Abstract
Since NHL is radiosensitive, total body irradiation (TBI) has been used as part of HDT and ASCT for NHL. However, due to short-term and long-term toxicity associated with TBI, alternative regimens have been developed. We have reported that Zevalin at conventional and high doses can be given in combination with HDT and ASCT in patients (pts) with poor-risk or relapsed B-cell NHL without additional toxicity. Given the efficacy of Zevalin in FL and DLBCL, we retrospectively evaluated the outcome of HDT and ASCT in pts with FL and DLBCL who received Zevalin-based HDT regimens (Z-ASCT) and compared to those receiving TBI-based regimen (TBI-ASCT)Between 1/2000 to 1/2006, 187 pts with FL grade I/II (30), FL grade III (20) and DLBCL (137) underwent HDT and ASCT, 62 received Z-ASCT while 125 received TBI-ASCT. For Z-ASCT, pts < 60 years old without prior radiotherapy (RT) received high-dose Zevalin in combination with high-dose etoposide and cyclophosphamide while pt > 60 yrs or with prior RT received conventional dose Zevalin plus high-dose BEAM. TBI-ASCT was performed during the same period for the following reasons: ineligible for Z-ASCT, pt refusal, physician preference and protocol closure. The pt characteristics between the two groups were similar with respect to histology, disease status, prior regimens, bulky disease, B symptoms and performance status. However, the median age was younger for TBI-ASCT (49 vs. 53, p=0.01) and there were more chemo-resistant pts in the Z-ASCT group (p=0.01).
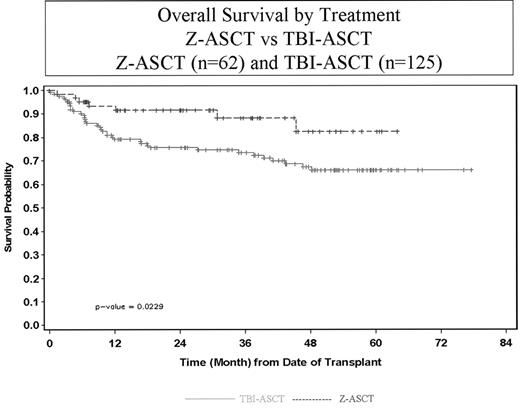
Results: At a median follow-up of 28 months (range 2–64) for Z-ASCT and 38 months (range 1–78) for TBI-ASCT, the 2-year overall survival (OS) and disease-free survival (DFS) were 91% (95% CI, 82–96) and 74% (95% CI, 64–82), respectively for Z-ASCT, and 76% (95% CI, 69–80) and 72% (95% CI, 65–77), respectively for TBI-ASCT(Figure 1). OS remained significantly different when first complete remission pts were excluded from analysis (p=0.019). Multivariate models were generated for the primary endpoints of the study (OS and DFS). The results of these analyses showed that the risk of death and/or relapse was less among the Z-ASCT pts after adjusting for baseline differences (ie. Age, performance status and chemosensitivity status at transplant), and other factors (i.e., disease status at transplant, number of previous chemotherapies) previously shown to be associated with survival/disease free survival post transplant (OS: p<0.01 | DFS: p<0.10).
Conclusion: Zevalin in combination with HDT followed by ASCT was associated with significantly improved survival in pts with poor-risk or relapsed/refractory FL and DLCBL when compared to TBI-ASCT. Further studies and longer follow-up are required to evaluate the long-term efficacy and safety of Zevalin in the HDT/ASCT setting.
Disclosures: The study is sponsored by Biogen/IDEC.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal