Erythroid progenitors have the potential to proliferate rapidly in response to environmental stimuli. This process is referred to as stress erythropoiesis, with erythropoietin (EPO) playing central roles in its promotion. In this study, we wanted to elucidate the molecular mechanisms governing the regulation of stress erythropoiesis and the maintenance of red-cell homeostasis. This was achieved by our development of a noninvasive real-time monitoring system for erythropoiesis using transgenic mouse lines expressing luciferase under the control of the mouse Gata1 hematopoietic regulatory domain (G1-HRD-luc) or human β-globin locus control region (Hbb-LCR-luc). Optical bioluminescence images revealed that the luciferase was specifically expressed in spleen and bone marrow and was induced rapidly in response to anemia and hypoxia stimuli. The G1-HRD-luc activity tracked the emergence and disappearance of proerythroblast-stage progenitors, whereas the Hbb-LCR-luc activity tracked erythroblasts and later stage erythroid cells. Increased plasma EPO concentration preceded an increase in G1-HRD-luc, supporting our contention that EPO acts as the key upstream signal in stress erythropoiesis. Hence, we conclude that G1-HRD-luc and Hbb-LCR-luc reporters are differentially activated during stress erythropoiesis and that the transgenic mouse lines used serve as an important means for understanding the homeostatic regulation of erythropoiesis.
Introduction
The lifespan of circulating erythroid cells is limited, rendering continuous erythropoiesis a necessity throughout the life of the animal. Erythroid progenitors have the potential to proliferate rapidly in response to anemia and hypoxia stimuli, a process referred to as stress erythropoiesis and mediated by erythropoietin (EPO).1,2 EPO is a glycoprotein hormone,3 and its expression is induced by various erythropoietic stimuli. During erythropoiesis, hematopoietic stem cells (HSCs) first give rise to common myeloid progenitors (CMPs) with the potential to differentiate toward erythroid, megakaryocytic, and granulocytic/monocytic cell lineages.4 CMP progenitors differentiate into bipotential megakaryocytic and erythroid progenitors (MEPs).4 The earliest erythroid-committed progenitors are those corresponding to erythroid burst-forming units (BFU-Es), which further differentiate through erythroid colony-forming units (CFU-Es) and proerythroblasts into erythroblasts. These erythroid progenitors, which account for a rare population of hematopoietic cells under steady state conditions, have the potential to proliferate rapidly in response to acute erythropoietic stimuli.5-7 In particular, the number of CFU-Es increases markedly during stress erythropoiesis. The receptor for erythropoietin (EpoR), which is expressed abundantly in CFU-E stage progenitors,8 plays a crucial role in promoting the erythropoietic response.9,10
Erythropoietic stimuli result in tissue hypoxia and induce EPO gene expression through the activation of hypoxia-inducible transcription factors. EPO–EpoR signaling results in the survival, proliferation, and differentiation of erythroid progenitors (for a review, see Jelkmann11 ). In addition, recent studies reveal that a variety of regulatory molecules, such as glucocorticoid hormone receptor,6 retinoblastoma tumor suppressor,12 BMP4/Madh5,13 and α4 integrin,14 are involved in stress erythropoiesis either independently of or dependent on the EPO–EpoR signaling pathway. Interactions among erythroid progenitors and their microenvironments play critical roles in promoting the acute erythropoietic response.13,14 To decipher this complex regulatory network of erythropoietic homeostasis, the roles that individual regulatory factors play in erythropoiesis must be elucidated. Similarly, it is important to evaluate precisely their in vivo erythropoietic status.
In this study, we generated transgenic lines of mice, which allowed us to evaluate erythropoiesis in living mice. We made use of firefly luciferase as a reporter gene because the expression of luciferase can be monitored noninvasively and quantitatively as a bioluminescent signal with an optical in vivo imaging system (for a review, see Massoud and Gambhir15 ). For example, a luciferase reporter mouse with the promoter of the murine vascular endothelial growth factor receptor 2 gene has been reported as a useful model for monitoring angiogenesis in living mice.16 In vivo imaging of an activated estrogen receptor revealed a tissue-specific response of the transgene to the estrogen signal, and the study successfully and simultaneously demonstrated differences in the hormone dependence of estrogen receptor activation among several tissues in the same animal.17
Two erythroid-oriented gene regulatory domains were used in the generation of transgenic mouse lines for evaluating erythropoiesis—the hematopoietic regulatory domain of the mouse Gata1 gene (G1-HRD) and the human β-globin locus control region (Hbb-LCR) linked to the β-globin promoter.14,15,18 GATA-1 is a transcription factor whose expression is restricted to erythroid cells, megakaryocytes, mast cells, eosinophils, and Sertoli cells in the testis.18-22 The 8-kb region of the murine Gata1 gene, spanning 3.9 kb 5′ of the proximal first exon to the second exon, provides a necessary and sufficient gene segment for recapitulating Gata1 gene expression in erythroid-lineage cells.23-25 Firefly luciferase reporter lines generated with G1-HRD and Hbb-LCR are referred to as G1-HRD-luc and Hbb-LCR-luc, respectively.
Here we present data demonstrating that G1-HRD-luc activity in spleen is markedly increased in response to various erythropoietic stresses. The Hbb-LCR-luc mice showed a delayed induction of luciferase expression on acute anemia compared with G1-HRD-luc mice. The expression of G1-HRD-luc corresponds tightly to the emergence and disappearance of erythroid progenitors and proerythroblasts during acute erythroid expansion. In contrast, Hbb-LCR-luc expression corresponds to erythroblasts and cells in much later stages. The data also revealed an increased plasma EPO concentration that preceded G1-HRD-luc activation during erythroid cell expansion. These results thus provide compelling evidence that the EPO–EpoR system primarily responds to erythropoietic stresses, followed by the induction of GATA-1 expression in erythroid masses in the body. The G1-HRD-luc and Hbb-LCR-luc mouse models offer sensitive, noninvasive, real-time monitoring of erythropoiesis in vivo.
Materials and methods
Generation of transgenic mice
To make the luciferase reporter driven by G1-HRD, firefly luciferase cDNA was cloned into the pIE3.9int vector, a plasmid containing an 8.0-kb region of G1-HRD.23 Construction of G1-HRD-GFP was previously described.24 These constructs were linearized with SalI to release the G1-HRD-luc or G1-HRD-GFP transgene cassette for generating transgenic mice. To generate Hbb-LCR-luc mice, a firefly luciferase cDNA fragment was cloned into the pEV3 vector containing human β-LCR and the β-globin promoter.26,27 These DNA fragments were injected into fertilized BDF-1 eggs by the standard procedure. Because the black coat of BDF-1 mice reduces bioluminescent signals, transgenic founders were crossed with ICR mice to establish transgenic lines with white coats. For screening G1-HRD-luc and Hbb-LCR-luc transgenes, tail DNA was extracted and polymerase chain reaction (PCR) was performed using the primer pair fireluc For (5′-GGGCATTTCGCAGCCTACCGTGGTGTT-3′) and firelucRev (5′-GGGGAGCGCCACCAGAAGCAATTTCGTGTA-3′). For screening the G1-HRD-luc/GFP dual reporter mice, PCR detection of GFP cDNA was also performed as previously described.24
In vivo and ex vivo imaging of luciferase activity
In vivo bioluminescence imaging was performed with an IVIS imaging system (Xenogen, Alameda, CA). G1-HRD-luc and Hbb-LCR-luc transgenic mice were anesthetized with isofluorane and injected intraperitoneally with 75 mg/kg D-luciferin. Ten minutes after luciferin injection, mice were imaged for 5 to 10 seconds. Photons emitted from specific regions were quantified with Living Image software (Xenogen). Ex vivo imaging was performed by imaging, in 300 μg/mL luciferin, the organs isolated from G1-HRD-luc mice humanely killed immediately after the injection of luciferin at a concentration of 75 mg/kg.
Stimulation of erythropoiesis
Phenylhydrazine (PHZ; 60 mg/kg) was injected intraperitoneally for 2 consecutive days. Phlebotomy was performed 4 times to remove 0.3 mL blood at 12-hour intervals from the retro-orbital venous plexus with the use of heparin-coated microtubes. For hypoxic exposure, mice were kept in an isolated chamber (Natsume, Tokyo, Japan) for 4 hours. In this chamber, room air was partially replaced with nitrogen gas to keep the oxygen concentration at 6%. Human recombinant EPO (a generous gift from Chugai Pharmaceutical, Tokyo, Japan) was injected subcutaneously at a concentration of 300 U/kg for 5 consecutive days to induce erythropoiesis.
Cell sorting analysis
Sorting and analysis of cells were performed using FACSVantage (Becton Dickinson, San Jose, CA). Mononuclear cell (MNC) suspensions from the spleen were prepared and sorted into GFP-negative, GFP-low, and GFP-high fractions. To separate early erythroid progenitors (EEPs) and late erythroid progenitors (LEPs), MNC suspensions from the spleen were incubated with biotinylated monoclonal antibodies recognizing Mac-1, Gr.1, Ter119, B220, CD4, and CD8. MNCs bound to these antibodies were removed by magnetic separation using streptavidin-conjugated magnetic beads (Polysciences, Warrington, PA). Unbound cells were collected and stained with phycoerythrin-conjugated anti-CD71 and allophycocyanin-conjugated anti–c-Kit antibodies. All antibodies were obtained from BD PharMingen (San Diego, CA). The luciferase activity of each fraction was determined with a dual-luciferase reporter assay system (Promega, Madison, WI) according to the manufacturer's instructions.
Hematocrit and reticulocyte counts and plasma EPO concentration
Peripheral blood (20-50 μL) was obtained from the tail for hematocrit and reticulocyte counts. Reticulocytes were counted by measuring the number of reticulocytes and erythroid cells in blood smears stained with new methylene blue. Plasma EPO concentration was measured using an EPO enzyme-linked immunoabsorbent assay (ELISA) kit (Roche, Basel, Switzerland) according to the manufacturer's instructions.
RNA blot analysis
Total RNA was isolated from the spleen with Isogen (Nippon Gene, Toyama, Japan) according to the manufacturer's instruction. Fifteen micrograms purified total RNA was denatured and separated on a 1% agarose gel containing formaldehyde, followed by capillary transfer onto a nylon membrane (Zeta Probe Blotting Membrane; Bio-Rad Laboratories, Mississauga, ON, Canada). Fragments of GATA-1 and G3PDH cDNA were labeled with 32P-dCTP and the Rediprime labeling system (Amersham Biosciences, Amersham, United Kingdom) and were used as probes. Hybridization and washing were performed according to the instructions for the blotting membrane (Zeta Probe; Bio-Rad Laboratories).
Results
Generation and bioluminescence imaging of G1-HRD-luc mice
To monitor stress erythropoiesis noninvasively in vivo, we established 4 lines of G1-HRD-luc transgenic mice. Luciferase expression was examined in adult mice with the optical bioluminescence imaging system. Even when the mice were examined under unstressed, normal conditions, we could observe bioluminescent signals from the transgene, specifically in hematopoietic tissues including spleen and bone marrow (femur, spine, and sternum; Figure 1A). This analysis demonstrated that a mass of erythroid-lineage cells expressing the luciferase reporter and, hence, GATA-1 resided in these tissues at levels detectable by the imaging system. To confirm that these tissues actually expressed luciferase in vivo, we executed ex vivo tissue analyses and found that most signals originated from the spleen and bone marrow (Figure 1B). Although the nuclear protein GATA-1 was not found in enucleated erythrocytes, firefly luciferase produced from G1-HRD-luc transgene localized and remained in the cytoplasm of mature erythrocytes even after enucleation, albeit at lower levels. Therefore, weaker signals were observed in circulating mature erythroid cells in blood vessels through the depths of the skin. Indeed, cell extracts prepared from circulating erythrocytes of G1-HRD-luc mice showed luciferase activity in vitro (data not shown). Curiously, we occasionally observed predominant signals from lower portions of the body only in male mice (Figure 1A). The signal had never appeared in the comparable image of female mice. We considered that the signal originated from circulating erythroid cells in the testis because ex vivo analysis after the deprivation of peripheral blood with saline reflux revealed a marked reduction of the signal (data not shown). In this assay system, signals from intraperitoneal organs tended to diminish in subcutaneous tissues and muscles, whereas those from regions with abundant blood flow and less hair coat occasionally appeared to predominate. We also found that shaving mice before imaging produced sharper images, though white coats minimally affected signal intensity. Although bioluminescence detection was somehow affected by these factors, the overall expression profile was in very good agreement with findings of our previous histologic examinations of transgenic lines of mice that express β-galactosidase driven by the G1-HRD.23
Bioluminescence imaging of G1-HRD-luc mouse. (A) G1-HRD-luc expression under steady state conditions. The relative light intensity emitted from the mouse was quantified by imaging analysis software according to the color scale (photons per second) shown on the right. Bioluminescent images (top panels) and their corresponding black-and-white photographs (bottom panels) of the lateral (left), dorsal (middle), and ventral (right) views are shown. Spl indicates spleen; F, femur; Spi, spine; St, sternum; T, testis. (B) Ex vivo bioluminescent images of isolated bone marrow (left) and spleen (right) from G1-HRD-luc mice. For the imaging of bone marrow, both ends of the 2 femoral bones were cut and put into luciferin. Bioluminescent image (top panel) and its corresponding black-and-white photograph (bottom panel) are shown.
Bioluminescence imaging of G1-HRD-luc mouse. (A) G1-HRD-luc expression under steady state conditions. The relative light intensity emitted from the mouse was quantified by imaging analysis software according to the color scale (photons per second) shown on the right. Bioluminescent images (top panels) and their corresponding black-and-white photographs (bottom panels) of the lateral (left), dorsal (middle), and ventral (right) views are shown. Spl indicates spleen; F, femur; Spi, spine; St, sternum; T, testis. (B) Ex vivo bioluminescent images of isolated bone marrow (left) and spleen (right) from G1-HRD-luc mice. For the imaging of bone marrow, both ends of the 2 femoral bones were cut and put into luciferin. Bioluminescent image (top panel) and its corresponding black-and-white photograph (bottom panel) are shown.
Induction of G1-HRD-luc activity represented an increase in erythroid progenitors and proerythroblasts
We then examined G1-HRD-luc expression during acute erythroid expansion by treating G1-HRD-luc transgenic mice with PHZ to induce acute hemolytic anemia. The luciferase signal was significantly induced 3 days after PHZ administration; marked induction was observed in the spleen, and less significant induction was observed in the bone marrow (Figure 2A-B). This is in contrast to the expression of G1-HRD-luc under steady state condition (Figure 1A; luciferase signal from bone marrow was comparable to or even greater than that from spleen). In fact, in the PHZ-induced acute anemia condition, luciferase activity was amplified by more than 40-fold in the spleen. Conversely, signals from circulating erythrocytes remained at low levels. We thus suggest that the G1-HRD-luc mouse is an excellent model for monitoring erythropoiesis in response to acute anemia.
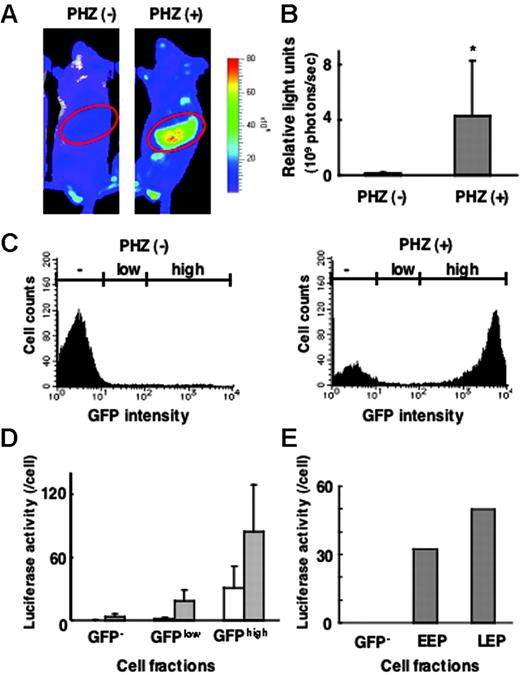
Correlation between G1-HRD-luc and G1-HRD-GFP expression in normal and anemic mice. (A) Bioluminescent images of G1-HRD-luc/G1-HRD-GFP mice treated with PHZ (right) or vehicle (left). Mice were injected intraperitoneally with PHZ or vehicle on days 1 and 2 and imaged on day 5. Areas encircled on the images represent the areas, including the spleen, processed for quantitative analysis. (B) Quantification of the luciferase signal from areas including the spleen, as shown in panel A. Mean signal intensities from PHZ-treated (n = 3) and untreated (n = 3) mice on day 5 are shown. *P < .5 compared with untreated control. (C-E) G1-HRD-luc/G1-HRD-GFP mice treated with PHZ or vehicle were killed on day 5, and spleen cells were examined. (C) GFP intensities of splenic mononuclear cells prepared from PHZ-treated (right panel) and untreated (left panel) mice. (D) Luciferase activities of whole-cell extracts from splenic mononuclear cells in GFP–, GFPlow, and GFPhighfractions. Each bar represents the average luciferase activity divided by the cell number in PHZ-treated (▦, n = 3) and untreated (□,n = 3) cells. Error bars indicate SD. (E) Luciferase activities in GFP–, EEP, and LEP fractions from untreated splenic mononuclear cells.
Correlation between G1-HRD-luc and G1-HRD-GFP expression in normal and anemic mice. (A) Bioluminescent images of G1-HRD-luc/G1-HRD-GFP mice treated with PHZ (right) or vehicle (left). Mice were injected intraperitoneally with PHZ or vehicle on days 1 and 2 and imaged on day 5. Areas encircled on the images represent the areas, including the spleen, processed for quantitative analysis. (B) Quantification of the luciferase signal from areas including the spleen, as shown in panel A. Mean signal intensities from PHZ-treated (n = 3) and untreated (n = 3) mice on day 5 are shown. *P < .5 compared with untreated control. (C-E) G1-HRD-luc/G1-HRD-GFP mice treated with PHZ or vehicle were killed on day 5, and spleen cells were examined. (C) GFP intensities of splenic mononuclear cells prepared from PHZ-treated (right panel) and untreated (left panel) mice. (D) Luciferase activities of whole-cell extracts from splenic mononuclear cells in GFP–, GFPlow, and GFPhighfractions. Each bar represents the average luciferase activity divided by the cell number in PHZ-treated (▦, n = 3) and untreated (□,n = 3) cells. Error bars indicate SD. (E) Luciferase activities in GFP–, EEP, and LEP fractions from untreated splenic mononuclear cells.
We previously generated G1-HRD-GFP mice and classified erythroid progenitors into 2 subgroups with the use of GFP expression.24 Cells that can be purified into a Lin–/c-Kit+/GFP+/CD71+ fraction corresponded to CFU-Es and proerythroblasts; therefore, this fraction is referred to as the late erythroid progenitor (LEP) fraction. The Lin–/c-Kit+/GFP+/CD71– fraction corresponded to immature erythroid progenitor cells, including BFU-Es, and is referred to as the early erythroid progenitor (EEP). These differentiation stages were carefully determined by morphologic analysis, reverse transcription–polymerase chain reaction (RT-PCR), and colony assays. We also observed that the LEP cell population was expanded in the spleens of mice treated with PHZ. Therefore, we hypothesized that the increased G1-HRD-luc reporter signal most likely reflected the rapid induction of erythropoiesis in the spleens of anemic mice.
To verify the linearity between the reporter gene expression and its detection, we set out to examine whether the G1-HRD-luc activity level correlated with the light intensity of G1-HRD-GFP in the spleen. For this purpose, we generated 2 additional transgenic lines of mice harboring G1-HRD-luc/G1-HRD-GFP dual reporters by simultaneous injection of the 2 reporter constructs into fertilized eggs. In fluorescence-activated cell sorter (FACS) analyses of splenic MNCs from the mice, we found that GFP intensity was markedly increased in response to PHZ treatment, consistent with our previous results with G1-HRD-GFP mice24 (Figure 2C). Then we divided the spleen MNCs into 3 fractions according to GFP intensity (GFPhigh, GFPlow, and GFP– fractions; Figure 2C). Each fraction was collected separately, and luciferase activity in the cellular extract was determined biochemically. Luciferase activity correlated well with GFP intensity, both in the PHZ-treated and in untreated mice (Figure 2D).
In the previous study of G1-HRD-GFP mice, we also found that the intensity of GFP in the EEP fraction was lower than that in the LEP fraction.24 Consistent with this observation, G1-HRD-luc activity of the EEP fraction was lower than that of the LEP fraction in the healthy spleen (Figure 2E).
An intriguing observation was that, when we compared luciferase activity on a per-cell basis, luciferase activity increased by less than 3-fold within the individual cells of the GFPhigh fraction and by less than 10-fold in the GFPlow fraction during PHZ-induced anemia (Figure 2D). This result is in clear contrast to the massive induction of luciferase activity in the spleen en bloc. We conclude that the increased number of LEP cells mainly contributes to the increased luciferase light intensity in response to acute anemia but that the contribution of the increased Gata1 gene expression in individual cells is relatively minor.
Tracking erythroblasts and later stage cells with Hbb-LCR-luc
To examine whether luciferase expression driven by other erythroid-specific gene regulatory regions can also be used for monitoring stress erythropoiesis, we generated transgenic lines of mice expressing luciferase under the control of human β-LCR27 linked to the β-globin gene promoter (Hbb-LCR-luc mice). Under nonanemia conditions, the Hbb-LCR-luc expression observed was similar to that of G1-HRD-luc (Figure 3A), indicating that β-LCR directs erythroid-specific reporter gene expression in vivo.
Delayed induction of Hbb-LCR-luc compared with G1-HRD-luc in response to anemia. (A) Bioluminescent images of G1-HRD-luc (top panels) and Hbb-LCR-luc (bottom panels) mice after 4 serial blood deprivations with 12-hour intervals. The day 0 image was taken before phlebotomy. (B) Average change in photon output from areas including the spleen of G1-HRD-luc (▴) and Hbb-LCR-luc (⋄) mice. Four to 6 mice were examined in each group. The intensity of the bioluminescent signal of day 0 was set to 1. Error bars indicate SD. *P < .05 compared with day 0. (C) EPO concentrations in the plasma of G1-HRD-luc (▦) and Hbb-LCR-luc mice (□) on the first and fourth blood deprivation (n = 6 in each group). Error bars indicate SD.
Delayed induction of Hbb-LCR-luc compared with G1-HRD-luc in response to anemia. (A) Bioluminescent images of G1-HRD-luc (top panels) and Hbb-LCR-luc (bottom panels) mice after 4 serial blood deprivations with 12-hour intervals. The day 0 image was taken before phlebotomy. (B) Average change in photon output from areas including the spleen of G1-HRD-luc (▴) and Hbb-LCR-luc (⋄) mice. Four to 6 mice were examined in each group. The intensity of the bioluminescent signal of day 0 was set to 1. Error bars indicate SD. *P < .05 compared with day 0. (C) EPO concentrations in the plasma of G1-HRD-luc (▦) and Hbb-LCR-luc mice (□) on the first and fourth blood deprivation (n = 6 in each group). Error bars indicate SD.
When anemia was induced by phlebotomy, Hbb-LCR-luc activity was induced, but the onset and peak of the induction were clearly delayed compared with those of the G1-HRD-luc activity (Figure 3A-B). The induction of G1-HRD-luc activity occurred rapidly and reached a maximum level on day 1 (the day after completion of the series of 4 blood removals at 12-hour intervals). On the other hand, the induction of Hbb-LCR-luc activity occurred gradually, and the high level was sustained to day 5 (Figure 3B). However, the magnitude of the induction was smaller in Hbb-LCR-luc than in G1-HRD-luc mice. Plasma EPO concentration was observed at similar levels in G1-HRD-luc and Hbb-LCR-luc mice at the first and fourth phlebotomies (Figure 3C). These results indicate that G1-HRD-luc tracked erythroid progenitors in the early stages of differentiation, whereas Hbb-LCR-luc monitored erythroblasts appearing in the much later stages of differentiation. Based on these observations, we conclude that the tracking of early erythroid progenitors with G1-HRD-luc enables us to detect and analyze the immediate early response of erythropoiesis in vivo.
Increase of G1-HRD-luc signal from spleen during PHZ-induced anemia
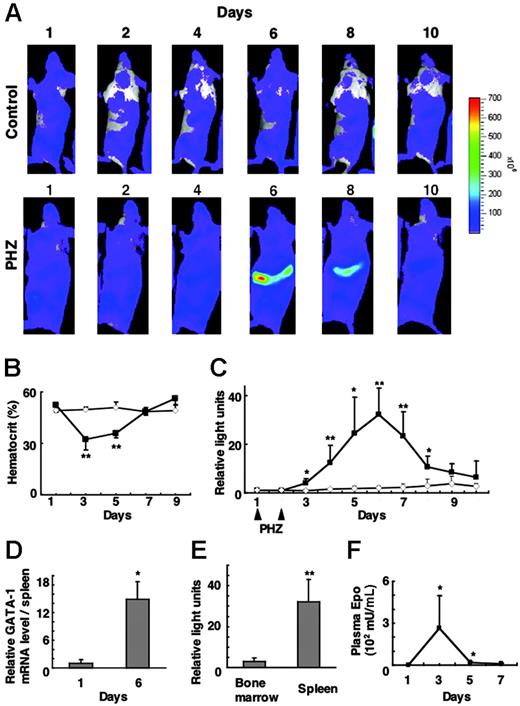
To monitor G1-HRD-luc activity during stress erythropoiesis, we acquired serial images over the course of 10 days after the administration of PHZ (Figure 4A). The hematocrit of these mice dropped to as low as 30% on day 3 and recovered by day 7 (Figure 4B). Luciferase activity in the spleen increased from day 3, peaked on day 6, and decreased to near basal level by day 10 (Figure 4A-C). Luciferase activity in the spleen was more than 30-fold greater than basal level on day 6; this G1-HRD-luc induction correlated well with the increased GATA-1 mRNA level in the spleen as determined by RNA blot analysis (Figure 4D).
G1-HRD-luc expression after PHZ treatment. (A) Serial imaging of G1-HRD-luc mice after PHZ treatment. Mice were treated with PHZ or vehicle on days 1 and 2. Bioluminescent images were taken of mice treated with PHZ (bottom panels) or vehicle (top panels) from day 1 to day 10. (B) Average change in the hematocrit of PHZ-treated (▴, n = 5) and vehicle-treated (⋄, n = 6) mice. (C) Average change in the photon output from PHZ-treated (▴,n = 6) or PBS-treated (⋄, n = 5) spleens. G1-HRD-luc activity on day 1 was set to 1 in each group. (D) Abundance of GATA-1 mRNA in the spleen of PHZ-treated mice (n = 4) on days 1 and 6. GATA-1 mRNA levels in the spleen were quantified by Northern blot analysis and normalized to the level of G3PDH mRNA (n = 4). (E) Induction of G1-HRD-luc activity in PHZ-treated mice from areas including femoral bone marrow (n = 4) and spleen (n = 7). Bioluminescent signals on day 6 were quantified and divided by those on day 1. (F) EPO concentrations in plasma prepared from PHZ-treated mice (n = 3) from days 1 to 7. Error bars indicate SD. *P < .05 and **P < .01 compared with untreated control.
G1-HRD-luc expression after PHZ treatment. (A) Serial imaging of G1-HRD-luc mice after PHZ treatment. Mice were treated with PHZ or vehicle on days 1 and 2. Bioluminescent images were taken of mice treated with PHZ (bottom panels) or vehicle (top panels) from day 1 to day 10. (B) Average change in the hematocrit of PHZ-treated (▴, n = 5) and vehicle-treated (⋄, n = 6) mice. (C) Average change in the photon output from PHZ-treated (▴,n = 6) or PBS-treated (⋄, n = 5) spleens. G1-HRD-luc activity on day 1 was set to 1 in each group. (D) Abundance of GATA-1 mRNA in the spleen of PHZ-treated mice (n = 4) on days 1 and 6. GATA-1 mRNA levels in the spleen were quantified by Northern blot analysis and normalized to the level of G3PDH mRNA (n = 4). (E) Induction of G1-HRD-luc activity in PHZ-treated mice from areas including femoral bone marrow (n = 4) and spleen (n = 7). Bioluminescent signals on day 6 were quantified and divided by those on day 1. (F) EPO concentrations in plasma prepared from PHZ-treated mice (n = 3) from days 1 to 7. Error bars indicate SD. *P < .05 and **P < .01 compared with untreated control.
We then quantified luciferase signals from the femoral bone marrow. G1-HRD-luc induction in the bone marrow was far less significant than that in the spleen over the course of this study. Peak induction of G1-HRD-luc in the femoral bone marrow was less than 10% that in the spleen (Figure 4E), confirming findings of earlier studies showing that spleen is the major hematopoietic tissue responding to erythropoietic stress in mice.5,28
We also examined plasma EPO concentration by ELISA on days 1, 3, 5, and 7. Blood sampling itself can induce erythropoiesis, so EPO concentrations were examined using peripheral blood from sex- and age-matched wild-type mice treated simultaneously with PHZ. Interestingly, plasma EPO showed a peak concentration on day 3, which preceded the induction of G1-HRD-luc (Figure 4F). The EPO concentration rapidly decreased to near basal level by day 5, which preceded the peak of G1-HRD-luc induction. These results support the hypothesis that EPO induction is the earliest event in stress erythropoiesis and that it is followed by an induction in G1-HRD-luc.
Hypoxia rapidly induced G1-HRD-luc expression in the spleen
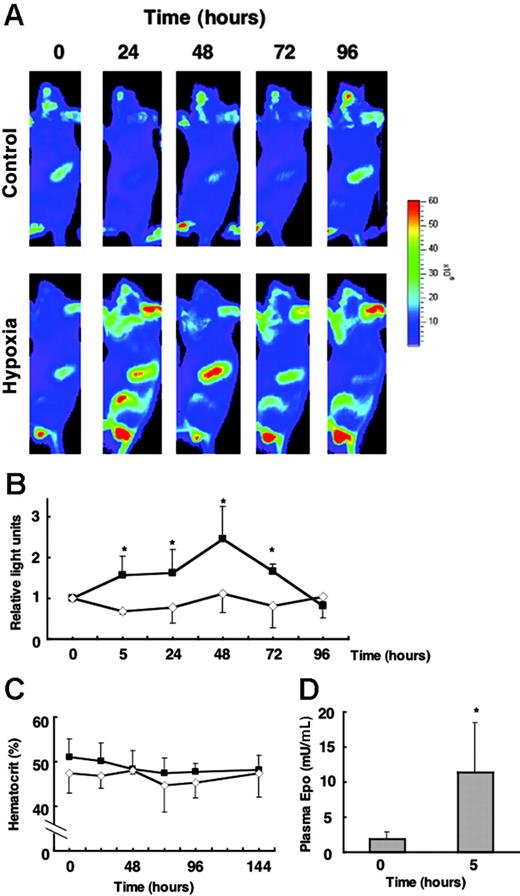
We next examined whether the induction of G1-HRD-luc is observed under hypoxia conditions. Mice were exposed to 6% O2 for 4 hours and were then returned to room air. The beginning of hypoxia exposure was set at the 0-hour time point, and G1-HRD-luc activity was periodically examined throughout 96 hours (Figure 5A-B). The plasma EPO concentration was increased by approximately 5.5-fold of the basal level at 5 hours (Figure 5D). The G1-HRD-luc signal from the spleen was increased from 5 hours and peaked at 48 hours (Figure 5B). The induction of EPO concentration after hypoxia (5.5-fold) was much smaller than that by PHZ administration (Figure 4F). Accordingly, the magnitude of the induction was only 2.5-fold of the basal level, even at 48 hours, which was much smaller than what we observed in the other studies, such as phlebotomy and PHZ administration. To detect such small induction, these images were taken at a lower range of scale than in other experiments (Figure 5A). Therefore, the signals from circulating blood cells in testis, nose, and ear appeared to be prominent. However, reproducible and significant induction was observed only in the spleen. No apparent change in hematocrit was observed from analysis to 144 hours (Figure 5C). Therefore, a possible explanation is that the increase of luciferase activity in the spleen was caused by increased transcription of the reporter gene per cell.
G1-HRD-luc expression in response to hypoxic exposure. (A) G1-HRD-luc mice were exposed to 6% oxygen for 4 hours and then returned to room air. Bioluminescent images were taken from 5 to 96 hours after hypoxic exposure began. The 0-hour image was taken just before exposure to hypoxia. Bioluminescent images overlaid with photographs of the lateral view are shown. (B) Average change in photon output from spleens exposed to hypoxia (▴,n = 4) and normoxia (⋄,n = 4). G1-HRD-luc activity in the spleen before the hypoxic exposure (0 hour) was set to 1. (C) Average change in the hematocrit of hypoxia-exposed (▴, n = 6) and normoxiaexposed (⋄,n = 4) mice. (D) EPO concentrations in the plasma before (0 hours) and 5 hours after hypoxic exposure (0 hours, n = 3; 5 hours, n = 4). Error bars indicate SD. *P < .05 compared with untreated control.
G1-HRD-luc expression in response to hypoxic exposure. (A) G1-HRD-luc mice were exposed to 6% oxygen for 4 hours and then returned to room air. Bioluminescent images were taken from 5 to 96 hours after hypoxic exposure began. The 0-hour image was taken just before exposure to hypoxia. Bioluminescent images overlaid with photographs of the lateral view are shown. (B) Average change in photon output from spleens exposed to hypoxia (▴,n = 4) and normoxia (⋄,n = 4). G1-HRD-luc activity in the spleen before the hypoxic exposure (0 hour) was set to 1. (C) Average change in the hematocrit of hypoxia-exposed (▴, n = 6) and normoxiaexposed (⋄,n = 4) mice. (D) EPO concentrations in the plasma before (0 hours) and 5 hours after hypoxic exposure (0 hours, n = 3; 5 hours, n = 4). Error bars indicate SD. *P < .05 compared with untreated control.
To examine whether erythroid cells are increased in the late stage of differentiation, we exposed the Hbb-LCR-luc mice to hypoxia. Interestingly, Hbb-LCR-luc activity failed to chase up the increase of G1-HRD-luc activity but stayed at a consistent level during the analysis (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article). This result indicates that transient exposure to hypoxia may not be sufficient to increase the number of erythroblasts in the late stage of differentiation. Taken together, our data suggest that the induction of G1-HRD-luc activity in this hypoxia experiment is largely affected by transcriptional activation of the reporter gene per cell rather than by the increase in the number of erythroid progenitors.
Exogenous administration of EPO induced G1-HRD-luc expression in the spleen
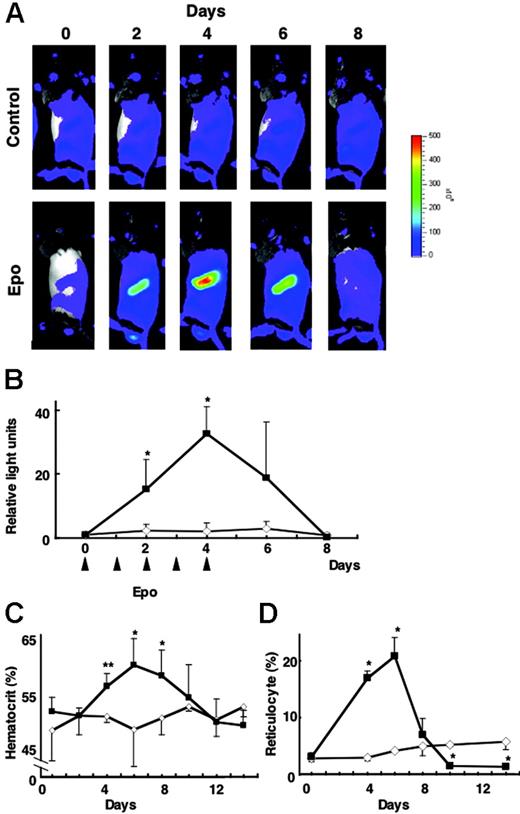
We finally examined whether exogenous EPO administration induces G1-HRD-luc activity in the absence of tissue hypoxia. After the administration of EPO (300 U/kg each on days 0-4), G1-HRD-luc activity was significantly induced in the spleen (Figure 6A-B). Quantitative analysis showed that the luciferase signal was more than 30-fold greater than that at the basal level on day 4 (Figure 6B). After the period of EPO administration (days 0-4), G1-HRD-luc expression returned to basal level by day 8. Hematocrit and reticulocyte counts increased in response to EPO and peaked on day 6—a delay of 2 days compared with the 4-day peak observed with G1-HRD-luc activity (Figure 6C-D). In contrast to PHZ administration, which induced G1-HRD-luc activity in 2 days, in this EPO administration experiment virtually no latent period was observed between the administration of EPO and the induction of G1-HRD-luc. These results further support the hypothesis that EPO acts as a key upstream signal in G1-HRD-luc induction and erythropoiesis. Maintenance of erythroid homeostasis must therefore be attained through the EPO sensing–signaling pathway.
G1-HRD-luc activity during EPO treatment. (A) Serial imaging of G1-HRD-luc during EPO treatment. Mice were treated with EPO or saline for 5 consecutive days. Bioluminescent images were taken from mice treated with EPO (bottom panels) or saline (top panels) for 8 consecutive days. (B-D) Changes in G1-HRD-luc activity (B), hematocrit (C), and reticulocyte counts (D) in EPO-treated (▴) or saline-treated (⋄) spleens (n = 3 in each group) over a period of days. G1-HRD-luc activity on day 0 was set to 1 in each group. Error bars indicate SD. *P < .05 and **P < .01 compared with untreated control.
G1-HRD-luc activity during EPO treatment. (A) Serial imaging of G1-HRD-luc during EPO treatment. Mice were treated with EPO or saline for 5 consecutive days. Bioluminescent images were taken from mice treated with EPO (bottom panels) or saline (top panels) for 8 consecutive days. (B-D) Changes in G1-HRD-luc activity (B), hematocrit (C), and reticulocyte counts (D) in EPO-treated (▴) or saline-treated (⋄) spleens (n = 3 in each group) over a period of days. G1-HRD-luc activity on day 0 was set to 1 in each group. Error bars indicate SD. *P < .05 and **P < .01 compared with untreated control.
Discussion
Transgenic lines of mice harboring a reporter gene linked to gene-regulatory domains controlling spatio-temporal or inducible expression of lineage-specific genes are a useful means for studying a variety of biologic processes in vivo. Similarly, the use of an optical bioluminescence imaging system extends our understanding of the multiple biologic events occurring in living subjects. In this study, we developed an in vivo system for monitoring stress erythropoiesis with the use of G1-HRD-luc and Hbb-LCR-luc reporter transgenic mice in combination with the optical bioluminescence imaging technique. This system enabled us to execute noninvasive, sensitive, and real-time monitoring of the increase and extinction of erythroid-lineage cells.
This novel system clearly illustrates a critical point, which is that the activities of the 2 erythroid-specific gene reporters are differentially controlled in the stress response in vivo. We found that G1-HRD-luc activity in spleen was induced dynamically and rapidly in response to various erythropoietic stresses but that inducible expression of Hbb-LCR-luc clearly occurred later than that of the G1-HRD-luc in response to anemia. These results indicate that G1-HRD-luc activity tracks the appearance and expansion of erythroid progenitors and proerythroblasts and that Hbb-LCR-luc activity tracks those of erythroblasts and later stage cells. These observations are in good agreement with previous findings demonstrating that the transgene expressing GATA-1 under the control of Hbb-LCR is lethal because of defective erythroid cell maturation,27 whereas transgenic mice expressing GATA-1 under the control of G1-HRD show no hematologic abnormality.25 These data serve as a prime example that temporal-specific gene regulation plays an important role in fundamental biologic responses.
The idea of using the regulatory domain of the mouse Gata1 gene for monitoring erythropoiesis stemmed from the finding that GATA-1 is most abundantly expressed in LEP cells, which correspond to CFU-Es and proerythroblasts.24 It has been well established that the CFU-E compartment expands dramatically during stress erythropoiesis.9,10 CFU-Es express a high density of EpoRs on their surfaces and are the most EPO-sensitive cells.8 Indeed, the administration of EPO to nonanemic animals induces G1-HRD-luc activity, and massive increases of LEP cells contribute to this dynamic induction of G1-HRD-luc activity. In contrast, G1-HRD-luc activity per cell showed only a small induction in the isolated GFPhigh cells of PHZ-induced anemic mice, suggesting a limited contribution of Gata1 gene activation to the increase of G1-HRD-luc activity. We also found that though Hbb-LCR-luc activity and hematocrit remained unchanged, G1-HRD-luc activity increased 2.5-fold after hypoxia exposure. This observation implies that the Gata1 gene might be activated by hypoxia-inducible transcription factors. However, our data from transient transfection assays suggest that G1-HRD-luc may not be a direct target of these transcription factors (data not shown), and the molecular basis of the hypoxia induction of G1-HRD-luc remains to be elucidated.
Although this study demonstrated the dynamic induction of G1-HRD-luc activity in the spleen, the specific roles played by GATA-1 during stress erythropoiesis have not been clarified. Loss-of-function studies have demonstrated that GATA-1 is essential for embryonic erythropoiesis (for a review, see Ferreira et al29 ). Targeted disruption30 and hematopoietic promoter-specific knock-down mutation31 of the Gata1 gene, which is on the X-chromosome, proved lethal in mutant mice hemizygous for Gata1 at the stage of yolk sac hematopoiesis because of defective primitive erythropoiesis. Recently, perturbation in the regulation of stress erythropoiesis was reported in GATA-1low mice, which were generated by a massive deletion of the Gata1 genomic locus.7,32 This deletion resulted in an 80% reduction of GATA-1 mRNA in erythroid cells. Surprisingly, however, the GATA-1low mice recovered from PHZ-induced anemia even faster than wild-type mice, suggesting that a high level of GATA-1 expression might not be necessary for promoting erythroid cell proliferation. Thus, the functional contribution of GATA-1 to the erythropoiesis operating in adult hematopoietic tissues largely remains to be clarified.
GATA-1 appears to regulate a variety of erythroid genes, including those for globin, heme biosynthetic enzymes, and erythroid membrane proteins (for reviews, see Weiss and Orkin33 and Ohneda and Yamamoto34 ). These target gene arrays seem reasonable if we consider the following points. First, to achieve an expanded capacity for oxygen transport, which is the ultimate end of stress erythropoiesis, proper formation of hemoglobin is necessary. Indeed, many heme biosynthesis enzymes are regulated by GATA-1 in hematopoietic tissues.35-38 Second, once erythroid cells start circulating, they must pass through capillary vessels by continuous deformation in their shape. Structural integrity of the erythroid membrane is required for such malleability in shape. We recently found that the transgenic expression of GATA-1 lacking the N-terminal zinc finger inhibits the endogenous function of GATA-1 and leads to fragile erythroid cells and hemolytic syndrome in adult mice.39 Data also indicate that the role of GATA-1 is essential for mature erythroid cell functions. Taken together, we assume that rapid induction of Gata1 gene expression in the erythroid mass during stress erythropoiesis may be crucial to erythroid progenitors for acquiring proper erythroid cell function without delay to respond adequately to tissue hypoxia.
To take advantage of this in vivo bioluminescence imaging system, which allowed monitoring of reporter activity simultaneously in multiple tissues, we wanted to compare the induction of G1-HRD-luc activity in the hematopoietic tissue in spleen and femoral bone marrow in all experiments conducted for the study. However, despite careful examination, bioluminescent signals from bone marrow tended to vary among mice probably because of the interruption of the signals by bone and muscle. Nonetheless, we obtained solid data demonstrating that the increase in G1-HRD-luc activity in response to PHZ was more than 10 times greater in spleen than in bone marrow. Consistent with these results, previous studies indicated that mouse spleen rather than bone marrow plays prominent roles in acute erythroid expansion.5,7 To date, 2 models have been proposed for the origin of erythroid progenitors during the acute erythropoietic response in the spleen. One is that the EPO–EpoR signaling pathway induces the migration of erythroid progenitors from the bone marrow to the spleen. The other is that the early progenitors reside in the spleen. Supporting the former model, stem cell factor (SCF) and c-Kit signaling affect the homing behavior of the erythroid progenitors. 5 On the contrary, supporting the latter model, BMP4 signaling has been shown to be critical for the initial response to stress erythropoiesis; only spleen progenitors can respond to BMP4. 13 Although the optical imaging system is not sensitive enough to detect the migration of early progenitors from bone marrow to spleen, it is possible to examine expression of G1-HRD-luc in mutant lines of mice that harbor attenuated stress erythropoiesis. Because G1-HRD-luc is active in early hematopoietic progenitors, including BFU-Es, we hypothesized that this is one of the most effective approaches for addressing the molecular basis of the early erythropoietic response.
In summary, we have generated G1-HRD-luc and Hbb-LCR-luc mouse models for monitoring erythropoiesis in living mice and demonstrated a rapid and dynamic induction of reporter gene expression in an erythroid mass in response to erythropoietic stresses. Our data demonstrate that both erythroid-specific gene regulatory regions were differentially activated during stress erythropoiesis. Studies using these reporter mouse lines would greatly facilitate a draft of the overall picture of the homeostatic regulation of erythropoiesis.
Prepublished online as Blood First Edition Paper, March 14, 2006; DOI 10.1182/blood-2005-10-4064.
Supported by grants from the Japan Science and Technology Corporation–Exploratory Research for Advanced Technology (ERATO) Environmental Response Project (M.Y.) and from the Ministry of Education, Science, Sports, and Culture of Japan (M.Y., K.O.).
M.S., K.O., O.O., S.P., and M.Y. designed the research; M.S., K.O., S.H.-O., and S.T. performed the research; M.S. and K.O. contributed vital new reagents or analytic tools; M.S., K.O., and M.Y. analyzed the data; and M.S., K.O., and M.Y. wrote the paper.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Norio Suzuki and Toshiharu Yamashita for helpful discussion, Yuko Kikuchi, Reiko Kawai, Emi Suzuki, and Yumiko Yamada for technical assistance, and Tania O'Connor and Jon Maher for critical reading of the manuscript. We also thank Shigeaki Watanabe and Junji Ozawa for technical suggestions.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal