Abstract
In a clinical study of recombinant adeno-associated virus-2 expressing human factor IX (AAV2-FIX), we detected 2 impediments to long-term gene transfer. First, preexisting anti-AAV neutralizing antibodies (NABs) prevent vector from reaching the target tissue, and second, CD8+ T-cell responses to hepatocyte-cell surface displayed AAV-capsid–terminated FIX expression after several weeks. Because the vector is incapable of synthesizing viral proteins, a short course of immunosuppression, until AAV capsid is cleared from the transduced cells, may mitigate the host T-cell response, allowing long-term expression of FIX. To evaluate coad-ministration of immunosuppression, we studied AAV8 vector infusion in rhesus macaques, natural hosts for AAV8. We administered AAV8-FIX in 16 macaques via the hepatic artery and assessed the effects of (1) preexisting anti-AAV8 NABs, (2) a standard T-cell immunosuppressive regimen, and (3) efficacy and safety of AAV8-FIX. We found that low titers (1:5) of preexisting NABs abrogate transduction, whereas animals with undetectable NABs are safely and effectively transduced by AAV8-FIX. Coadministration of mycophenolate mofetil and tacrolimus with vector does not induce toxicity and does not impair AAV transduction or FIX synthesis. These findings enable a clinical study to assess the effects of immunomodulation on long-term FIX expression in patients with hemophilia B.
Introduction
Adenoassociated virus (AAV)–mediated, liver-directed gene transfer has shown considerable promise as a treatment for a wide range of genetic diseases in canine and murine models, including hemophilia,1,2 glycogen storage disease,3 hypercholesterolemia,4 phenylketonuria,5 and Fabry disease.6 However, in the only attempt thus far to extend this approach to humans (individuals with severe hemophilia B), we observed only short-term transgene expression, maintained at a stable level for 4 weeks, then declining to baseline by 10 weeks after vector infusion.7 This phenomenon is in sharp contrast to the long-term expression seen in all other species studied including mice, rats, rabbits, dogs (> 5 years), and nonhuman primates (NHPs).1,8-10 In humans, the loss of factor IX (FIX) expression was accompanied by an asymptomatic, self-limited elevation in transaminases, and a documented cytotoxic T-cell response to AAV capsid peptides7 (K.A.H and G.F.P., unpublished data, May 2006). The implication is that hepatocytes displaying AAV capsid sequences complexed to major histocompatibility complex class I molecules on the surface of the transduced cells were destroyed by capsid-specific CD8+ T cells.7 Because the AAV capsid is not encoded in the vector and is present only transiently before being degraded, one potential solution to mitigate the host T-cell response is transient immunosuppression.
A second critical observation in the clinical study was that, whereas AAV2-FIX achieved therapeutic levels of FIX in the setting of low preexisting neutralizing antibody (NAB) titers to AAV2, the same dose of vector resulted in undetectable FIX levels in the setting of high-titer preexisting NABs. This observation suggests an inhibitory effect of AAV2 NAB on liver transduction by AAV2 vector infused via the hepatic artery,7 which is consistent with a finding in SCID mice reconstituted with pooled human intravenous immunoglobulin containing anti-AAV2 antibodies.11
To assess the effects of preexisting immunity on AAV transduction of liver, we administered AAV-FIX to 16 rhesus macaques via the hepatic artery. We used AAV8 because rhesus macaques are the natural hosts for AAV812 and thus some animals have preexisting NABs to this serotype. In addition, we assessed whether a commonly used T-cell immunosuppressive (IS) regimen alters the characteristics of AAV transduction in NHP liver.
An additional goal of this study was to assess AAV8 efficacy in a large animal model. A number of liver-targeted mouse studies have shown that AAV8 pseudo-typed vectors are more efficient in mediating gene transfer, with a 1- to 2-log increase in transduction efficiency compared to AAV2 vectors.4,12-15 However, evidence for greater efficacy and safety of AAV8 in large-animal models is sparse. Previous NHP studies have been hampered by small numbers of animals.9,10,15,16 Therefore, this study is also designed to characterize comprehensively the efficacy and safety of AAV8-FIX in rhesus macaques and to determine whether the greater efficacy achieved in mice can be reproduced in the large animal model.
Materials and methods
Animals
Male rhesus macaques were purchased from a purpose-bred colony in China and housed at Charles River Laboratory, Sierra Biomedical Division. The study protocol was approved by the institutional Animal Care Committee, and was conducted in accordance with the United States Department of Agriculture Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals.33 Prior to the studies, macaques underwent complete physical examinations and evaluation of clinical pathology parameters. Macaques were screened and were negative for tuberculosis, simian retrovirus, simian T-cell leukemia virus, and simian immunodeficiency virus.
Vector infusion procedure
The abdomen was opened by a midline incision extending from the xiphoid to the pubis. The points of origin of the celiac, hepatic, right gastric, gastroduodenal, and cystic arteries were identified, isolated, and mobilized with temporary ligatures (except for hepatic artery). A 30-gauge needle attached to a syringe infusion pump was inserted into the hepatic artery, and the vectors were infused over a 5-minute period. At the conclusion of the infusion, the needle was removed, hemostasis was ensured by direct application of pressure for 3 minutes, all ligatures were removed, and the abdominal wound was closed in layers.
Transient immunomodulation therapy
Immunosuppressants were administered twice per day orally via the nasogastric tube. Initial treatment on day –3 consisted of 0.25 mg/kg tacrolimus (FK506) and 25 mg/kg mycophenolate mofetil (MMF). The subsequent dose levels of each immunosuppressant were adjusted, ranging from 0.25 to 1 mg/kg for tacrolimus and from 25 to 100 mg/kg for MMF weekly until week 6 so as to maintain the target trough levels within the range of 5 to 10 ng/mL for tacrolimus and 2 to 3.5 μg/mL for mycophenolic acid (MPA, the active metabolite of MMF). The serum levels of MPA were determined by high-performance liquid chromatography (HPLC), and FK506 in EDTA-whole blood by HPLC/tandem mass spectrometry at Mayo Medical Laboratories (Rochester, MN), once or twice per week.
Vector construct
AAV vector construct encoding human FIX (hFIX) has been previously described.17 Briefly, the expression of the hFIX minigene was under the control of a liver-specific promoter, the human α1-antitrypsin promoter containing the hepatic control region from the apolipoprotein E (ApoE) gene. The transgene cassette was flanked by AAV2 inverted terminal repeats (ITRs) and was packaged in capsids from either AAV2 or AAV8.12 Vectors were produced by the triple transfection method in 293 cells and purified using an enhanced CsCl density gradient purification.18 The vector genome was quantified by quantitative polymerase chain reaction (QPCR) against a linearized plasmid standard.19 The purity was verified by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining. The vector preparations contained 1.1 EU/mL or less of endotoxin and were at least 99.8% monomeric as determined by dynamic light scattering.
AAV8-neutralizing antibody titer assay
Test macaque serum (15 μL) was serially diluted in mouse serum, then mixed with 15 μL AAV8-LacZ vector (2 × 109 vg), and incubated at 37°C for 1 hour. Primary rhesus macaque hepatocytes (CellzDirect, Pittsboro, NC) were rinsed and then 10 μL medium (DMEM/1% ITS + Premix [BD Biosciences, San Jose, CA]/100 nM dexamethasone [Sigma, St Louis, MO]) containing wild-type Ad5 at 200 particles/cell was added, followed by addition of the AAV8-lacZ/testing serum mixture at 1 × 104 vg AAV8-lacZ/cell. The cells were then incubated at 37°C for 2 hours before addition of 50 μL medium and further incubated at 37°C for 24 hours. LacZ-positive cells were stained, counted, and compared to those of AAV8-lacZ mixed with normal mouse serum to calculate the percent of inhibition of AAV8-lacZ transduction. The neutralizing antibody titer was determined as the highest serum dilution at which 50% or more inhibition occurred.
ELISA for hFIX in macaque plasma
For enzyme-linked immunosorbent assay (ELISA), Immulon 96-well plates were coated with monoclonal anti-hFIX heavy-chain antibody (Haematologic Technologies, Essex Junction, VT) diluted 1:3000 in 0.1 M carbonate coating buffer (pH 9.6). Plates were washed with 0.05% Tween-20/PBS, blocked with 2 mg/mL BSA in PBS, incubated with diluted macaque plasma samples, and detected by 1:1000 dilution of affinity purified HRP-conjugated goat anti-hFIX antibody (1 mg/mL, Cedarlane Labs, Hornby, ON, Canada). Levels of hFIX were determined by OD490 and quantified against the linear standard curve generated with serially diluted purified human plasma FIX (Calbiochem, San Diego, CA). The set of hFIX-specific antibodies was chosen, after extensive screening of commercially available antibodies, because of their minimal cross-reactivity to rhesus FIX, possibly due to the recognition of species-specific conformation of the FIX heavy chain. The limit of detection was 39 ng/mL hFIX.
Bethesda assay
The Bethesda assay was performed as previously described.20 Briefly, test macaque plasma was first inactivated by heating at 56°C for 30 minutes, then incubated with normal human plasma for 2 hours at 37°C. The residual hFIX activity was determined by activated partial thromboplastin time (aPTT) assay. One Bethesda unit (BU) was defined as the reciprocal of the dilution of test plasma at which 50% of hFIX activity was inhibited. The limit of detection was 0.5 BU.
Immunofluorescence staining
Immunofluorescence (IF) staining for AAV8 capsid proteins was performed on serial cryosections (12 μm) of rhesus liver. Capsids were denatured and blotted with mouse monoclonal anti-AAV2 antibody clone B1 (RDI Research Diagnostics, Concord, MA), which cross-reacts to the 8–amino-acid epitope at the C-terminus common to VP1, VP2, and VP3 proteins of AAV8. The secondary antibody used was goat anti–mouse Alexa fluor 488 (green; Molecular Probes, Eugene OR). Nuclei were counterstained with DAPI (Molecular Probes). Images were captured by a Quips imaging system equipped with a Plan-Neofluar 40×/0.75 NA objective lens (Applied Imaging, Santa Clara, CA) and mounted on a Zeiss Axioskop microscope (Carl Zeiss, Thornwood, NY). The images were not further processed.
DNA fluorescence in situ hybridization
DNA fluorescence in situ hybridization (FISH) was performed on serial cryosections (12 μm) of rhesus liver. DNA was denatured and hybridized with a cocktail of 0.2 μM 5′ HRP-labeled 30-mer oligos comprising hFIX sequences. FISH was detected using FITC-labeled tyramide signal amplification reagent (TSA-FITC; Perkin Elmer Life Sciences, Boston, MA). Nuclei were counterstained with DAPI as described in the previous section.
Southern blotting
Ten micrograms of BamHI- and BglII-digested macaque liver gDNAs were hybridized with a P32-labeled BamHI-BglII DNA fragment (3064 bp) purified from plasmid pAAV-hFIX16. Standards were generated with BamHI-BglII–digested pAAV-hFIX16 and spiked in pooled liver gDNA from naive macaques at 10 to 0.5 copy/diploid genome.
Real-time QPCR
Real-time QPCR was performed as previously described19 on gDNAs isolated from various tissues of rhesus macaques. Copy numbers of hFIX DNA were quantified by QPCR with primers and a probe annealing to exons 5 and 6 of the hFIX gene, avoiding the intron present in genomic FIX but absent in transgene FIX, thus minimizing cross-reaction to the endogenous macaque FIX gene.
Results
AAV8-FIX infused into the hepatic artery under direct visualization at a dose of 5 × 1012 vg/kg results in therapeutic levels of hFIX in the circulation
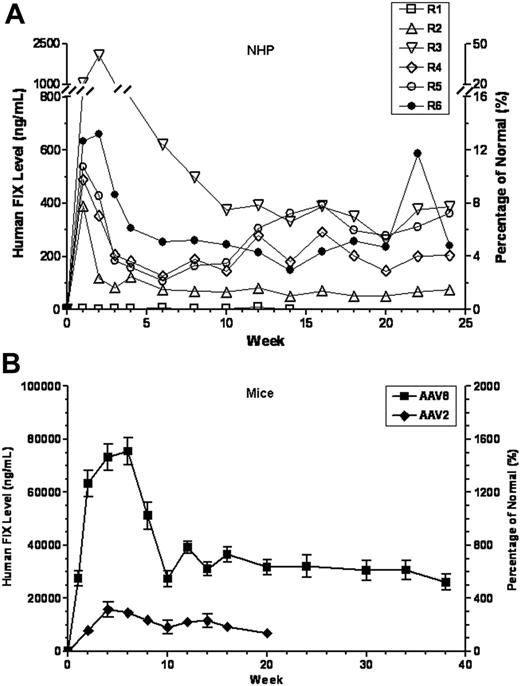
Six rhesus macaques with undetectable preexisting anti-AAV8 antibodies (group 1 in Table 1) were infused via direct injection of the hepatic artery with 5 × 1012 vg/kg of an AAV8 vector expressing hFIX (AAV8-hFIX). All animals tolerated vector infusion, at a rate of 0.2 mL/kg/min, uneventfully. Animals reached plateau levels of FIX expression at 4 to 10 weeks after vector infusion and were followed for a total of 25 weeks (Table 1; Figure 1A). In 5 of the 6 animals, the plateau levels were clearly in a therapeutic range (1.5%-9.6% of normal hFIX levels), whereas one animal (R1) showed subtherapeutic plateau FIX levels (< 1%). Transgene copy number in liver was determined by Southern blotting and correlated with plateau FIX levels (Table 1). Note that animal R1, which had no detectable circulating hFIX, also had low transgene copy number, albeit above baseline (compare to group 2 in Table 1). R1 also showed a high proportion of vector in the spleen (Table 2), suggesting technical misplacement of the injection catheter. For the remaining animals, the temporal pattern of transgene expression is characteristic of AAV8-mediated expression in liver and has been reported previously,15,21 that is, a high peak immediately after vector infusion, possibly due to rapid uncoating of AAV8 vectors, followed by a sharp decline to a plateau (Figure 1A; Table 1), attributable to the instability of reannealed but not stabilized vector DNAs.15,21
Summary of rhesus macaques treated with AAV8-hFIX vectors
Animal no. according to group . | Preexisting AAV8 NAB . | Vector dose, vg/kg . | Peak hFIX level,*ng/mL (% of normal) . | Steady-state hFIX level,*ng/mL (% of normal) mean ± SEM . | hFIX DNA in liver,†copy/diploid genome, mean ± SEM . |
|---|---|---|---|---|---|
| Efficacy study‡ | |||||
| R1 | < 1:1 | 5 × 1012 | < 39 (< 0.7) | < 39 (< 0.7) | 0.5 ± 0.2 |
| R2 | < 1:1 | 5 × 1012 | 390 (7.8) | 75 ± 6 (1.5 ± 0.1) | 3.3 ± 1.2 |
| R3 | < 1:1 | 5 × 1012 | 2074 (41.5) | 479 ± 61 (9.6 ± 1.2) | 8.5 ± 2.5 |
| R4 | < 1:1 | 5 × 1012 | 488 (9.8) | 208 ± 18 (4.2 ± 0.4) | 7.5 ± 2.3 |
| R5 | < 1:1 | 5 × 1012 | 538 (10.8) | 271 ± 29 (5.4 ± 0.6) | 5.1 ± 1.5 |
| R6 | < 1:1 | 5 × 1012 | 660 (13.2) | 312 ± 42 (6.2 ± 0.8) | 5.1 ± 1.8 |
| R7 | 1:5 | 5 × 1012 | < 39 (< 0.7) | < 39 (< 0.7) | 0.12 ± 0.01 |
| R8 | 1:5 | 5 × 1012 | < 39 (< 0.7) | < 39 (< 0.7) | 0.01 ± 0.03 |
| R9 | 1:5 | 5 × 1012 | < 39 (< 0.7) | < 39 (< 0.7) | 0.10 ± 0.05 |
| R10 | 1:5 | 5 × 1012 | < 39 (< 0.7) | < 39 (< 0.7) | 0.15 ± 0.01 |
| Immunosuppression study | |||||
| No IS regimen | |||||
| R11 | < 1:1 | 2 × 1013 | 1386 (27.7) | 206 ± 22 (4.1 ± 0.4) | ND |
| R12 | < 1:1 | 2 × 1013 | 697 (13.9) | 520 ± 48 (10.4 ± 1.0) | 1.1 ± 0.7 |
| R13 | < 1:1 | 2 × 1013 | 3396 (67.9) | 778 ± 147 (15.6 ± 2.9) | ND |
| IS regimen§ | |||||
| R14 | < 1:1 | 2 × 1013 | 2669 (53.4) | 920 ± 316 (18.4 ± 6.3) | 3.7 ± 2.4 |
| R15 | < 1:1 | 2 × 1013 | 480 (9.6) | 370 ± 30 (7.4 ± 0.6) | ND |
| R16 | < 1:1 | 2 × 1013 | 305 (6.1) | 150 ± 9 (3.0 ± 0.2) | ND |
Animal no. according to group . | Preexisting AAV8 NAB . | Vector dose, vg/kg . | Peak hFIX level,*ng/mL (% of normal) . | Steady-state hFIX level,*ng/mL (% of normal) mean ± SEM . | hFIX DNA in liver,†copy/diploid genome, mean ± SEM . |
|---|---|---|---|---|---|
| Efficacy study‡ | |||||
| R1 | < 1:1 | 5 × 1012 | < 39 (< 0.7) | < 39 (< 0.7) | 0.5 ± 0.2 |
| R2 | < 1:1 | 5 × 1012 | 390 (7.8) | 75 ± 6 (1.5 ± 0.1) | 3.3 ± 1.2 |
| R3 | < 1:1 | 5 × 1012 | 2074 (41.5) | 479 ± 61 (9.6 ± 1.2) | 8.5 ± 2.5 |
| R4 | < 1:1 | 5 × 1012 | 488 (9.8) | 208 ± 18 (4.2 ± 0.4) | 7.5 ± 2.3 |
| R5 | < 1:1 | 5 × 1012 | 538 (10.8) | 271 ± 29 (5.4 ± 0.6) | 5.1 ± 1.5 |
| R6 | < 1:1 | 5 × 1012 | 660 (13.2) | 312 ± 42 (6.2 ± 0.8) | 5.1 ± 1.8 |
| R7 | 1:5 | 5 × 1012 | < 39 (< 0.7) | < 39 (< 0.7) | 0.12 ± 0.01 |
| R8 | 1:5 | 5 × 1012 | < 39 (< 0.7) | < 39 (< 0.7) | 0.01 ± 0.03 |
| R9 | 1:5 | 5 × 1012 | < 39 (< 0.7) | < 39 (< 0.7) | 0.10 ± 0.05 |
| R10 | 1:5 | 5 × 1012 | < 39 (< 0.7) | < 39 (< 0.7) | 0.15 ± 0.01 |
| Immunosuppression study | |||||
| No IS regimen | |||||
| R11 | < 1:1 | 2 × 1013 | 1386 (27.7) | 206 ± 22 (4.1 ± 0.4) | ND |
| R12 | < 1:1 | 2 × 1013 | 697 (13.9) | 520 ± 48 (10.4 ± 1.0) | 1.1 ± 0.7 |
| R13 | < 1:1 | 2 × 1013 | 3396 (67.9) | 778 ± 147 (15.6 ± 2.9) | ND |
| IS regimen§ | |||||
| R14 | < 1:1 | 2 × 1013 | 2669 (53.4) | 920 ± 316 (18.4 ± 6.3) | 3.7 ± 2.4 |
| R15 | < 1:1 | 2 × 1013 | 480 (9.6) | 370 ± 30 (7.4 ± 0.6) | ND |
| R16 | < 1:1 | 2 × 1013 | 305 (6.1) | 150 ± 9 (3.0 ± 0.2) | ND |
ND indicates not determined.
Circulating hFIX levels in rhesus plasma were determined by ELISA (see “Materials and methods”), the limit of detection is 39 ng/mL.
Mean copy numbers of hFIX transgene DNA in liver as determined by Southern blotting of gDNAs from different hepatic lobes.
Additional 5 rhesus macaques which had anti-AAV8 titers ∼1:25 served as uninjected controls. The controls were housed in the same room and blood analyses and liver biopsies were performed along with the study subjects to monitor for any spontaneous variables. In all cases, the controls remained healthy with normal laboratory findings (data not shown).
Six-week oral treatment (twice/d) of immunosuppressants: tacrolimus and mycophenolate mofetil (see “Materials and methods”).
Biodistribution of AAV8-hFIX vector DNA in rhesus macaques 6 months following direct hepatic artery injection
Macaque no. . | Vector dose, vg/kg . | Preexisting AAV8 NAB titer . | hFIX DNA copy/diploid genome* . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Heart . | Kidney . | Liver . | Lung . | Spleen . | Testis . | Thymus . | ||||||
| Naive | 0 | NA | 0.0001 | 0.0000 | 0.0006 | 0.0000 | 0.0000 | 0.0001 | 0.0001 | ||||||
| R1 | 5 × 1012 | < 1:1 | 0.0002 | 0.0001 | 0.7606 | 0.0001 | 0.4566 | 0.0000 | 0.0003 | ||||||
| R2 | 5 × 1012 | < 1:1 | 0.0178 | ND | 4.7552 | 0.0021 | 0.0376 | 0.0017 | 0.0003 | ||||||
| R3 | 5 × 1012 | < 1:1 | 0.0342 | 0.0993 | 15.8653 | 0.0012 | 0.3650 | 0.0030 | 0.0002 | ||||||
| R4 | 5 × 1012 | < 1:1 | 0.0135 | 0.0189 | 6.6727 | 0.0001 | 1.9020 | 0.0167 | 0.0002 | ||||||
| R5 | 5 × 1012 | < 1:1 | 0.0038 | 0.0127 | 5.8211 | 0.0030 | 0.0735 | 0.0003 | 0.0000 | ||||||
| R6 | 5 × 1012 | < 1:1 | 0.0282 | 0.0213 | 5.7351 | 0.0062 | 0.1504 | 0.0022 | 0.0000 | ||||||
| R10 | 5 × 1012 | 1:5 | ND | 0.0001 | 0.1566 | 0.0000 | 0.0191 | 0.0002 | 0.0000 | ||||||
| R12‡ | 2 × 1013† | < 1:1 | 0.0807 | 0.0393 | 1.0671 | 0.0706 | 0.1626 | 0.0170 | 0.0200 | ||||||
| R14§ | 2 × 1013† | < 1:1 | 0.1442 | 0.1233 | 3.7224 | 0.0790 | 0.5691 | 0.0189 | 0.0000 | ||||||
Macaque no. . | Vector dose, vg/kg . | Preexisting AAV8 NAB titer . | hFIX DNA copy/diploid genome* . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Heart . | Kidney . | Liver . | Lung . | Spleen . | Testis . | Thymus . | ||||||
| Naive | 0 | NA | 0.0001 | 0.0000 | 0.0006 | 0.0000 | 0.0000 | 0.0001 | 0.0001 | ||||||
| R1 | 5 × 1012 | < 1:1 | 0.0002 | 0.0001 | 0.7606 | 0.0001 | 0.4566 | 0.0000 | 0.0003 | ||||||
| R2 | 5 × 1012 | < 1:1 | 0.0178 | ND | 4.7552 | 0.0021 | 0.0376 | 0.0017 | 0.0003 | ||||||
| R3 | 5 × 1012 | < 1:1 | 0.0342 | 0.0993 | 15.8653 | 0.0012 | 0.3650 | 0.0030 | 0.0002 | ||||||
| R4 | 5 × 1012 | < 1:1 | 0.0135 | 0.0189 | 6.6727 | 0.0001 | 1.9020 | 0.0167 | 0.0002 | ||||||
| R5 | 5 × 1012 | < 1:1 | 0.0038 | 0.0127 | 5.8211 | 0.0030 | 0.0735 | 0.0003 | 0.0000 | ||||||
| R6 | 5 × 1012 | < 1:1 | 0.0282 | 0.0213 | 5.7351 | 0.0062 | 0.1504 | 0.0022 | 0.0000 | ||||||
| R10 | 5 × 1012 | 1:5 | ND | 0.0001 | 0.1566 | 0.0000 | 0.0191 | 0.0002 | 0.0000 | ||||||
| R12‡ | 2 × 1013† | < 1:1 | 0.0807 | 0.0393 | 1.0671 | 0.0706 | 0.1626 | 0.0170 | 0.0200 | ||||||
| R14§ | 2 × 1013† | < 1:1 | 0.1442 | 0.1233 | 3.7224 | 0.0790 | 0.5691 | 0.0189 | 0.0000 | ||||||
NA indicates not applicable; ND, not determined.
As determined by QPCR, according to a standard curve generated with linearized plasmid DNA pAAV-hFIX. The limit of detection is 8 × 10-5 copy/diploid genome. Endogenous macaque FIX DNA was not detected since the primer and probe are specific to hFIX spanning exons 5 and 6, excluding the intervening intron.
Biodistribution determined at 9 months following direct hepatic artery injection.
Without immunosuppressive treatment.
With immunosuppressive treatment.
Therapeutic levels of circulating hFIX achieved in AAV8-naive mice and rhesus monkeys. Levels of hFIX were determined by ELISA and presented on the left y-axis, and the corresponding percentage of normal FIX levels (5000 ng/mL) is presented on the right y-axis. (A) AAV8-naive monkeys received 5 × 1012 vg/kg AAV8-hFIX vectors via direct intrahepatic artery injection. (B) C57Bl/6 mice received 1 × 1011 vg/mouse (ie, 4 × 1012 vg/kg) AAV8-or AAV2-hFIX by direct intraportal vein injection. Results presented here are means ± SEM from all data collected from 3 independent experiments comparing 3 lots of AAV8 and 2 lots of AAV2 vectors. Each time point consists of a minimum of 3 mice or a maximum of 21 mice.
Therapeutic levels of circulating hFIX achieved in AAV8-naive mice and rhesus monkeys. Levels of hFIX were determined by ELISA and presented on the left y-axis, and the corresponding percentage of normal FIX levels (5000 ng/mL) is presented on the right y-axis. (A) AAV8-naive monkeys received 5 × 1012 vg/kg AAV8-hFIX vectors via direct intrahepatic artery injection. (B) C57Bl/6 mice received 1 × 1011 vg/mouse (ie, 4 × 1012 vg/kg) AAV8-or AAV2-hFIX by direct intraportal vein injection. Results presented here are means ± SEM from all data collected from 3 independent experiments comparing 3 lots of AAV8 and 2 lots of AAV2 vectors. Each time point consists of a minimum of 3 mice or a maximum of 21 mice.
Efficacy of AAV8-hFIX is not scalable from mice to rhesus macaques
To compare FIX expression achieved by AAV8 vector in NHPs versus mice, we injected a comparable dose (4 × 1012 vg/kg) of the same lots of vector into 21 C57Bl/6 mice via portal vein (Figure 1B). AAV8-treated mice displayed a pattern of FIX expression similar to that in NHP, that is, an early high peak followed by a lower plateau, but the mean plateau level was much higher at 33 608 ± 2306 ng/mL (672% ± 46% of normal) than that (4.5% ± 1.4%) seen at a slightly higher dose (5 × 1012 vg/kg) in NHPs. Therefore, the AAV8 vector is approximately 2-logs more efficacious in mice than in NHPs.
Biodistribution study shows vector is found primarily in the liver and to a lesser extent in the spleen but not to any appreciable degree in other tissues examined
Twenty-five weeks after vector infusion, animals were humanely killed, tissues harvested, and transgene copy number determined in a QPCR assay (Table 2). In all 6 animals, vector DNA was found predominantly in the liver but was also detected in spleen, at levels ranging from less than 1% to about 50% of those in liver. The presence of detectable vector DNA in other tissues including heart and kidney is not surprising, and likely reflects hematogenous spread of vector from the infusion site in the hepatic artery. The minute quantity of vector DNA detected in the testes is consistent with findings that we have reported previously with AAV2 in mice and in humans.7,22
Vector is evenly distributed throughout the liver
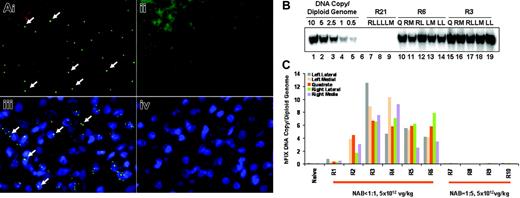
Multiple approaches were taken to assess the distribution of infused vector in the liver. First, animals underwent a liver biopsy at 4 weeks after vector infusion, and liver sections were analyzed by both DNA FISH for vector DNA and immunofluorescence staining for capsid protein. Both were found to be distributed generally evenly in the liver (Figure 2A). Second, when animals were killed at 25 weeks, gDNAs were isolated from individual hepatic lobes and transgene copy numbers determined by Southern blotting analysis (Figure 2B). All 6 animals (R1-R6) showed less than 2-fold differences in levels of transgene DNA among different hepatic lobes (Figure 2C). On average, macaques R2, R3, R4, R5, and R6 contained 3.3 ± 1.2, 8.5 ± 2.5, 7.5 ± 2.3, 5.1 ± 1.5, and 5.1 ± 1.8 copies of hFIX DNA/diploid genome, respectively (Table 1). Note that these results were comparable to those obtained by QPCR analysis (Table 2). Therefore, hepatic artery delivery of AAV8-hFIX resulted in widespread and relatively uniform hepatic distribution of vector in NHPs. IF staining for vector capsid was also carried out on liver sections obtained at 25 weeks, but was uninformative for vector distribution, because it was entirely negative (data not shown).
Preexisting AAV8 NABs abrogate AAV8-mediated liver transduction, reducing transgene copy number in liver by up to 100-fold and resulting in undetectable levels of transgene expression
To assess the impact of preexisting anti-AAV8 antibodies on liver transduction by AAV8-FIX delivered via hepatic artery, we infused the same dose of vector in 4 additional rhesus macaques that had AAV8 NAB titers of 1:5 (group 2 in Table 1). None of the 4 animals showed circulating FIX levels above the limit of detection (0.7%) following vector infusion. Furthermore, the transgene copy numbers in liver (0.01-0.15 copies/diploid genome, determined by Southern blotting; Table 1; Figure 2C) were also significantly lower than those exhibited by AAV8-naive animals (0.5-8.5 copies/diploid genome; group 1 in Table 1; Figure 2C). QPCR analysis of other tissues for macaque R10, containing the highest transgene copy number in liver among animals in this group, showed that the antibody was highly effective at blocking transduction of other tissues as well (Table 2). Thus, preexisting AAV8 antibodies at neutralizing titers as low as 1:5 effectively neutralized 5 × 1012 vg/kg AAV8 vector delivered through the circulation.
Vector infusion does not result in transaminitis
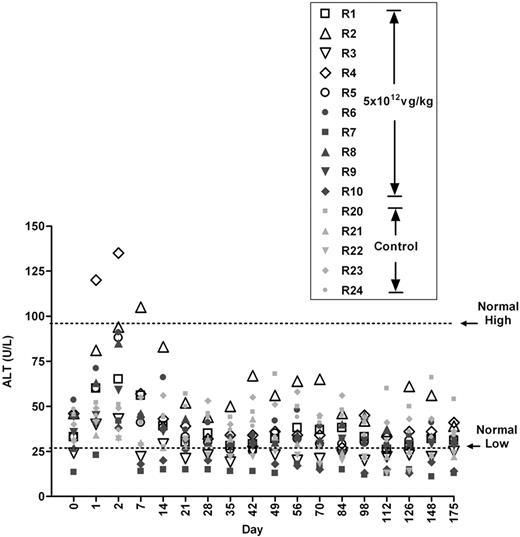
One of the purposes of this study was to determine whether we could recapitulate the transient transaminitis resulting in the destruction of the transduced hepatocytes seen in the human clinical study with AAV2. We hypothesized that, in humans, a memory T-cell response to AAV2 capsid was responsible for the induction of transaminitis.7 We further hypothesized that rhesus macaques, as the natural hosts for AAV8, may possess AAV8-specific memory T cells. However, as is apparent from Figure 3, and subsequently shown at a higher dose as well (Figure 4A), no elevation in transaminases was observed in any of the 16 animals infused with AAV8-hFIX.
Determination of hFIX gene transfer efficiency and distribution in liver. Monkeys that received 5 × 1012 vg/kg vector were biopsied at week 4 and humanely killed at week 25. (A) DNA FISH for hFIX (i and ii), and immunofluorescent staining for AAV-8 capsid protein (iii and iv) were performed on liver biopsies from an AAV8-injected animal (R3, i and iii) and from a noninjected control animal (R21, ii and iv). The detecting probe in DNA FISH and the detecting antibody in IF were labeled with Alexa fluor 488, so that positive signals appear as green punctuated dots (some identified by arrows in i and iii). Blue signal represents nuclei counterstained with DAPI (iii and iv). (B) Southern blotting on gDNAs isolated from individual lobes of rhesus liver collected at necropsy. LL indicates left lateral lobe; LM, left medial lobe; Q, quadrate lobe; RL, right lateral lobe; and RM, right medial lobe. Standards were generated with BamHI/BglII-digested plasmid DNA pAAV-hFIX spiked in pooled liver gDNAs from naive macaques at specified quantities. (C) Quantification of the copy numbers of transgene DNA per diploid genome in individual lobes of liver in each monkey as determined by Southern blotting analysis. Individual subjects, their preexisting AAV8 neutralizing titers, and the doses of AAV8-hFIX vector administered are as labeled.
Determination of hFIX gene transfer efficiency and distribution in liver. Monkeys that received 5 × 1012 vg/kg vector were biopsied at week 4 and humanely killed at week 25. (A) DNA FISH for hFIX (i and ii), and immunofluorescent staining for AAV-8 capsid protein (iii and iv) were performed on liver biopsies from an AAV8-injected animal (R3, i and iii) and from a noninjected control animal (R21, ii and iv). The detecting probe in DNA FISH and the detecting antibody in IF were labeled with Alexa fluor 488, so that positive signals appear as green punctuated dots (some identified by arrows in i and iii). Blue signal represents nuclei counterstained with DAPI (iii and iv). (B) Southern blotting on gDNAs isolated from individual lobes of rhesus liver collected at necropsy. LL indicates left lateral lobe; LM, left medial lobe; Q, quadrate lobe; RL, right lateral lobe; and RM, right medial lobe. Standards were generated with BamHI/BglII-digested plasmid DNA pAAV-hFIX spiked in pooled liver gDNAs from naive macaques at specified quantities. (C) Quantification of the copy numbers of transgene DNA per diploid genome in individual lobes of liver in each monkey as determined by Southern blotting analysis. Individual subjects, their preexisting AAV8 neutralizing titers, and the doses of AAV8-hFIX vector administered are as labeled.
Expression of an hFIX transgene does not result in inhibitor formation
In this study, none of the 16 macaques (13 treated with vector alone, 3 treated with vector and transient immunosuppression) developed inhibitory antibodies to hFIX, as determined by both ELISA and Bethesda assays (data not shown). This is in contrast to a previous report in which rhesus macaques treated with an adenoviral vector expressing hFIX developed high titer antibodies to the foreign (human) transgene.23
Transient immunosuppression with MMF and tacrolimus does not alter characteristics of AAV-mediated liver transduction
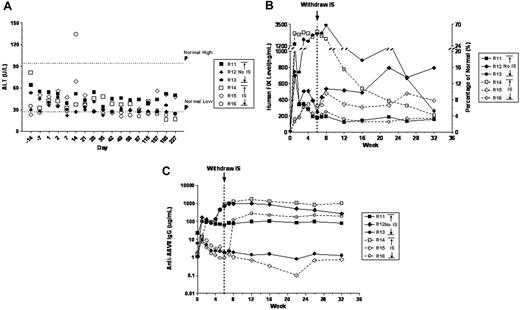
In the clinical study with AAV2-hFIX, we detected a T-cell response to AAV capsid that resulted in the destruction of transduced cells (manifested as a transient elevation in transaminases and concomitant loss of FIX expression).7 Because there was no documented T-cell response to FIX, we hypothesized that it might be possible to suppress the T-cell response to the transiently present AAV capsid and thus preserve FIX-producing hepatocytes, resulting in long-term FIX expression. A short course of immunosuppression in concert with vector infusion could block the capsid-directed T-cell response until AAV capsid has been degraded and cleared from the cells. We therefore conducted a comparative study in 6 rhesus macaques, and coadministered AAV8-FIX with or without transient immunosuppression, with 3 animals in each group (Table 1; Figure 4). We selected animals with low or undetectable NAB titers to AAV8, so that the liver could be transduced. We increased the vector dose by 4-fold to 2 × 1013 vg/kg and sought to determine if the IS regimen would alter the characteristics of AAV-mediated liver transduction. For immunosuppression, we chose a regimen consisting of MMF and tacrolimus (FK506), which are commonly used, with an excellent safety profile, to block T-cell responses in renal transplantation.24 However, because the mechanism of action of MMF is to selectively inhibit inosine monophosphate dehydrogenase, which inhibits the de novo pathway of guanosine nucleotide synthesis, and because a necessary prerequisite for AAV transduction is synthesis of the second strand of DNA, which requires GTP, it is conceivable that MMF could reduce AAV transduction efficiency. The IS regimen was administered from days –3 to 45 before and after vector infusion, whereas AAV8-hFIX vector was infused on day 1 at an infusion rate of 0.46 mL/kg/min.
All animals tolerated vector infusion well. Animals in the No IS group remained healthy, with normal serum chemistry values (including ALT/AST; Figure 4A) and hematologic parameters (data not shown) throughout the study, indicating that AAV8 vector at doses as high as 2 × 1013 vg/kg is safe and does not induce transaminitis in AAV8-naive rhesus macaques. Animals in the IS group also maintained normal ALT/AST values (Figure 4A), despite minor changes in clinical pathology parameters, including the following: (1) animal R16 had increased glucose levels in weeks 4 to 7, which were consistent with known side effects of tacrolimus; (2) all animals showed reduction in lymphocytes and total white blood cells on days 2 to 22. Most importantly, the coadministration of MMF and tacrolimus had no negative impact on AAV8 liver transduction. The expression pattern as well as the plateau levels of circulating hFIX in the animals from the IS group (3%-18.3%) and the No IS group (4.1%-15.6%) showed no statistically significant differences (P = .9251; Table 1; Figure 4B). Gene copy number was determined by QPCR in only one animal from each group, but these also showed no substantive difference (1.1 copy/diploid genome in R12, No IS; 3.7 in R14, IS), consistent with the expression data.
Monitoring levels of serum ALT in monkeys that received 5 × 1012 vg/kg of vector. Normal high and low are means from a normal reference range established at the study facility. Values from noninjected control monkeys (R20-R24) from the same colony are also shown.
Monitoring levels of serum ALT in monkeys that received 5 × 1012 vg/kg of vector. Normal high and low are means from a normal reference range established at the study facility. Values from noninjected control monkeys (R20-R24) from the same colony are also shown.
Coadministration of AAV8-hFIX and immunosuppressants was well tolerated in rhesus macaques. Six AAV8-naive macaques were assigned to 2 groups. The No IS group consisted of R11, R12, and R13 and received 2 × 1013 vg/kg AAV8-hFIX16. The IS group consisted of R14, R15, and R16 and received the same dose of the vector in combination with MMF and tacrolimus. (A) Serum ALT levels. Dotted lines represent the normal reference range at the study facility. (B) Levels of hFIX detected in macaque plasma by ELISA (left y-axis) and their corresponding percentage of normal FIX levels (5000 ng/mL; right y-axis). Dotted line indicates when immunomodulatory therapy was stopped. (C) The relative levels of anti-AAV8 IgG detected in rhesus macaque plasma by ELISA before and after AAV8-hFIX infusion. Dotted line indicates when immunomodulatory therapy was stopped.
Coadministration of AAV8-hFIX and immunosuppressants was well tolerated in rhesus macaques. Six AAV8-naive macaques were assigned to 2 groups. The No IS group consisted of R11, R12, and R13 and received 2 × 1013 vg/kg AAV8-hFIX16. The IS group consisted of R14, R15, and R16 and received the same dose of the vector in combination with MMF and tacrolimus. (A) Serum ALT levels. Dotted lines represent the normal reference range at the study facility. (B) Levels of hFIX detected in macaque plasma by ELISA (left y-axis) and their corresponding percentage of normal FIX levels (5000 ng/mL; right y-axis). Dotted line indicates when immunomodulatory therapy was stopped. (C) The relative levels of anti-AAV8 IgG detected in rhesus macaque plasma by ELISA before and after AAV8-hFIX infusion. Dotted line indicates when immunomodulatory therapy was stopped.
Withdrawal of immunosuppression leads to a rise in anti-AAV8 antibody titers but no anti-FIX antibody formation
In these experiments, the IS regimen did not affect the antibody response to AAV8 capsid. Two of the 3 animals in the No IS group showed a significant increase in anti-AAV8 IgG levels over their respective pretreatment baseline following vector infusion, whereas a third macaque R13 had no discernible increase in AAV8 antibody levels (Figure 4C) despite significant hFIX expression (Figure 4B) and thus successful vector infusion. In comparison, one of the 3 animals in the IS group (R14) developed a strong anti-AAV8 IgG response despite the IS treatment, whereas the other 2 macaques did not (Figure 4C). The indication that MMF and tacrolimus may have transiently suppressed an anti-AAV8 antibody response came from macaque R16, in which the withdrawal of immunosuppression at week 6 led to a 2-log increase in anti-AAV8 IgG levels within 2 weeks (Figure 4C). This marked rise in antibody titer at 6 weeks indicates the presence of AAV8 capsid antigen, either in the form of capsid or presented on the surface of antigen-presenting cells. None of the animals developed inhibitory antibodies to hFIX (data not shown).
Discussion
We showed previously that recombinant AAV2 vectors can transduce human liver and that therapeutic dose ranges in humans were accurately predicted by preclinical testing in the dog model.7 Unexpectedly, though, expression at therapeutic levels in humans was short-lived and appeared to be terminated by a cytotoxic T-cell response to transduced hepatocytes that had not been observed in other species7 (and G.F.P. and K.A.H., unpublished data, May 2006). A detailed analysis of peripheral-blood mononuclear cells (PBMCs) from an infused human subject revealed a T-cell response to AAV capsid sequences, but not to FIX, leading to the hypothesis that transient immunosuppression could block the capsid-directed T-cell response until capsid had been degraded and cleared from the hepatocytes, thus permitting long-term FIX expression.
As a genetically engineered viral vector, recombinant AAV2 has been successful at transducing both skeletal muscle and liver in humans.7,25,26 However, as a defective virus to which most humans have had prior exposure, its transduction is hindered by host memory immune response, a problem substantial though not insurmountable, and a problem that is addressed in the current study. From an immunologic standpoint, the vector presents 2 classes of antigens—the viral capsid proteins that are not encoded in the vector and de novo synthesized transgene product, FIX. Consequently, the host can mount both B-cell and T-cell responses to each of these antigens. The NHP studies reported here bear on the T- and B-cell responses to the vector capsid and also on the B-cell response to FIX.
It was expected that high titers (≥ 1:500) of preexisting antibodies to the AAV capsid would have the ability to effectively neutralize vectors infused via the circulation, thus blocking transduction of hepatocytes in NHPs.15,27 However, it was surprising to realize the inhibitory potency of NABs at low titers, as illustrated by our prior clinical study,7 the current NHP study, and a passive immunity study in mice.11 In this study, NHPs with NAB titers as low as 1:5 showed a complete absence of FIX expression (< 0.7%; Table 1) and absence of vector DNAs in all tissues examined (Table 2). This is consistent with findings in the clinical trial,7 where 2 subjects infused with the same dose of AAV2-hFIX displayed maximum levels of circulating FIX that were inversely correlated to their pretreatment AAV2 NAB titers (11% in subject with AAV NAB < 1:2, and 2% in subject with AAV NAB 1:177 ).
Preexisting NABs to AAV2 are easily screened for, and other solutions, including use of alternate serotypes, use of balloon catheter-mediated delivery techniques that minimize exposure of vector to flowing blood,28 or plasmapheresis to reduce antibody titers, may allow their inhibitory effects to be circumvented. Moving down the phylogenetic tree, to AAVs that infect lower mammals or nonmammalian species, may also avoid the antibody cross reactivity.29
A preexisting capsid-specific CD8+ T-cell response, on the other hand, has the capacity to drive an adverse event, namely, hepatotoxicity, which, though self-limited in the human studies, is nonetheless a concern, because the destruction of AAV2-transduced hepatocytes will result in the loss of FIX expression.7 Because AAV2 capsid is present in cells only transiently before being degraded and cleared, our long-term goal is to determine whether a well-established anti–T-cell regimen, administered transiently with rAAV2 vector, can block the CD8+ T-cell response to capsid and thus permit sustained expression of FIX. As a prelude to human studies, we therefore analyzed in NHPs the safety of coadministration of IS drugs with an AAV vector.
We chose AAV8 as the vector in NHPs, in an attempt to develop a model of the hepatotoxicity and loss of FIX expression seen in humans. Because NHPs are natural hosts for AAV8, it seemed plausible that animals infused with an AAV8 vector might activate a memory T-cell response to the vector capsid. The result from the study, however, demonstrates that even high doses of AAV8-hFIX vectors failed to provoke transaminitis in any of the 16 treated animals. It is possible that in macaques with AAV8-specific memory T cells, AAV8-hFIX vectors were completely neutralized by preexisting antibodies before they could transduce hepatocytes and induce transaminitis (group 2, Tables 1, 2). If so, the difficulty in identifying macaques with prior exposure to AAV8 (thus primed T cells) and low titers of antibodies (low enough to allow vector transduction) hampers our efforts to recapitulate the host T cell-mediated immune response to AAV-transduced hepatocytes that has been observed in humans.7
Because we were unable to trigger transaminitis in NHPs, perhaps because the ideal animals (low NAB titer but positive memory T-cell response to AAV8 capsid) had not been included in our study, we cannot assess the efficacy of the IS regimen in blocking the late-occurring transaminitis. However, our data can address safety and can also assess whether the IS regimen we selected interferes with AAV-mediated liver transduction.
Coadministration of AAV8-hFIX and the IS regimen of MMF and tacrolimus appeared safe. There were no untoward side effects, and importantly, no compromise of transduction efficiency in the immunosuppressed versus the nonimmunosuppressed animals, as evidenced by similar plateau levels of FIX and similar transgene copy number in both groups (Table 1; Figure 4B). In addition, the regimen was safe in that it did not result in formation of inhibitory antibodies to hFIX in the rhesus macaques. This is a point that deserves emphasis. A particular advantage of AAV as a gene delivery vehicle for genetic disease is that expression from an AAV vector in the liver appears to induce tolerance to the transgene product,30 through a mechanism dependent on CD4+CD25+ regulatory T cells.31 The infusion of an AAV vector expressing hFIX into rhesus macaques mimics the situation in hemophilic patients because the FIX proteins of the 2 species differ slightly (at 11/461 residues), much as a patient with hemophilia has a coding sequence that differs from wild-type hFIX. That rhesus macaques can form NABs to hFIX has been demonstrated previously by infusion of an adenoviral vector expressing the human transgene into rhesus macaques (3 of 3 animals formed antibodies to hFIX23,32 ). In the current study, neither the animals infused with AAV-hFIX alone (0 of 13) nor those infused with vector and IS regimen (0 of 3) developed antibodies to hFIX. These data are consistent with the notion that expression of a transgene in liver from an AAV vector induces tolerance to the transgene product,30 and further indicate that the IS regimen chosen does not interfere with induction of tolerance to the transgene product.
It should be noted that the anti–T-cell regimen used here does not reliably block B-cell response to capsid (Figure 4C). MMF and tacrolimus in concert suppress cytotoxic T-cell responses, but only MMF has an effect on B-cell proliferation, because it inhibits de novo guanosine nucleotide synthesis, a pathway commonly required for both T- and B-cell proliferation. Tacrolimus specifically inhibits T-cell activation by inhibiting IL-2 production and exerts no effect on B-cell proliferation. Thus, the rise in anti-AAV titers for animals on an IS regimen is not surprising, especially because the MMF dose used in this study was not optimized for suppression of B-cell proliferation. The fact that one of the animals had a marked increase in titer after IS was withdrawn at 6 weeks after injection does serve as an indicator that capsid antigen was still present on antigen-presenting cells that could be recognized by helper T cells at that time point, resulting in the boost of B-cell proliferation and antibody production. We interpret this to mean that the IS regimen would be required for longer than 6 weeks for this particular serotype. We do not believe that the B-cell response to capsid on this IS regimen predicts or represents the T-cell response, the end point of interest.
Finally, 2 other findings should be underscored. First, the data contribute to a growing body of evidence that the superiority, by logs in some reports, of AAV8 pseudo-typed vectors in mice, does not translate to large animals. Thus, in this report, 5 × 1012 vg/kg AAV8-hFIX produced steady-state levels of hFIX in rhesus macaques (n = 6) at levels 2-log less than levels seen with a comparable dose in C57Bl/6 mice (n = 21) (4.5% ± 1.4% versus 672% ± 46%). The hFIX levels achieved by AAV8-hFIX in macaques reported here are comparable to those achieved with similar doses of AAV2-hFIX in NHPs (0.8%-10% of normal).9,16 This difference needs to be borne in mind when designing human trials, because, for AAV2, dosing in large animals has been a better predictor of levels of expression in humans than have dosing studies in mice.7
Second, we demonstrate for the first time that AAV vector infused via the hepatic artery distributes relatively evenly within the liver. These data are of interest for investigators considering follow-up liver biopsies in subjects infused with AAV to the liver.
At this time, the critical issue in AAV-mediated gene transfer to human liver is whether long-term expression can be achieved. Our previous work demonstrates that AAV2 can transduce human liver and lead to expression of FIX at therapeutic levels.7 The duration of expression appears to be shortened by a T-cell response to AAV2 capsid, an antigen that is transiently present after vector infusion. We demonstrate here that coadministration of an extensively used IS regimen with AAV8-FIX does not lead to toxicity and does not interfere with AAV transduction of hepatocytes. From an immunologic perspective, the IS regimen is not associated with formation of inhibitory antibodies to hFIX. However, the NHP model, as studied herein, does not allow us to determine whether the anti–T-cell regimen we used can block the presumed memory T-cell response to capsid-presenting hepatocytes because we were unable to trigger such a response in the NHPs studied. It is possible that we will be able to use the NHP model to demonstrate safety but that efficacy in blocking an unwanted CD8+ T-cell response in this particular setting can only be assessed in humans.
Prepublished online as Blood First Edition Paper, July 25, 2006; DOI 10.1182/blood-2006-04-017913.
Supported in part by Bayer HealthCare. K.A.H. and F.M. were supported by the National Institutes of Health (NIH), National Heart, Lung and Blood Institute (NHLBI), Programs of Excellence in Gene Therapy (PEGT) grant U01 HL66948, NIH NHLBI grand P01 HL078810, and Howard Hughes Medical Institute.
The authors declare no competing financial interests.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
G.F.P. is currently at Bayer HealthCare Pharma, Berkeley, CA 94710; e-mail: glenn.pierce.b@bayer.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal