Comment on Kalfa et al, page 3637
A paper by Kalfa and colleagues in this issue of Blood shows that Rac GTPases regulate actin assembly in mouse red cells, overturning previous assumptions that actin filaments are stable structural elements in the spectrin-actin membrane skeleton.
It has been an article of faith for more than 20 years that the short actin filaments in the red cell membrane skeleton function principally as core structural elements in junctional complexes that serve to link the spectrin molecules at their ends, forming the vertices of the hexagonal spectrin-actin network.1 This idea is derived from the precisely controlled length distribution of the actin filaments to 12 to 18 subunits, the lack of a significant free actin monomer concentration in the cytosol of mature human red cells (< 10 μg/mL), and the presence of caps at both filament ends that prevent growth or shrinkage. These are adducin, which caps the barbed end, and tropomodulin, which caps the pointed end, blocking subunit addition or dissociation and preventing actin filament turnover.2 These observations have led to the belief that actin filaments do not turn over in the red cell membrane skeleton.
This presumed stability of the actin in the red cell contrasts with the regulated and dynamic actin assembly and disassembly in other cell types, where the transmittance of extracellular signals to the actin cytoskeleton is accomplished through the Rho family proteins, Rac, Rho, and Cdc42.3 The function of these proteins is to serve as molecular switches, which activate a variety of actin regulators, including Arp2/3, formins, and cofilins. The mechanisms by which the Rho proteins activate these downstream targets are manifold, and include signaling through WASP, p21-activated kinase (PAK), Rho kinases (RhoK), protein kinase Cs (PKCs), and IQGAP. The ultimate result of this plethora of signals is constant: in all cases of Rho-family activation, actin dynamics is increased.
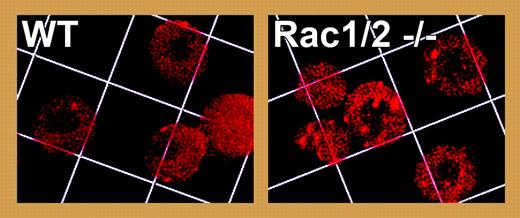
To study Rac signaling in red cells, Kalfa and colleagues have analyzed a hematopoietic-specific conditional knock-out for Rac1 in a Rac2-null genetic background. Mice whose red cells lack Rac1 and Rac2 develop a microcytic hemolytic anemia with bizarrely shaped microspherocytes, fragmented cells, and increased reticulocytosis. Rac1/2 knock-out red cells are less deformable, and their membrane skeletons are grossly disorganized, with gaps and aggregates in the actin filament network (see figure), increased levels of actin, and reduced levels of spectrin on the membrane. Notably, a striking increase in phosphorylation of adducin is observed at Ser724, a PKC site in the C-terminal actin-capping domain, and the phospho-adducin is released from the membrane skeleton of Rac1/2 knock-out red cells. In vitro, phosphorylation of adducin reduces its barbed-end capping activity and its ability to recruit spectrin to actin filaments.4 Thus, in the absence of Rac activity we can speculate that PKC phosphorylation of adducin and its dissociation from the membrane skeleton leads to uncapping of barbed ends and promotion of actin assembly, followed by filament rearrangements and destabilization of the spectrin-actin membrane skeleton.FIG1
Disorganization and aggregation of actin in Rac1/2 double knock-out red cells revealed by staining for actin filaments with rhodamine-phalloidin. Grid square = 7.3 μm. See the complete figure in the article beginning on page 3637.
Disorganization and aggregation of actin in Rac1/2 double knock-out red cells revealed by staining for actin filaments with rhodamine-phalloidin. Grid square = 7.3 μm. See the complete figure in the article beginning on page 3637.
The roles for actin filament assembly and turnover in the other cellular processes in which the rho family proteins are involved, such as cell migration, vesicular transport, and cell polarization are obvious.3 What actin dynamics would accomplish in the red cell is less clear. One possibility raised by the data from Kalfa and colleagues is that Rac signals and their accompanying actin dynamics are required during red cell maturation to correctly assemble the mature membrane skeleton. Alternatively, regulated turnover of the short actin filaments may help confer the remarkable deformability and dynamic plasticity of red cell shapes under shear stress in the circulation. In any case, it is clear that the red cell still has many lessons to teach us about actin dynamics and its regulation. Furthermore, this study points to likely roles of Rho-family proteins in the molecular mechanisms underlying abnormal red cell shape and deformability, and highlights these and other signaling proteins as potential new targets in treatment of hemolytic anemias.
The authors declare no competing financial interests. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal