Abstract
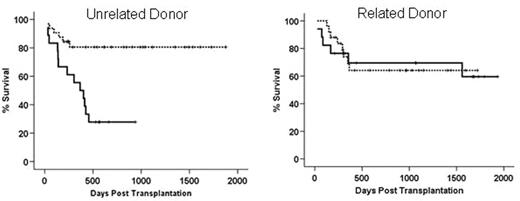
Genetic polymorphisms of various donor and host genes have been found to be associated risk factors for graft versus host disease and transplant related mortality. Most of these genes are involved in the regulation of immune response and inflammatory reactions towards pathogens. The heme oxygenase (HO) protein is the rate limiting enzyme in heme degradation to biliverdin, free iron and carbon monoxide. Its inducible form I (HO-I) is constitutively expressed in the spleen due to the abundance of its substrate. It is inducible in a wide range of tissues upon exposure to cellular stress factors such as irradiation, bacterial lipopolysaccharide, proinflammatory cytokines and heat shock. Further, HO-I is involved in regulating inflammatory response and has been described as a “protective gene” in solid organ transplantation. In man the promoter region of HO-I displays length polymorphism due to a variable number of gt repeats within a 500bp region upstream of the TATA box. Individuals exhibiting 25 or less gt repeats express HO-I upon cellular stress at a higher level than individuals with more than 30 gt repeats. We retrospectively analysed length polymorphisms of 92 donor and host pairs undergoing allogeneic stem cell transplantation for various malignancies. We show here that donor promoter length polymorphism leading to low expression of HO-1 (>30 gt repeats) is associated with improved overall survival, relative to donors with ≤ 30 repeats (80.6% versus 54.4%, p=0.023). When subgrouped, having HLA identical siblings as donors had no influence on outcome (see figure below, right panel) whereas having matched unrelated donors strongly influenced overall survival (82.6 vs. 44.2%, p=0.015). Combination of high HO-1 expressing unrelated donors and high expressing hosts displayed a 27.8% survival (left panel, solid line vs.81.3% for individuals with one allele >30 repeats dotted line). Polymorphisms of the recipient HO-1 genes did not influence outcome after transplantation. When matched unrelated donors were used, the incidence of grade III gut GvHD was significantly higher when (p=0.032) and overall GvHD (≥ grade III) displayed a trend towards significance (p=0.098). Transplant related mortality was strongly influenced by having a donor who expresses HO-1 at a high level upon cellular stress (p=0.006). In a multivariate Cox regression analysis of pretransplant factors such as age, disease stage, sibling/unrelated donor etc. polymorphism in the donor HO-1 gene had a significant influence on overall survival (p=0.027). These data suggest a role for HO-I in GvHD pathophysiology and a possible role for HO-1 as a non MHC risk factor for unrelated transplantation.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal