,vi) bone marrows. Indirect immunolabeling of VWF by immunohistochemistry (described in “Materials and methods”) shows quantitative and qualitative alterations of WASp-deficient MKs and the abnormal presence of platelets in femoral bone marrow obtained from WASp-deficient mice in comparison with wild-type 129Sv mice. (i-ii) Normal distribution of MKs in mouse bone marrow is shown, whereas WASp-deficient bone marrow MKs appear more numerous (original magnification, × 200). (iii-iv) Typical morphology of MKs in wild-type bone marrow. Some WASp-deficient bone marrow MKs have lost their round shape, and they appear stretched with elongated cytoplasmic extensions resembling proplatelets (arrow) (original magnification, × 400). (v-vi) At a higher magnification, the MK appears well limited, with round shape. No labeling is detected in the extramegakaryocytic space. In contrast, WASp-deficient bone marrow MKs often exhibit an atypical shape with an eccentric position of the nucleus. Some labeling (arrow) is detected in the extramegakaryocytic space, suggesting the presence of in situ platelet production (original magnification, × 600).
In situ cytologic alterations of MKs in WASp-deficient bone marrow.(A) Semithin toluidine blue staining sections of wild-type and WASp-deficient bone marrows. Epon-embedded sections of wild-type bone marrow MKs examined by light microscopy show centrally located nuclei (i, arrows), whereas MKs in WASp-deficient bone marrow (ii, arrows) display eccentric moon crescent-like nucleus attesting for the cell structure disorganization (original magnification, × 200). (B) Immunohistochemical staining of VWF in wild-type (i,iii,v) and WASp-deficient (ii,iv,vi) bone marrows. Indirect immunolabeling of VWF by immunohistochemistry (described in “Materials and methods”) shows quantitative and qualitative alterations of WASp-deficient MKs and the abnormal presence of platelets in femoral bone marrow obtained from WASp-deficient mice in comparison with wild-type 129Sv mice. (i-ii) Normal distribution of MKs in mouse bone marrow is shown, whereas WASp-deficient bone marrow MKs appear more numerous (original magnification, × 200). (iii-iv) Typical morphology of MKs in wild-type bone marrow. Some WASp-deficient bone marrow MKs have lost their round shape, and they appear stretched with elongated cytoplasmic extensions resembling proplatelets (arrow) (original magnification, × 400). (v-vi) At a higher magnification, the MK appears well limited, with round shape. No labeling is detected in the extramegakaryocytic space. In contrast, WASp-deficient bone marrow MKs often exhibit an atypical shape with an eccentric position of the nucleus. Some labeling (arrow) is detected in the extramegakaryocytic space, suggesting the presence of in situ platelet production (original magnification, × 600).
In WASp-deficient bone marrow, MKs were more numerous compared with the normal mouse marrow as observed by conventional histology (data not shown).
Epon-embedded sections of wild-type bone marrow MKs (toluidine blue staining) showed centrally located nuclei (Figure 1Ai arrows). In contrast, the nuclei were eccentrically located at the cell periphery of MKs in WASp-deficient bone marrows (Figure 1Aii arrows).
Immunohistochemistry confirmed the increased number and abnormal structure of WASp-deficient MKs (Figure 1Bi,ii). In addition, short cytoplasmic extensions resembling proplatelets were found in the vicinity of some mature MKs, and numerous deposits of specific immunostaining were scattered in the hematopoietic space of the bone marrow, suggesting that local platelet production had occurred (Figure 1Biv,vi). In contrast, control bone marrow did not display any background staining (Figure 1Biii,v).
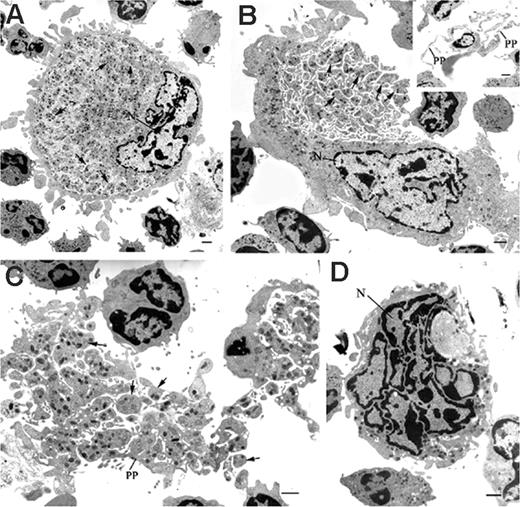
Representative electron-microscopic views of mature MKs in the bone marrow of WASp-deficient mice. Samples obtained from bone marrow of wild-type 129Sv and WASp-deficient mice were processed for electronic microscopy as described in “Materials and methods.” Ultrastructural analysis showed the abnormal presence of WASp-deficient MKs displaying morphologic features of maturing MKs. (A) Mature MK showing delineated platelet territories (arrows) (magnification, × 2200; bar, 1 μm). (B) Bone marrow MKs in the process of shedding platelets (arrows) (magnification, × 2200; bar, 1 μm). (Inset) Proplatelets (PP) extended from a mature MK within the bone marrow (magnification, × 1650; bar, 1 μm). (C) Platelets (P) and proplatelets (PP) released from MKs and scattered within the bone marrow (magnification, × 2950; bar, 1 μm). (D) Naked MK nucleus (N) found in the bone marrow space (magnification, × 3900; bar, 1 μm).
Representative electron-microscopic views of mature MKs in the bone marrow of WASp-deficient mice. Samples obtained from bone marrow of wild-type 129Sv and WASp-deficient mice were processed for electronic microscopy as described in “Materials and methods.” Ultrastructural analysis showed the abnormal presence of WASp-deficient MKs displaying morphologic features of maturing MKs. (A) Mature MK showing delineated platelet territories (arrows) (magnification, × 2200; bar, 1 μm). (B) Bone marrow MKs in the process of shedding platelets (arrows) (magnification, × 2200; bar, 1 μm). (Inset) Proplatelets (PP) extended from a mature MK within the bone marrow (magnification, × 1650; bar, 1 μm). (C) Platelets (P) and proplatelets (PP) released from MKs and scattered within the bone marrow (magnification, × 2950; bar, 1 μm). (D) Naked MK nucleus (N) found in the bone marrow space (magnification, × 3900; bar, 1 μm).
Electron microscopy demonstrated that mature WASp-deficient MKs exhibited delineated platelet territories within their cytoplasm more often than in control mice (Table 1; Figure 2A). In addition, numerous MKs in the process of shedding and release of platelets were observed in WASp-deficient bone marrows (Table 1; Figure 2B), and this aspect could not be seen in wild-type marrows. Proplatelets and platelets were also observed in WASp-deficient bone marrow either in clusters or scattered in the hematopoietic space (Table 1; Figure 2C), and these images were virtually absent from our control mice. Naked MK nuclei were also found in the WASp-deficient bone marrow space (Table 1; Figure 2D), again indicating that cytoplasmic shedding had occurred in situ. Naked nuclei were not observed in EM control marrow sections, suggesting that these are rare events in 129Sv mice marrow in contrast to Wasp-deficient mice marrow.
Percentage of different platelet formation stages on wild-type and WASp-deficient bone marrow EM sections
. | Control . | Wasp−/− . |
|---|---|---|
| Maturing MKs with no apparent platelet territories | 52 | 11 |
| MKs exhibiting platelet territories (Figure 2A) | 48 | 34 |
| MKs in the process of shedding (Figure 2B) | 0 | 25 |
| Isolated platelet clusters (Figure 2C) | 0 | 23 |
| Naked nuclei (Figure 2D) | 0 | 7 |
. | Control . | Wasp−/− . |
|---|---|---|
| Maturing MKs with no apparent platelet territories | 52 | 11 |
| MKs exhibiting platelet territories (Figure 2A) | 48 | 34 |
| MKs in the process of shedding (Figure 2B) | 0 | 25 |
| Isolated platelet clusters (Figure 2C) | 0 | 23 |
| Naked nuclei (Figure 2D) | 0 | 7 |
Bone marrow samples of wild-type 129Sv and WASp-deficient mice were processed for electronic microscopy. Different cytologic aspects of MK maturation together with platelet clusters and MK naked nuclei (illustrated in Figure 2) were then scored (219 events) in wild-type 129Sv and WASp-deficient bone marrow EM sections.
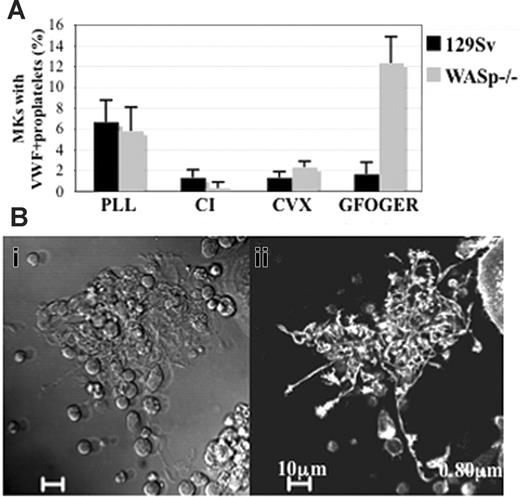
WASp is involved in inhibition of proplatelet formation induced by α2 integrin ligation. (A) The Lin- bone marrow cells harvested from wild-type 129Sv mice or WASp-deficient mice, grown for 72 hours in the presence of TPO were plated onto PLL-, CI-, CVX-, or GFOGER-coated coverslips for 2 hours. Adherent cells were fixed, permeabilized, and stained with anti-VWF and TRITC-phalloidin, and the percentage of MKs displaying VWF+ proplatelets was evaluated by counting at least 300 MKs showing VWF+ staining in each experiment. The graph represents the mean ± SD of the percentage of MKs showing VWF+ proplatelets in the indicated substrates in 3 independent experiments. (B) Phase-contrast (i) and diffuse F-actin staining (ii) images illustrate proplatelet formation in WASp-deficient MKs adherent to GFOGER peptide, the high-affinity substrate of α2 integrin. Bar, 10 μm.
WASp is involved in inhibition of proplatelet formation induced by α2 integrin ligation. (A) The Lin- bone marrow cells harvested from wild-type 129Sv mice or WASp-deficient mice, grown for 72 hours in the presence of TPO were plated onto PLL-, CI-, CVX-, or GFOGER-coated coverslips for 2 hours. Adherent cells were fixed, permeabilized, and stained with anti-VWF and TRITC-phalloidin, and the percentage of MKs displaying VWF+ proplatelets was evaluated by counting at least 300 MKs showing VWF+ staining in each experiment. The graph represents the mean ± SD of the percentage of MKs showing VWF+ proplatelets in the indicated substrates in 3 independent experiments. (B) Phase-contrast (i) and diffuse F-actin staining (ii) images illustrate proplatelet formation in WASp-deficient MKs adherent to GFOGER peptide, the high-affinity substrate of α2 integrin. Bar, 10 μm.
Loss of negative regulation of proplatelet formation by α2β1 integrin in WASp-deficient MKs
Recently, we showed that proplatelet formation of human MKs is inhibited by CI, the main component of ECM in the bone marrow.4 We investigated whether adhesion to CI would affect proplatelet formation in wild-type and WASp-deficient MKs derived from lineage-depleted (lin-) bone marrow. Cells harvested from 129Sv and WASp-deficient mice were cultured in the presence of TPO for 3 days, and plated for 2 hours on CI. Cells were then labeled by TRITC-phalloidin to visualize F-actin and VWF to confirm MK identity and discriminate between proplatelets and pseudopodal projections that could be due to cell spreading. First, we confirmed that as in WAS patients, WASp-deficient murine MKs formed proplatelets in liquid culture with the same efficiency as wild-type MKs. In addition, wild-type or WASp-deficient murine MKs seeded on CI produced only few MKs bearing proplatelets.
We previously demonstrated that CI mediates a very strong negative signal to proplatelet formation, and that a more discriminatory effect can be obtained by studying the response to ligation of the 2 main collagen receptors GPVI and α2β1 integrin.4 Wild-type and WASp-deficient MKs were thus plated on CVX14 or GFOGER peptide,15 2 high-affinity substrates for GPVI and α2β1 integrin, respectively. Adhesion to CVX mediated a very similar inhibition of proplatelet formation in wild-type and WASp-deficient MKs (Figure 3). Remarkably, we found that the proportion of WASp-deficient MKs forming VWF+ proplatelets on GFOGER peptide-coated surfaces increased significantly compared with wild-type-derived MKs (Figure 3A), and reached an even higher level than that observed in liquid culture. In addition, WASp-deficient MKs underwent striking morphologic alterations leading to the elaboration of long, branched proplatelets with diffuse actin staining, similar to those observed in liquid culture of wild-type MKs (Figure 3B). These findings indicate that inhibition of proplatelet formation in response to α2 integrin ligation is dependent on the presence of WASp.
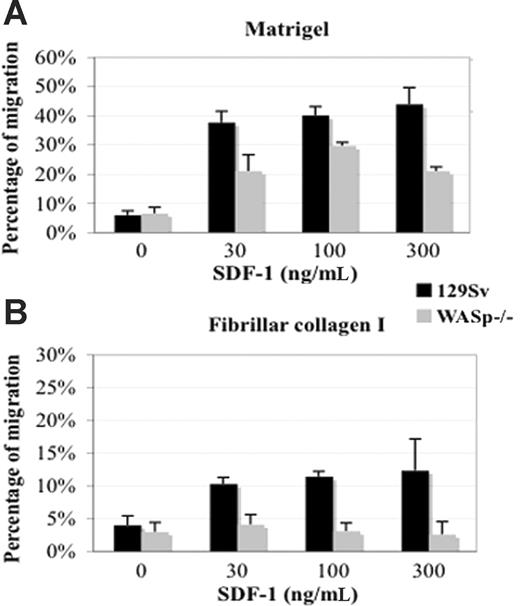
Decreased chemotactic migration of WASp-deficient CD41+MKs. Lin- bone marrow cells harvested from wild-type 129Sv and WASp-deficient mice were grown for 72 hours in the presence of TPO. Migration experiments were carried out for 4 hours in bare, matrigel-coated (A) or CI-coated (B) transwells as described in “Materials and methods.” SDF-1 was added to the lower compartment at the indicated concentrations. The percentage of migrated CD41+ cells was determined by flow cytometry and showed reduced migratory potential of CD41+ WASp-deficient MKs compared with CD41+ wild-type MKs in the presence of SDF-1. The data represent the percentage of migrated CD41+ cells compared with total CD41+ input cells. The graphs show the mean and SD of 2 independent experiments performed in triplicate.
Decreased chemotactic migration of WASp-deficient CD41+MKs. Lin- bone marrow cells harvested from wild-type 129Sv and WASp-deficient mice were grown for 72 hours in the presence of TPO. Migration experiments were carried out for 4 hours in bare, matrigel-coated (A) or CI-coated (B) transwells as described in “Materials and methods.” SDF-1 was added to the lower compartment at the indicated concentrations. The percentage of migrated CD41+ cells was determined by flow cytometry and showed reduced migratory potential of CD41+ WASp-deficient MKs compared with CD41+ wild-type MKs in the presence of SDF-1. The data represent the percentage of migrated CD41+ cells compared with total CD41+ input cells. The graphs show the mean and SD of 2 independent experiments performed in triplicate.
WASp-deficient MKs have a marked defect in migration in the presence of CI
WASp plays a crucial role in chemotactic migration and trafficking of several hematopoietic cell lineages.9 It has also been suggested that immunodeficiency in WAS might at least in part be related to migration defects of immune cells.18 To assess how migration of WASp-deficient MKs might be modulated by the bone marrow microenvironment including ECM and SDF-1 (the central chemokine in cytokine-independent regulation of thrombopoiesis),19 we tested the ability of wild-type and WASp-deficient MKs to migrate in vitro through a gradient of SDF-1 using ECM-coated transwell chambers. In the absence of SDF-1, only a few cells were able to migrate (Figure 4). When transwells were not coated, the migration efficiency of wild-type and WASp-deficient MKs was similar in the presence of SDF-1. In matrigel-coated chambers, addition of SDF-1 induced a higher migration of wild-type CD41+ MKs compared with that observed in CI-coated chambers (37.7% ± 3.8% and 10.3% ± 0.98%, respectively), at suboptimal doses of SDF-1 (30 ng/mL). Similar rates of cell migration were observed at 100 and 300 ng/mL. The migration efficiency of WASp-deficient MKs was significantly decreased on both matrigel-coated (Figure 4A) and CI-coated (Figure 4B) transwells. Thus, in the presence of CI or matrigel, WASp-deficient MKs have a marked defect in migration. Of importance, migration of WASp-deficient MKs was barely detectable on CI, suggesting a selective role of WASp on CI signaling. In combination with a loss of α2β1 integrin-mediated suppression of platelet production, these findings may explain the occurrence of ectopic and premature platelet shedding within the bone marrow microenvironment.
CI induced actin-rich podosome structures in wild-type, but not WASp-deficient, MKs
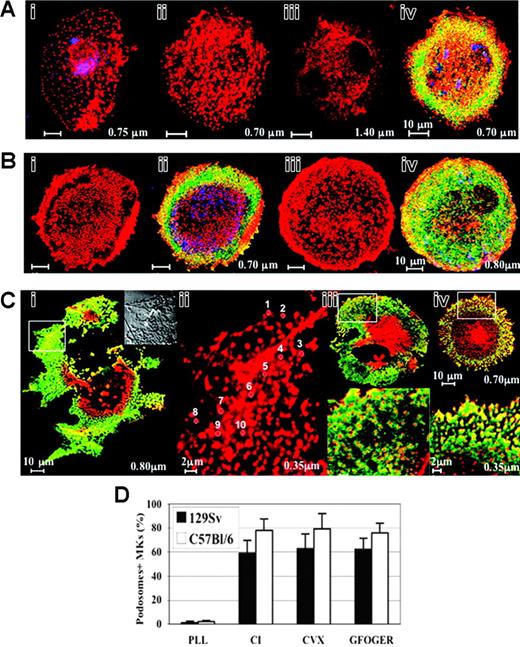
We hypothesized that alterations of the actin cytoskeleton may underlie the observed differences in the migration behavior of WASp-deficient MKs. Thus, we investigated cytoskeletal actin reorganization induced by adhesion to ECM components in wild-type and WASp-deficient MKs. Cells harvested from wildtype and WASp-deficient mice were cultured in the presence of TPO for 3 days and plated on CI, fibronectin, fibrinogen, matrigel, or PLL for 30 minutes, 1 hour, or 2 hours. Unexpectedly, on CI-coated slides, actin dotlike structures reminiscent of podosomes were noticeable at 30 minutes (Figure 5Ai) and were prominent in 59% ± 10% of MKs within 2 hours (Figure 5Aii,iv,D). The MK identity of cells was verified by staining for VWF (blue pseudocolor). Confocal sections revealed punctate actin structures that were restricted to the ventral MK surface (Figure 5Aii: section = 0.70 μm) and were not observed in upper sections (Figure 5Aiii: section = 1.40 μm). Punctate actin structures at the cell margin were surrounded by vinculin rings (green), which are characteristic of podosome organization (Figure 5Aiv). Of interest, podosomes were not observed when cells were seeded on PLL, matrigel, fibrinogen, or fibronectin-coated slides (data not shown). Thus, podosomes in murine MKs were not spontaneously assembled as in monocytic-derived cells and were specifically induced by CI, likely through its main receptors GPVI and/or α2β1 integrin.
CI-induced podosome assembly in primary murine MKs. (A) CI-induced podosome assembly in 129Sv-derived MKs: the Lin- bone marrow cells harvested from 129Sv mice were grown for 72 hours in the presence of TPO, seeded on coverslips coated with CI for 30 minutes (i) or 2 hours (ii-iv), processed for staining with rhodamine-phalloidin (red) and antivinculin (green) to label F-actin and vinculin, respectively, and analyzed by confocal microscopy. Labeling with anti-VWF (blue pseudocolor) shows that the observed cells are, indeed, MKs. Podosomes are readily discernible 30 minutes following adhesion to CI (i), and their assembly is optimal within 2 hours (ii,iv). Optical sections from the bottom (ii: section = 0.70 μm) to the top of the cell (iii: section = 1.40 μm) illustrate ventral localization of punctate F-actin structures. Vinculin rings were noticeable mainly at the cell margin (iv). Bar, 10 μm. (B) Adhesion to the high-affinity substrates of GPVI or α2β1 integrin CVX (i-ii) or GFOGER peptide (iii-iv), respectively, induced similarly the formation of actin-rich podosome structures with vinculin rings in 129Sv-derived MKs at the ventral side of the cells mainly at the cell periphery. Bar, 10 μm. (C) CI induced podosome assembly in C57Bl/6-derived similarly to 129Sv-derived MKs: vinculin (green) delineates the core of F-actin structures mainly in the cell margin (i). Bar, 10 μm. The inset is a phase contrast of a 5-fold magnification of the area marked by a rectangle. Dark dots corresponding to actin punctate structures are located in the leading edges and the tips of lamellae. (ii) High-power view of actin-rich podosome-like structures in the same area (section = 0.35 μm). Actin dots are circled, individually numbered (for example: 1 to 10) and measured using CLSM5 Zeiss Browse Image software (diameter of circles: no. 1 = 0.44 μm; no. 2 = 0.45 μm; no. 3 = 0.54 μm; no.4 = 0.41; no. 5 = 0.45; no. 6 = 0.55; no. 7 = 0.55; no. 8 = 0.44; no. 9 = 0.55; and no. 10 = 0.41 μm). Bar, 2 μm. (iii-iv) Podosome assembly induced by adhesion to CVX (iii) or GFOGER (iv) substrates is illustrated. The magnified region confined in a white square (0.35 μm, bottom panel) illustrates the ringlike shape of vinculin lining actin dots mainly at the cell periphery. Bar, 2 μm. (D) CI-induced podosome assembly is similarly induced by GPVI and α2β1 integrin in primary murine MKs: quantification of the experiments illustrated in panels A-C. Note that only a negligible proportion of MKs show podosome structures on PLL substrate. At least 300 MKs showing podosome-like structures were counted in each experiment. Bars represent the mean ± standard deviation of 3 independent experiments. The results are not significantly different between C57Bl/6 and 129Sv mice in all tested conditions.
CI-induced podosome assembly in primary murine MKs. (A) CI-induced podosome assembly in 129Sv-derived MKs: the Lin- bone marrow cells harvested from 129Sv mice were grown for 72 hours in the presence of TPO, seeded on coverslips coated with CI for 30 minutes (i) or 2 hours (ii-iv), processed for staining with rhodamine-phalloidin (red) and antivinculin (green) to label F-actin and vinculin, respectively, and analyzed by confocal microscopy. Labeling with anti-VWF (blue pseudocolor) shows that the observed cells are, indeed, MKs. Podosomes are readily discernible 30 minutes following adhesion to CI (i), and their assembly is optimal within 2 hours (ii,iv). Optical sections from the bottom (ii: section = 0.70 μm) to the top of the cell (iii: section = 1.40 μm) illustrate ventral localization of punctate F-actin structures. Vinculin rings were noticeable mainly at the cell margin (iv). Bar, 10 μm. (B) Adhesion to the high-affinity substrates of GPVI or α2β1 integrin CVX (i-ii) or GFOGER peptide (iii-iv), respectively, induced similarly the formation of actin-rich podosome structures with vinculin rings in 129Sv-derived MKs at the ventral side of the cells mainly at the cell periphery. Bar, 10 μm. (C) CI induced podosome assembly in C57Bl/6-derived similarly to 129Sv-derived MKs: vinculin (green) delineates the core of F-actin structures mainly in the cell margin (i). Bar, 10 μm. The inset is a phase contrast of a 5-fold magnification of the area marked by a rectangle. Dark dots corresponding to actin punctate structures are located in the leading edges and the tips of lamellae. (ii) High-power view of actin-rich podosome-like structures in the same area (section = 0.35 μm). Actin dots are circled, individually numbered (for example: 1 to 10) and measured using CLSM5 Zeiss Browse Image software (diameter of circles: no. 1 = 0.44 μm; no. 2 = 0.45 μm; no. 3 = 0.54 μm; no.4 = 0.41; no. 5 = 0.45; no. 6 = 0.55; no. 7 = 0.55; no. 8 = 0.44; no. 9 = 0.55; and no. 10 = 0.41 μm). Bar, 2 μm. (iii-iv) Podosome assembly induced by adhesion to CVX (iii) or GFOGER (iv) substrates is illustrated. The magnified region confined in a white square (0.35 μm, bottom panel) illustrates the ringlike shape of vinculin lining actin dots mainly at the cell periphery. Bar, 2 μm. (D) CI-induced podosome assembly is similarly induced by GPVI and α2β1 integrin in primary murine MKs: quantification of the experiments illustrated in panels A-C. Note that only a negligible proportion of MKs show podosome structures on PLL substrate. At least 300 MKs showing podosome-like structures were counted in each experiment. Bars represent the mean ± standard deviation of 3 independent experiments. The results are not significantly different between C57Bl/6 and 129Sv mice in all tested conditions.
We therefore sought to determine the respective roles of CI receptors in podosome assembly. Unlike primary human MKs,4 adhesion to CVX induced significant spreading, podosome formation, and stress fiber assembly (Figure 5Bi,ii,D). Adhesion to GFOGER peptide, the specific substrate of α2β1 integrin, induced podosome assembly to the same extent as CVX (Figure 5Biii,iv,D) independently of GPVI receptor. Furthermore, we found that podosome formation was not restricted to the mouse 129Sv background and was similarly induced by CI in C57Bl/6-derived MKs (Figure 5Ci,ii,iv,D). As noted in 129Sv-derived MKs, actin structures were discernible by phase contrast as dark dots at the leading edges and the tips of lamellae (Figure 5Ci inset), and their spatial distribution and average diameter (0.48 ± 0.06 μm, Figure 5Cii) resembled the phenotype noted previously for migrating macrophages and DCs. Adhesion to CVX (Figure 5Ciii,D) or GFOGER peptide (Figure 5Civ,D) induced also similar morphologic alterations to that observed in 129Sv-derived MKs. As illustrated in high magnification views (Figure 5Ciii-iv bottom panels), vinculin rings were evident mainly at the cell periphery.
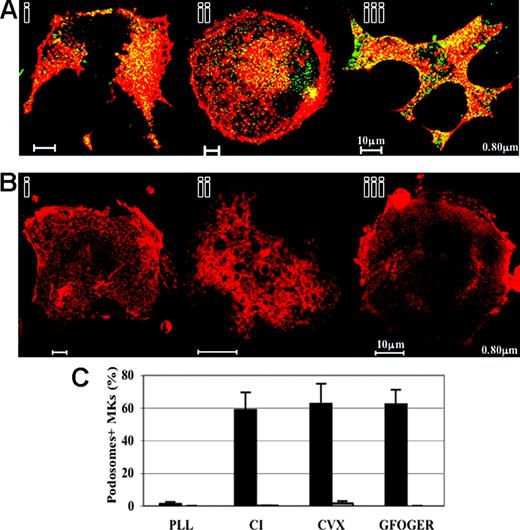
WASp has been shown to be critical for podosome formation in other hematopoietic-cell types in both human and murine models and has been found to localize to the core of podosomes in DCs, macrophages, and transformed fibroblasts.8 Costaining of actin and WASp showed colocalization of WASp and actin in the core of podosomes, mainly at the cell periphery of wild-type 129Sv MKs adherent to CI, CVX, or GFOGER peptide (Figure 6Ai, ii, and iii, respectively). To further investigate the role of WASp in podosome assembly, we analyzed actin cytoskeletal reorganization of WASp-deficient MKs. WASp-deficient MKs adherent to CI, CVX, or GFOGER peptide were completely devoid of podosomes (Figure 6Bi, ii, and iii, respectively, and Figure 6C), and exhibited diffuse vinculin staining (data not shown). The inability of MKs to form podosomes was coupled with other morphologic defects. The number of wild-type MKs seeded on CVX and exhibiting stress fibers (61% ± 2.8%) was significantly decreased compared with WASp-deficient MKs (26% ± 9.2%). Altogether, these data suggest that WASp deficiency leads to a defective MK actin cytoarchitecture. Failure to assemble podosomes may compromise normal adhesive interactions with ECM components and chemokine-induced MK migration.
Murine MKs do not display podosome structures in the absence of WASp. (A) WASp-deficient MKs were grown in parallel with C57Bl/6- and 129Sv- derived MKs and processed for immunofluorescence for WASp and F-actin as described in “Materials and methods.” F-actin (red) and WASp (green) are colocalized in the core of podosomes, mainly at the cell periphery of 129Sv-derived MKs adherent to CI (i), CVX (ii), or GFOGER peptide (iii). Bar, 10 μm. (B) WASp-deficient MKs adherent to either CI (i), CVX (ii), or GFOGER peptide (iii) do not show podosome structures. Cells extend lamellipodia, but show “cracked-like” F-actin shape (i-iii). Note that spreading and stress fiber assembly were also reduced in WASp-deficient MKs adherent to CVX (ii). Bar, 10 μm. (C) Podosome assembly is drastically impaired in WASp-deficient MKs in comparison with 129Sv-derived MKs. Bars represent the mean ± standard deviation of 3 independent experiments.
Murine MKs do not display podosome structures in the absence of WASp. (A) WASp-deficient MKs were grown in parallel with C57Bl/6- and 129Sv- derived MKs and processed for immunofluorescence for WASp and F-actin as described in “Materials and methods.” F-actin (red) and WASp (green) are colocalized in the core of podosomes, mainly at the cell periphery of 129Sv-derived MKs adherent to CI (i), CVX (ii), or GFOGER peptide (iii). Bar, 10 μm. (B) WASp-deficient MKs adherent to either CI (i), CVX (ii), or GFOGER peptide (iii) do not show podosome structures. Cells extend lamellipodia, but show “cracked-like” F-actin shape (i-iii). Note that spreading and stress fiber assembly were also reduced in WASp-deficient MKs adherent to CVX (ii). Bar, 10 μm. (C) Podosome assembly is drastically impaired in WASp-deficient MKs in comparison with 129Sv-derived MKs. Bars represent the mean ± standard deviation of 3 independent experiments.
Discussion
Microthrombocytopenia is an invariable characteristic of WASp-deficient disease in humans. The mechanisms of thrombocytopenia are not fully understood, but are thought predominantly to be due to accelerated platelet destruction in the spleen associated with an intrinsic defect of platelet production.6 WASp-deficient mice have only a moderate thrombocytopenia, which is not associated with reduced platelet size.13 Although splenectomy has not been performed in these animals to evaluate the role of platelet destruction in the mechanism of thrombocytopenia, it is possible that a defect in platelet biogenesis may be an important mechanism. Our data support this hypothesis by showing that WASp deficiency affects platelet production by disturbing the negative regulation of proplatelet formation mediated by CI through α2β1, and MK migration induced by SDF-1 in the presence of CI.
Previously, we did not observe significant cytologic differences at the ultrastructural level between normal and WASp-deficient human MKs grown in vitro. In addition, proplatelet formation occurs normally in liquid culture in the presence of TPO.12 As shown in this study, WASp-deficient murine MKs behaved similarly in vitro. However, in situ within the WASp-deficient mouse bone marrow, MKs were more numerous than in the controls, and were morphologically abnormal. This immediately suggested that the contribution of the bone marrow microenvironment is crucial for WASp-mediated effects on MKs. Moreover, proplatelet formation, true platelets, and naked MK nuclei were ectopically localized in the bone marrow of WASp-deficient mice. The increase in naked nuclei in the marrow could be explained both by the premature platelet production in the marrow and the defect in phagocytosis function of Wasp-deficient macrophages. Thus, WASp may be involved in the 2 main mechanisms, which suppress premature occurrence of proplatelet formation in the bone marrow microenvironment prior to platelet release into the blood stream (ie, MK migration and exit from the bone marrow2 ) and inhibition of proplatelet formation by the bone marrow environment (ECM4 and stromal cells5 ).
The chemokine SDF-1 has been shown to participate in the regulation of platelet formation, especially in the absence of TPO. SDF-1 appears to play an important role in allowing the interaction of MKs with endothelial cells that regulate MK terminal differentiation.19 In addition, SDF-1 controls the migration of hematopoietic cells and their retention in the bone marrow.20 It has been previously shown that WASp-deficient T cells and CD34+ cells have an altered migration in response to SDF-1.21,22 In contrast, human WAS MKs derived in vitro from CD34+ cells have a normal migratory response to SDF-1.12 We found a similar result for WASp-deficient murine MKs in the absence of ECM. In contrast, when transwells were coated with a complex ECM (matrigel) or CI to mimic a more physiologic substratum, murine WASp-deficient MKs exhibited a marked defect in migration. The mechanism for defective migration under these conditions is not fully resolved, but these findings are reminiscent of abnormalities reported in other cell lineages including DCs.23 As for DCs, we have demonstrated that primary wild-type murine MKs are able to assemble actin-rich classic podosome structures, which, interestingly, were selectively induced by adhesion to CI, and by engagement of either GPVI or α2β1 receptors. Ventral localization of podosomes8 and the condensation of vinculin24 surrounding the actin core suggest that podosomes may play a role in the dynamics of cell adhesion. In contrast to DCs, podosome assembly was temporally restricted to mature MKs (days 3 and 4 of culture), which may tie in with previous observations that differentiation of primary murine25 and human26 MKs is accompanied by an increase of GPVI expression and functional activation. In human platelets, WASp is selectively tyrosine-phosphorylated by CI,27 likely through GPVI receptor.28 The detailed role of α2β1 integrin and GPVI in WASp activation and recruitment to podosomes remains to be investigated in murine MKs. However, as shown for other WASp-deficient cell types,8 we have demonstrated here that WASp-deficient MKs were completely devoid of podosome structures, further indicating that WASp is a nonredundant regulator of podosome assembly in hematopoietic cells.
In accordance with our previous findings in primary human MKs,4 we have found that CI inhibits proplatelet formation in murine MKs. Surprisingly, we also found that WASp participates in suppression of proplatelet formation in MKs adhering to CI through a mechanism involving engagement of α2β1 integrin. Microtubule-based forces represent the main motor for proplatelet elongation, whereas a dynamic F-actin network is involved in the amplification and bifurcation of proplatelet ends to increase the yield of released platelets.29 WASp-induced podosome assembly may partly account for the negative regulation of proplatelet formation. In accordance with their ventral surface localization, WASp-induced podosomes provide localized adhesive contacts, which may generate mechanical constraints to the extension of proplatelets.
This study supports the notion that WASp participates as an intrinsic negative effector of megakaryocytopoiesis in vivo and suppresses the occurrence of premature platelet shedding within the bone marrow compartment. Whether these findings are applicable to human disease requires further investigation, particularly as there are presently several strands of evidence suggesting that human4 and murine megakaryopoiesis differ, such as in the reorganization of the actin cytoskeleton in response to CI, as shown in this study. These studies will be facilitated by the development of reagents that enable the selective down-regulation of WASp expression in normal hematopoietic cells, as recently demonstrated for human DCs.29 However, this study paves the way toward a better comprehension of the mechanisms of microthrombocytopenia in WAS patients and defines a novel role for WASp in suppression of premature platelet shedding.
Prepublished online as Blood First Edition Paper, March 7, 2006; DOI 10.1182/blood-2005-03-1219.
Supported by the European Commission (contract no. QLG1CT 1999-01090, WASPNEST program), La Ligue Nationale contre le cancer (équipe labellisée 2004), and a contract of the French Ministry of Research (ACI). S.S. was supported by fellowships from the European community and la Fondation de France; A.J.T., by the Wellcome Trust.
S.S. and A.F. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Dr Stephan Linder (Institut fur Prophylaxe und Epidemiologie der Kreislaufkrankheiten, Munich, Germany) for discussions about this work.
We would like to thank P. Ardouin and A. Rouches (Institut Gustave Roussy) for animal facilities.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal