Abstract
Drug development in human chronic lymphocytic leukemia (CLL) has been limited by lack of a suitable animal model to adequately assess pharmacologic properties relevant to clinical application. A recently described TCL-1 transgenic mouse develops a chronic B-cell CD5+ leukemia that might be useful for such studies. Following confirmation of the natural history of this leukemia in the transgenic mice, we demonstrated that the transformed murine lymphocytes express relevant therapeutic targets (Bcl-2, Mcl-1, AKT, PDK1, and DNMT1), wild-type p53 status, and in vitro sensitivity to therapeutic agents relevant to the treatment of human CLL. We then demonstrated the in vivo clinical activity of low-dose fludarabine in transgenic TCL-1 mice with active leukemia. These studies demonstrated both early reduction in blood-lymphocyte count and spleen size and prolongation of survival (P = .046) compared with control mice. Similar to human CLL, an emergence of resistance was noted with fludarabine treatment in vivo. Overall, these studies suggest that the TCL-1 transgenic leukemia mouse model has similar clinical and therapeutic response properties to human CLL and may therefore serve as a useful in vivo tool to screen new drugs for subsequent development in CLL.
Introduction
Chronic lymphocytic leukemia (CLL) has an initial asymptomatic phase of disease that slowly progresses with the development of an elevated lymphocyte count associated with enlarged lymph nodes, liver, and spleen. Treatment of human CLL is not curative, although conventional therapies such as fludarabine can reduce blood-lymphocyte counts and organomegaly and increase progression-free survival.1-3 The development of new therapies for CLL has been impaired by the inability to establish relevant human CLL cell lines and also to propagate primary tumor cells in vivo, and by the lack of a murine model that adequately represents the human disease. An appropriate animal model allows identification of problems such as high protein binding, first pass effect, and unfavorable biodistribution that may limit ultimate clinical effectiveness of a therapeutic agent. Generation of a relevant murine model to facilitate drug development in CLL would therefore represent a significant breakthrough for developing new therapeutic strategies in this disease.
TCL-1 is a 14-kDa protein present in both the cytoplasm and nucleus of immature normal T-cells, T-cell prolymphocytic leukemia (PLL), and a variety of B-cell lymphoproliferative disorders, including CLL.4,5 TCL-1 protein function has been studied predominately in T-cells, in which it has been demonstrated to bind to the pleckstrin homology domain of Akt and to enhance both AKT enzymatic activity and translocation from cytoplasm to nucleus.6-8 Introduction of tcl-1 under the control of a T-cell–specific lck gene promoter in transgenic mice results in a mature T-cell leukemia late in life that is similar to human T-cell PLL.9 In contrast, placement of tcl-1 under the control of a B-cell–specific IgVH promoter and IgH-Eμ enhancer results in a similar B-cell phenotype in which mice develop normally into adulthood, but then develop enlarged spleens, livers, and lymph nodes associated with high blood-lymphocyte counts. The transformed lymphocytes from the TCL-1 mice are G0-1 arrested, clonal, and express CD19+/CD5+/IgM+, as seen in human CLL.10 We therefore sought to determine if TCL-1 transgenic mice with established leukemia could serve as a preclinical tool for drug development in CLL. We herein demonstrate both the expression of proteins involved in the pathogenesis of CLL and sensitivity to agents used in the treatment of this disease.
Materials and methods
Mice, cell isolation, cell culture, and pharmaceutical reagents
All animal experiments were carried out under protocols approved by the Ohio State University Institutional Laboratory Animal Care and Use Committee. Homozygous TCL-1 transgenic mice (background strain C3H/B6) used for these experiments have been previously described.10 Breeding pairs were provided to our group as a generous gift from C.M.C. Mice were housed in a clean environment and supplied with sterile food and water ad libitum. In vitro studies used single-cell suspensions of tumor cells, derived from spleens of TCL-1 transgenic mice with established leukemia, following CD19-positive selection using magnetic-activated cell sorting (MACS) beads and columns (Miltenyi Biotec, Auburn, CA). Control normal B cells were derived from CD19-selected spleen cells of wild-type C3H/B6 mice (Jackson Laboratories, Bar Harbor, ME). Cells were cultured using methods previously described by our group.11 Fludarabine was obtained from Berlex Oncology (Montville, NJ), paclitaxel was obtained from EMD Biosciences (San Diego, CA), and flavopiridol was from the National Cancer Institute (Bethesda, MD). OSU03012 was synthesized as previously described.12
Immunophenotyping studies
Cells isolated from murine spleen or peripheral blood were collected to confirm both surface antigen expression and clonality. Cells were stained with antibodies specific for murine CD5, CD19, Igκ, Igλ, and IgM (BD Biosciences, San Jose, CA). Flow cytometry was carried out on a Beckman Coulter EPICS XL (Fullerton, CA).
Immunoblot analysis
Whole cellular lysates were prepared as previously described by our group with the addition of the phosphatase inhibitors sodium orthovanadate (1 mM) and microcystin LR (1 μM) (both from Sigma, St Louis, MO) to the lysis buffer.13 Antibodies used included Bcl-2 (Santa Cruz Biotechnology, Santa Cruz, CA); AKT, phospho-AKT, PDK1, and phospho-PDK1 (Cell Signaling, Beverly, MA); Mcl-1 (Abgent, San Diego, CA); DNMT1 (New England Biolabs, Ipswich, MA), and horseradish peroxidase–conjugated secondary antibodies (BioRad, Hercules, CA). Samples (50 μg/lane) were separated on 8% to 14% polyacrylamide gels and transferred onto 0.2 μm nitrocellulose membranes (Schleicher & Schuell, Keene, NH). Following antibody incubations, proteins were detected with chemiluminescent substrate (Pierce, Rockville, IL).
Cytotoxicity determination by MTT assay
MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide) assays were performed as described previously.14 Briefly, 1 × 106 leukemic cells were incubated for 24 or 72 hours in drug or vehicle control. MTT reagent (Sigma) was then added and plates were incubated for an additional 24 hours before washing with protamine sulfate in phosphate-buffered saline and measuring by spectrophotometry at 540 nm in a Labsystems 96-well plate reader (Fisher Scientific, Pittsburgh, PA). Data were plotted and values were calculated using GraphPad software (San Diego, CA).
p53 mutational analysis
Mutations of the p53 gene were assessed by initially extracting genomic DNA using the QIAmp kit according to the manufacturer's instructions (Qiagen, Valencia, CA). Each p53 exon was amplified individually from genomic DNA and subjected to automated sequencing by standard methods in the Ohio State University (OSU) Sequencing Core facility. The sequence was compared with the reported p53 sequence (GenBank accession no.AB01781531 ).
Statistics
To evaluate the efficacy of fludarabine in the TCL-1–established leukemia mouse model, an initial assumption was made that all mice treated with saline would die at approximately week 8, based on our observations. With a lack of censoring, to detect an increase in survival of 50%, a sample size of 10 mice per group was shown to be sufficient to allow a power of 0.9 with an overall 2-sided alpha of 0.05. The overall survival was defined from the date of randomization to date of death (event) or last follow-up (censored). The survival curves and medians were calculated within subgroups with the method of Kaplan-Meier.15 The log-rank test was used to compare differences between estimated survival curves.
Results
TCL-1 leukemia clinical characteristics
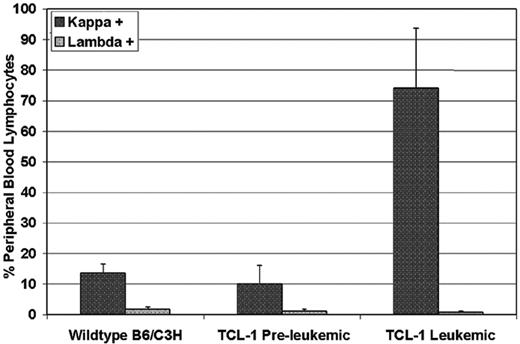
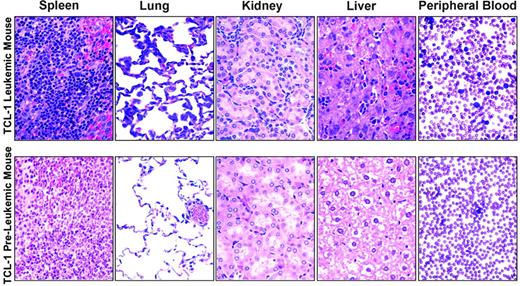
To determine the natural history of the TCL-1 transgenic mice previously described, we serially followed 22 mice from birth until death. In these mice, we noted the development of a clonal leukemia initially manifested by peripheral-blood lymphocytosis or splenomegaly at a median of 10.95 months (range, 8.72 to 13.28 months). Figure 1 shows results from flow cytometry analysis of peripheral blood from leukemic TCL-1 transgenic mice revealing a 7-fold increase in circulating Igλ+ B cells in the peripheral blood compared with the nonleukemic TCL-1 transgenic or wild-type mice (74.0%, 10.1%, and 13.5%, respectively). Consistent with the previous report on the clonal origin of the leukemic splenocytes from TCL-1 transgenic mice,10 we failed to see increased Igλ+ B cells in the peripheral blood obtained from leukemic mice compared with preleukemic and wild-type mice (0.747%, 1.12%, and 1.79%, respectively). Death in these same mice was noted at a median of 11.93 months (range, 7.28-16.10 months). In contrast to other murine models of acute leukemia, weight loss and other signs of animal stress were not present in most of the TCL-1 transgenic mice prior to death. Autopsies performed in mice with established leukemia demonstrated enlarged spleens (sometimes up to 25 times normal size) with significant hyperplasia of the white pulp and infiltration of lymphocytes to liver, lungs, and kidney, as shown in Figure 2. In addition, blood smears showed significant increases in lymphocyte numbers. All of these findings are consistent with those observed at autopsy in patients with CLL.16 A larger second series of mice was assessed and demonstrated active leukemia in 103 of 108 mice dying. The cause of death in the 5 animals not having evidence of leukemia is uncertain. Finally, we assessed the status of the p53 gene in 14 mice with TCL-1 leukemia and demonstrated wild-type sequence in all. This is similar to that observed in human CLL, in which fewer than 10% of untreated cases initially exhibit mutated p53.17 Thus, the TCL-1 leukemia model provides a natural history of murine leukemia with features similar to the more aggressive IgVH-unmutated human CLL.
TCL-1 transgenic mouse expresses targets relevant to therapeutic agents in CLL
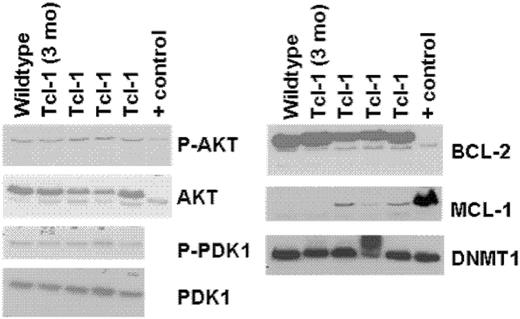
In human CLL and other types of leukemia, a variety of therapeutic agents have moved forward to the clinic that target antiapoptotic proteins (Bcl-2, Mcl-1), survival kinases (AKT, PDK1), and genes that maintain methylation (DNA methyltransferase-1). In anticipation of testing such therapeutic agents currently being examined by our group (flavopiridol, OSU03012, 17AAG, and decitabine), we sought to determine if these target proteins were expressed in CD19+ leukemic cells from the TCL-1 transgenic mice. Figure 3 demonstrates that TCL-1 leukemia cells express Bcl-2, Mcl-1, and DNMT1, with corresponding phosphorylation of AKT and PDK1. Thus, the TCL-1 transgenic leukemic cells have multiple demonstrable targets relevant to human CLL and therapeutics currently being examined in this disease.
Selective expansion of Igκ, but not Igλ, light chain–positive B cells in the peripheral blood of TCL-1 transgenic leukemic mice compared with preleukemic TCL-1 or B6/C3H wild-type mice. Each bar represents the average of 3 mice. Error bars show standard deviation.
Selective expansion of Igκ, but not Igλ, light chain–positive B cells in the peripheral blood of TCL-1 transgenic leukemic mice compared with preleukemic TCL-1 or B6/C3H wild-type mice. Each bar represents the average of 3 mice. Error bars show standard deviation.
Hematoxylin and eosin–stained tissues and blood smears from TCL-1 leukemic and preleukemic mice. Images were visualized using an Olympus BX41 microscope (Olympus, Melville, NY) as well as 40 ×/0.65 numeric aperture (NA) and 60 ×/0.80 NA plan objectives for tissue sections and blood smears, respectively. Photographs were taken using an Olympus C-3040 camera, and images were acquired and processed using Adobe Photoshop (Adobe Systems, San Jose, CA) in the OSU Mouse Phenotyping Shared Resource facility.
Hematoxylin and eosin–stained tissues and blood smears from TCL-1 leukemic and preleukemic mice. Images were visualized using an Olympus BX41 microscope (Olympus, Melville, NY) as well as 40 ×/0.65 numeric aperture (NA) and 60 ×/0.80 NA plan objectives for tissue sections and blood smears, respectively. Photographs were taken using an Olympus C-3040 camera, and images were acquired and processed using Adobe Photoshop (Adobe Systems, San Jose, CA) in the OSU Mouse Phenotyping Shared Resource facility.
Murine TCL-1 leukemias have in vitro sensitivity to fludarabine
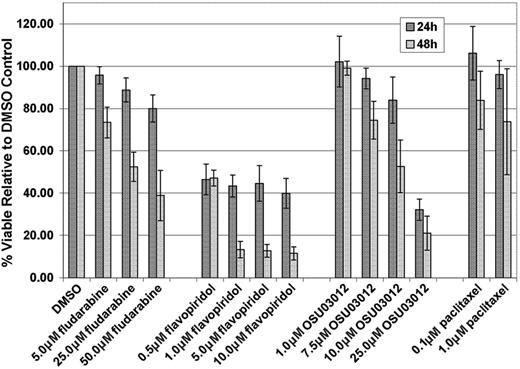
Fludarabine is currently the most common frontline therapy for CLL, and human CLL cells are sensitive to this agent in vitro.1-3 In addition, we and others have demonstrated that human CLL cells are sensitive to other novel molecules in clinical or preclinical development for CLL, including OSU0301211 and flavopiridol.13 We therefore sought to determine if similar in vitro cytotoxicity was observed with these agents against murine TCL-1 leukemia cells at concentrations and incubation times previously shown to be effective in human CLL. TCL-1 leukemia cells derived from 3 mice with established disease were incubated in various concentrations of fludarabine or OSU03012 for 24 and 48 hours, and with flavopiridol for 4 hours followed by an additional 20 or 44 hours in media without drug. These results, shown in Figure 4, demonstrate that fludarabine, OSU03012, and flavopiridol are highly cytotoxic toward primary TCL-1 leukemia cells in vitro, using doses and times at which our group has previously demonstrated comparable toxicity in human CLL cells.11,13 Paclitaxel, an agent shown to be ineffective in CLL,18 has very little cytotoxic activity in the TCL-1 mouse leukemia cells using physiologically relevant doses.19
Immunoblots of whole-cell lysates from spleens of TCL-1 leukemic, nonleukemic, and wild-type (C3H/B6) mice. Positive control for Bcl-2, Mcl-1, and DNMT1 is whole cellular lysate from Jurkat cell line. Positive control for AKT is from the 697 cell line.
Immunoblots of whole-cell lysates from spleens of TCL-1 leukemic, nonleukemic, and wild-type (C3H/B6) mice. Positive control for Bcl-2, Mcl-1, and DNMT1 is whole cellular lysate from Jurkat cell line. Positive control for AKT is from the 697 cell line.
Murine TCL-1 leukemia responds to treatment with fludarabine
We next sought to determine if fludarabine, a therapy commonly used in CLL, could diminish clinical evidence of disease as measured by serial leukocyte counts, spleen size assessment, and ultimately survival. A total of 20 transgenic mice with active leukemia (lymphocyte counts greater than 20 × 109/L or enlarged spleens) were randomly assigned to treatment with low-dose fludarabine (34 mg/kg intraperitoneally on days 1-5 every month) or saline control with the same schedule of administration. The study continued for a total of 80 days, at which time all of the saline-injected control animals had died, but 4 of the fludarabine-treated animals remained alive. Figure 5A demonstrates the modest but significant (P = .046) survival advantage observed with fludarabine compared with saline treatment. This mimics findings with fludarabine in human CLL, in which early application of this therapy also shows a modest trend toward prolonged survival.3 The average blood-lymphocyte changes over time are shown in Figure 5B. Overall, this demonstrates that the lymphocyte count progressively increases over time in saline-treated animals, whereas it declines temporarily in response to fludarabine. In a subset of mice, response as measured by decrease in blood-lymphocyte count was noted immediately after each fludarabine treatment, but counts subsequently rose before the next cycle of therapy was administered (Figure 5C). Eventually, the lymphocytes became completely resistant to fludarabine treatment, and death followed soon thereafter. This pattern is similar to that observed in fludarabine-refractory human CLL. Overall, these data demonstrate that TCL-1 murine leukemia has a therapeutic profile in response to fludarabine that is similar to what is observed in human CLL.
Analysis of in vitro cytotoxicity by fludarabine, flavopiridol, OSU03012, and paclitaxel on splenic B cells from TCL-1 leukemic mice. Error bars indicate SD.
Analysis of in vitro cytotoxicity by fludarabine, flavopiridol, OSU03012, and paclitaxel on splenic B cells from TCL-1 leukemic mice. Error bars indicate SD.
Survival analysis and white blood cell counts of TCL-1 leukemic mice after fludarabine treatment. (A) Kaplan-Meier survival curve of TCL-1 leukemic mice treated with fludarabine (34 mg/kg intraperitoneally). (B) White blood cell count as determined on blood smear of TCL-1 leukemic mice treated with fludarabine (34 mg/kg intraperitoneally) versus saline control. (C) White blood cell count as determined on blood smear of 1 TCL-1 leukemic mouse treated with fludarabine (34 mg/kg intraperitoneally). Arrows indicate day 1 of 5 days of fludarabine treatment.
Survival analysis and white blood cell counts of TCL-1 leukemic mice after fludarabine treatment. (A) Kaplan-Meier survival curve of TCL-1 leukemic mice treated with fludarabine (34 mg/kg intraperitoneally). (B) White blood cell count as determined on blood smear of TCL-1 leukemic mice treated with fludarabine (34 mg/kg intraperitoneally) versus saline control. (C) White blood cell count as determined on blood smear of 1 TCL-1 leukemic mouse treated with fludarabine (34 mg/kg intraperitoneally). Arrows indicate day 1 of 5 days of fludarabine treatment.
Discussion
We have described the use of a new animal model to systematically study currently used as well as novel therapeutic agents relevant to the treatment of CLL. The leukemia of the TCL-1 transgenic mouse model, initially described by Bichi and colleagues, has a prolonged natural history and biochemical similarities to human CLL.10 In addition, preliminary studies done by others have demonstrated that the clonal leukemic cells in this mouse model of CLL have IgVH-unmutated disease associated with a T-cell immune defect similar to that observed in human CLL.20,21 Herein, we demonstrate that several therapeutic targets that are potentially relevant to the pathogenesis of human CLL, including Mcl-1, Bcl-2, PDK1, AKT, and DNMT1, are expressed in the CD19+ transformed lymphocytes. We have demonstrated that the TCL-1 leukemic cells have wild-type p53 and are sensitive in vitro to several therapeutic agents active in vitro and/or in vivo in human CLL, including fludarabine22 and flavopiridol,3,23 as well as a novel preclinical agent, OSU03012.11 Subsequent in vivo studies using TCL-1 transgenic mice with established leukemia demonstrated that fludarabine treatment resulted in decreased blood tumor cells and spleen size, and ultimately prolonged survival. Mice with TCL-1 leukemia that initially gained clinical benefit to fludarabine therapy subsequently became resistant to this and ultimately died from leukemia, an outcome commonly noted in human CLL. This series of studies suggest that the TCL-1 transgenic mouse model is also similar to human CLL in its therapeutic response, and could be used as a preclinical tool to evaluate new drugs being considered for clinical development in CLL.
The importance of this mouse model to drug development in CLL is significant, as until recently there have been no suitable animal models for this disease. A high frequency of lymphoproliferative disorders have been reported in New Zealand (NZ) mouse strains.24 For example, Okada et al25 have described a NZ black (NZB) strain with clonal populations of CD5-positive B cells arising in the peritoneal cavity. Unfortunately, little research has supported this as a viable model for drug discovery. A report from Su et al26 describes the transplantation of B-1 cells from the NZ white (NZW) strain of mouse into a severe combined immunodeficiency (SCID) mouse, resulting in clinical characteristics similar to human CLL with Richter tranformation. However, as the recipient animals do not have mature T or B cells, the usefulness of this observation to human CLL therapeutic development is limited. Xenograft models using immunodeficient mice and peripheral-blood mononuclear cells (PBMCs) from patients with CLL have had varying results. Some studies have reported high incidence of lymphoproliferative disease not arising from the CLL.27,28 Recently, Shimoni and colleagues29,30 reported the adoptive transfer of PBMCs from patients with CLL into lethally irradiated Balb/C mice that were radioprotected with bone marrow from immunodeficient mice. While this is an intriguing finding, this method presents substantial challenges with regard to feasibility and reproducibility, factors critical to the utility of a model for drug development. Finally, given the lack of representative Epstein-Barr virus (EBV)–negative CLL cell lines, pursuit of cell line xenograft studies, as generally done in solid tumors and acute leukemia, is less relevant.
Limitations of the model described herein are the extended period of time before TCL-1 transgenic mice develop leukemia, making the reproduction of experiments time-consuming, and the lack of predictability of death using traditional features such as body weight, animal appearance, leukocyte count, and presence of enlarged lymph nodes and spleen. We have attempted to shorten the time to disease development by administering TCL-1 leukemia cells intraperitoneally to nonleukemic mice in repeated experiments. Engraftment of leukemia cells occurred at an average of 5 months, with all engrafted animals subsequently developing fatal leukemia or lymphoma. However, this engraftment occurred in just 62% of mice, and time to disease development was variable. To diminish allogeneic rejection in an attempt to improve the engraftment efficiency, we are currently backcrossing the TCL-1 strain onto a pure B6 genetic background. Relative to the cause of death commonly observed in leukemic mice, Figure 2 provides evidence that organ infiltration may contribute to the eventual demise of these animals, as is also observed in autopsies of patients with CLL.16 Recent work using this TCL-1 transgenic mouse model has also shown profound B-cell–and T-cell–immune defects similar to that observed in human CLL, which might also contribute to unrecognized infectious morbidity.20,21 Defining the normal B-cell defect and response to common encapsulated organisms is under investigation by several groups at this time. Despite these limitations, we believe this model represents an extremely useful tool for drug development in CLL.
Other potential applications of this mouse model exist, such as serial assessment of pharmacodynamic target modulation in vivo following treatment with therapeutic agents. However, as shown in Figure 3, overexpression of many of these are not present as in CLL, making validation of the specific target in this leukemia essential before active exploration of therapies targeting each of these. Another potential application is the characterization of resistance mechanisms of known and new therapeutic agents. Similar to most human CLL, tumor cells in the TCL-1 mouse have wild-type p53. Our in vivo experiment with fludarabine described here demonstrates that the TCL-1 mouse blood-lymphocyte count initially declines with therapy, after which fludarabine resistance develops. This offers the opportunity to study paired de novo and drug-resistant TCL-1 leukemia tumor cells from blood and spleen to identify relevant mechanisms of drug resistance, such as mutation of p53, in an in vivo system.
In summary, we have demonstrated that the TCL-1 transgenic model of leukemia has properties similar to human CLL. Given the absence of useful murine models to study new therapeutic agents in human CLL, we offer that this model will serve as a useful new tool to test pharmacology, to validate relevant targets, to identify optimal schedule of administration, and to understand relevant mechanisms of drug resistance in CLL.
Prepublished online as Blood First Edition Paper, May 2, 2006; DOI 10.1182/blood-2005-12-011213.
Supported by the National Cancer Institute (P01 CA95426), the American Cancer Society, The Leukemia and Lymphoma Society, and The D. Warren Brown Foundation. J.C.B. is a clinical scholar of the Leukemia and Lymphoma Society of America. A.J.J. is supported by the American Cancer Society–IDEC/Genentech/Ronald Levy Postdoctoral Fellowship.
C.M.C. has declared a financial interest in Crogen, a company whose patented mouse strain was used in the present work.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal