The best strategy for incorporating imatinib in front-line treatment of Ph+ acute lymphoblastic leukemia (ALL) has not been established. We enrolled 92 patients with newly diagnosed Ph+ALL in a prospective, multicenter study to investigate sequentially 2 treatment schedules with imatinib administered concurrent to or alternating with a uniform induction and consolidation regimen. Coadministration of imatinib and induction cycle 2 (INDII) resulted in a complete remission (CR) rate of 95% and polymerase chain reaction (PCR) negativity for BCR-ABL in 52% of patients, compared with 19% in patients in the alternating treatment cohort (P = .01). Remarkably, patients with and without a CR after induction cycle 1 (INDI) had similar hematologic and molecular responses after concurrent imatinib and INDII. In the concurrent cohort, grades III and IV cytopenias and transient hepatotoxicity necessitated interruption of induction in 87% and 53% of patients, respectively; however, duration of induction was not prolonged when compared with patients receiving chemotherapy alone. No imatinib-related severe hematologic or nonhematologic toxicities were noted with the alternating schedule. In each cohort, 77% of patients underwent allogeneic stem cell transplantation (SCT) in first CR (CR1). Both schedules of imatinib have acceptable toxicity and facilitate SCT in CR1 in the majority of patients, but concurrent administration of imatinib and chemotherapy has greater antileukemic efficacy.
Introduction
Adult patients with Philadelphia chromosome (Ph)-positive acute lymphoblastic leukemia (ALL) have a significantly inferior treatment outcome than patients who are Ph negative.1-5 This is partly due to a lower complete remission rate following induction chemotherapy but more importantly to a significantly shorter remission duration, which ranges from 9 to 16 months. Intensification of chemotherapy failed to improve overall treatment results despite CR rates of up to 90%.5 As the probability of long-term survival with chemotherapy alone is less than 10%, allogeneic stem cell transplantation (SCT) in first complete remission (CR1) is generally accepted as the treatment of choice.6-11 However, only 20% to 60% of patients actually undergo alloSCT in large prospective studies. Moreover, even after allogeneic SCT in CR1 the probability of relapse for Ph+ALL patients is approximately 30%, which together with a high transplant-related mortality of 20% to 40% highlights the limitations of current therapy.12-13 In childhood ALL, the level of residual disease that is still detectable prior to transplantation correlates with outcome after SCT.14-15 Thus, treatment approaches that enhance the antileukemic efficacy without incurring excessive toxicity prior to transplantation may lead to improved overall outcome.
The ABL tyrosine kinase inhibitor imatinib (Glivec, Gleevec; Novartis, Basel, Switzerland) selectively targets the molecular mechanism underlying Ph-positive leukemias16 and was shown in early phase 1 and 2 studies to have significant, albeit nonsustained, activity against relapsed or refractory Ph+ALL.17-19 Based on the premise that imatinib would be more effective during first-line as opposed to salvage treatment of Ph+ALL, entailing a lower incidence of resistance, the clinical value of imatinib in newly diagnosed Ph+ALL has received increasing attention. Preclinical in vitro data suggest that imatinib may augment the activity of cytotoxic drugs commonly used in treatment of ALL and chronic myelogenous leukemia (CML).20-22 In the clinical setting, the potential of combination therapy to either enhance treatment efficacy or potentially aggravate therapy-associated toxicity prompted several ALL study groups to explore different schedules of imatinib in conjunction with a variety of chemotherapy regimens.23-26 Together, these studies show that incorporation of imatinib in the first-line treatment of adult Ph+ALL is associated with a hematologic CR rate consistently higher than 90%, irrespective of the schedule with which imatinib and chemotherapy were administered. While the results of these studies compare favorably with the 70% to 90% CR rate generally achieved by the respective chemotherapy regimens alone, it is unclear whether concurrent or alternating schedules of imatinib and cytotoxic drugs differ in their relative efficacy and tolerability. Moreover, it is noteworthy that induction with imatinib monotherapy likewise results in CR rates of approximately 95%, identical to combination therapy but not associated with toxicity of chemotherapy.27-28 Thus, it has not yet been established how imatinib is best incorporated in the front-line treatment of Ph+ALL.
Here, we report the results of a prospective, multicenter clinical trial encompassing 92 patients with newly diagnosed Ph+ALL who received uniform induction and consolidation chemotherapy in conjunction with 1 of 2 schedules of imatinib that were investigated sequentially: while imatinib was administered alternating with the chemotherapy cycles in the first patient cohort, the second treatment schedule in which imatinib was administered concomitantly throughout induction phase 2 (INDII), first consolidation (C1), and up to SCT was introduced by amendment after sufficient safety and efficacy data were available from cohort 1. Besides assessing feasibility and toxicity, we compared the antileukemic activity of these 2 schedules in terms of CR rate, incidence of relapse prior to SCT, proportion of patients transferred to SCT, frequency of polymerase chain reaction (PCR) negativity, and BCR-ABL transcript levels (minimal residual disease = MRD) measured by quantitative real-time (RT)-PCR at predefined times between diagnosis and consolidation.
Patients, materials, and methods
Patients
Patients older than 18 years with newly diagnosed Ph+ALL or CML lymphoid blast crisis were eligible if they had an ECOG performance status of 0 to 2, adequate organ function, no life-threatening or uncontrolled infections, and had received no other therapy than specified by GMALL protocols 06/99 or 07/03 (Table 1). These protocols differed slightly only with respect to the dexamethasone dose during prephase and INDI and the timing of asparaginase during INDI. All patients gave written informed consent to participate in the study, and the study was reviewed and approved by a recognized ethics review committee at each trial center. The study was performed in accordance with the Declaration of Helsinki, as amended in Tokyo, Venice, and Hong Kong.
Chemotherapy according to the GMALL protocols 06/99 and 07/03
Treatment phase . | Dose . | Route (duration) . | Time administered . |
|---|---|---|---|
| Prephase | |||
| Dexamethasone | 10 mg/m2 | By mouth | Days 1-5* |
| Cyclophosphamide | 200 mg/m2 | Intravenously | Days 3-5 |
| MTX | 15 mg | Intrathecally | Day 1† |
| Remission induction (INDI) | |||
| Dexamethasone | 10 mg/m2 | By mouth | Days 6-7, 13-16* |
| Vincristine | 2 mg | Intravenously | Days 6, 13, 20 |
| Daunorubicin | 45 mg/m2 | Intravenously | Days 6 + 7, 13 + 14 |
| PEG-asparaginase | 1000 U/m2 | Intravenously (2 h) | Day 20* |
| G-CSF | 5 μg/kg | Subcutaneously | Starting day 6 |
| Remission induction (INDII) | |||
| Cyclophosphamide | 1000 mg/m2 | Intravenously | Days 26, 46 |
| AraC | 75 mg/m2 | Intravenously | Days 28-31, 35-38, 42-45 |
| 6-MP | 60 mg/m2 | By mouth | Days 26-46 |
| MTX | 15 mg | Intrathecally | Days 28, 35, 42 |
| G-CSF | 5 μg/kg | Subcutaneously | Until ANC > 1 × 109/L |
| CNS-irradiation | 24 Gy | NA | 12 days, during INDII |
| Consolidation | |||
| Dexamethasone | 10 mg/m2 | By mouth | Days 1-5 |
| Vindesine | 3 mg/m2 | Intravenously | Day 1 |
| MTX | |||
| Age up to 55 y | 1.5 g/m2 | Intravenously (24 h) | Day 1 |
| Age more than 55 y | 1.0 g/m2 | Intravenously (24 h) | Day 1 |
| VP16 | 250 mg/m2 | Intravenously (1 h) | Days 4 + 5 |
| AraC | |||
| Age up to 55 y | 2 g/m2 × 2 | Intravenously (3 h) | Day 5 |
| Age more than 55 y | 1 g/m2 × 2 | Intravenously (3 h) | Day 5 |
| G-CSF | 5 μg/kg | Subcutaneously | Starting day 7 |
| MTX, AraC, DEX | 15 mg/40 mg/4 mg | Intrathecally | Day 12 |
Treatment phase . | Dose . | Route (duration) . | Time administered . |
|---|---|---|---|
| Prephase | |||
| Dexamethasone | 10 mg/m2 | By mouth | Days 1-5* |
| Cyclophosphamide | 200 mg/m2 | Intravenously | Days 3-5 |
| MTX | 15 mg | Intrathecally | Day 1† |
| Remission induction (INDI) | |||
| Dexamethasone | 10 mg/m2 | By mouth | Days 6-7, 13-16* |
| Vincristine | 2 mg | Intravenously | Days 6, 13, 20 |
| Daunorubicin | 45 mg/m2 | Intravenously | Days 6 + 7, 13 + 14 |
| PEG-asparaginase | 1000 U/m2 | Intravenously (2 h) | Day 20* |
| G-CSF | 5 μg/kg | Subcutaneously | Starting day 6 |
| Remission induction (INDII) | |||
| Cyclophosphamide | 1000 mg/m2 | Intravenously | Days 26, 46 |
| AraC | 75 mg/m2 | Intravenously | Days 28-31, 35-38, 42-45 |
| 6-MP | 60 mg/m2 | By mouth | Days 26-46 |
| MTX | 15 mg | Intrathecally | Days 28, 35, 42 |
| G-CSF | 5 μg/kg | Subcutaneously | Until ANC > 1 × 109/L |
| CNS-irradiation | 24 Gy | NA | 12 days, during INDII |
| Consolidation | |||
| Dexamethasone | 10 mg/m2 | By mouth | Days 1-5 |
| Vindesine | 3 mg/m2 | Intravenously | Day 1 |
| MTX | |||
| Age up to 55 y | 1.5 g/m2 | Intravenously (24 h) | Day 1 |
| Age more than 55 y | 1.0 g/m2 | Intravenously (24 h) | Day 1 |
| VP16 | 250 mg/m2 | Intravenously (1 h) | Days 4 + 5 |
| AraC | |||
| Age up to 55 y | 2 g/m2 × 2 | Intravenously (3 h) | Day 5 |
| Age more than 55 y | 1 g/m2 × 2 | Intravenously (3 h) | Day 5 |
| G-CSF | 5 μg/kg | Subcutaneously | Starting day 7 |
| MTX, AraC, DEX | 15 mg/40 mg/4 mg | Intrathecally | Day 12 |
Days of administration of remission induction counted from start of prephase.
MTX indicates methotrexate; VP16, etoposide; AraC, cytarabine; 6-MP, 6-mercaptopurine; NA, not appliable; DEX, dexamethasone
Slight variations in number of applications and total dose (DEX) and timing (PEG-Asp)
Delay in case of high PB blast count
Study design
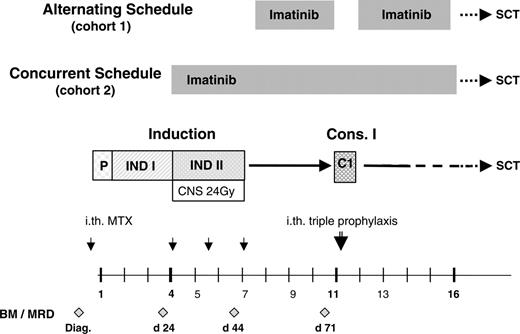
This prospective, multicenter phase 2 study investigated the safety and efficacy of imatinib given in conjunction with induction and early consolidation chemotherapy in adult Ph+ALL. The overall design of the schedules is depicted in Figure 1; the individual components of chemotherapy are listed in Table 1. Two administration schedules were tested in successive cohorts: the first cohort of 47 patients, enrolled between March 2001 and December 2002, received an alternating schedule in which imatinib was started after induction chemotherapy in patients who had achieved a complete remission (CR), for a 28-day cycle. Recovery of peripheral blood (PB) values to neutrophil counts (ANCs) of at least 1 × 109/L and platelet counts of at least 100 × 109/L and recovery from any toxicity of chemotherapy were required. A CR was required for eligibility in this initial group of patients because of the previously documented poor response to imatinib in patients who were refractory to chemotherapy.17-19 Imatinib was administered as a single daily oral dose of 400 mg or 600 mg. Consolidation chemotherapy (C1) (Table 1) was administered after this imatinib cycle, with the same requirement for recovery of PB values as described. After C1, patients could receive an optional imatinib cycle of up to 8 weeks duration to bridge the time to SCT.
Enrollment into the second cohort receiving simultaneous imatinib and chemotherapy was started in December 2002 after amendment of the protocol. The present analysis is based on all patients (n = 92) entered until July 2004. The amendment was designed to investigate whether treatment results achieved with the alternating schedule could be improved by administering imatinib simultaneously with chemotherapy. The treatment schedule is depicted in Figure 1. Patients were enrolled after completing the first 3 weeks of induction chemotherapy (INDI) (Table 1), irrespective of their response to therapy. The other eligibility criteria were unchanged. Imatinib (600 mg once a day) was started together with INDII (weeks 4-6; Table 1) and was continued without scheduled interruption for up to 8 weeks after the first consolidation cycle, to provide antileukemic treatment until patients underwent SCT.
Treatment
Remission induction chemotherapy (INDI: weeks 1 to 3; INDII: weeks 4 to 6) and consolidation cycle C1 of the GMALL protocols 06/99 and 07/03 are specified in Table 1. Consolidation 1 was started after recovery of ANCs to more than 1.0 × 109/L and of platelet counts to more than 100 × 109/L. Central nervous system (CNS) prophylaxis consisted of intrathecal injection of methotrexate (MTX, 15 mg) once during prephase and thrice during INDII, and a single intrathecal administration of MTX (15 mg), cytarabine (40 mg), and dexamethasone (4 mg) after C1; patients achieving a CR after INDI also received prophylactic cranial irradiation (24 Gy) parallel to INDII and thus concurrently with imatinib in cohort 2 (Figure 1).
Study design indicating the 2 schedules of imatinib administration in conjunction with the GMALL protocols 06/99 and 07/03. P indicates prephase; INDI, induction phase 1; INDII, induction phase 2; C1, consolidation phase 1; SCT, stem cell transplantation; CNS 24 Gy, CNS irradiation, scheduled by protocol in patients having achieved a CR after INDI; ith, intrathecal chemotherapy; MTX, methotrexate; BM, bone marrow analysis; MRD, minimal residual disease analysis; and diag, diagnosis.
Study design indicating the 2 schedules of imatinib administration in conjunction with the GMALL protocols 06/99 and 07/03. P indicates prephase; INDI, induction phase 1; INDII, induction phase 2; C1, consolidation phase 1; SCT, stem cell transplantation; CNS 24 Gy, CNS irradiation, scheduled by protocol in patients having achieved a CR after INDI; ith, intrathecal chemotherapy; MTX, methotrexate; BM, bone marrow analysis; MRD, minimal residual disease analysis; and diag, diagnosis.
The initial starting dose of imatinib with the alternating schedule was 400 mg, given orally as a single daily dose (n = 35); this was increased to 600 mg once a day (n = 12) by protocol amendment after the availability of sufficient safety data. In cohort 2, imatinib was started at 600 mg once a day. Imatinib was interrupted in the event of grade 3 or 4 nonhematologic toxicity until toxicity resolved to grade 1 or less and was then resumed at a reduced dose of 300 mg or 400 mg, depending on the starting dose. In the first cohort, imatinib was interrupted for grade III or IV neutropenia or thrombocytopenia and resumed after recovery of PB values to cytopenia grade I. In patients who received imatinib concurrently with chemotherapy (cohort 2) and experienced grade III or IV hematologic toxicity, imatinib was interrupted only when the duration of severe cytopenia was felt by the investigator to exceed the duration expected from chemotherapy alone, as prolonged cytopenias occur in a significant proportion of patients during remission induction.29 No dose-reduction was scheduled for anemia, except for grade 3 or 4 anemia resulting from an acute cause considered to be related to administration of imatinib (eg, gastrointestinal hemorrhage).
Supportive therapy was conducted according to the standard procedures of the individual participating centers. It was recommended that patients receive prophylactic G-CSF throughout induction therapy and after consolidation, as we and others have previously shown that G-CSF support decreases the frequency of severe infections during induction.29,30
Assessments
Bone marrow aspiration for cytology, immunophenotyping, quantification of BCR-ABL transcripts, and cytogenetic analysis was performed as part of the pretreatment analysis. Histology was required only in the event of a dry aspirate. Marrow aspirations were performed after INDI (day 24), after INDII (day 44), and prior to consolidation (C1) therapy (Figure 1). Complete blood counts were performed at least twice weekly, and more frequently if the ANCs were less than 1 × 109/L or platelet counts were less than 50 × 109/L. Clinical chemistry analyses were performed once weekly.
Analysis of minimal residual disease (MRD) was performed centrally in the study's reference laboratory in Frankfurt using quantitative (q) RT-PCR analysis for BCR-ABL transcripts in PB and BM samples as previously described, using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a housekeeping gene.31,32 A negative qRT-PCR was confirmed by a nested RT-PCR. In this communication, MRD assessments are based exclusively on the values obtained for BM samples.
Response criteria
Complete remission (CR) was defined as less than 5% blasts in a bone marrow of normal cellularity with ANCs of more than 1.5 × 109/L and platelet counts more than 100 × 109/L. A partial response (PR) required a response of the leukemic cells as in a CR but with incomplete recovery of PB counts, or a reduction of BM blasts to between 5% and 25%. Patients were considered refractory if blasts were not reduced to less than 25% in BM or if PB blasts or extramedullary disease had not been eliminated. Relapse was defined by recurrence of blasts exceeding 5% in BM or by extramedullary involvement in a patient with previously documented CR.
Statistical analysis
Differences in BCR-ABL transcript levels in patients from the same cohort were assessed by the Wilcoxon test; the Mann-Whitney test was applied to comparisons of BCR-ABL levels between patients cohorts. The proportion of patients with a given characteristic was compared by means of Fisher exact test. Remission duration and overall survival curves were plotted according to the methods of Kaplan and Meier, with differences between patient groups analyzed by the log-rank test, using the GraphPad Prism software package (GraphPad software, San Diego, CA).
Results
Patients
A total of 92 patients were enrolled, 47 in the alternating schedule cohort and 45 in the cohort receiving imatinib and chemotherapy in parallel (Figure 1); the median age was 46 years (range, 21-65 years) and 41 years (range, 19-63 years), respectively. Demographic data and disease characteristics of the patients are shown in Table 2. By trial design, the 2 cohorts differed with respect to disease status at the time of enrollment (Table 2; Figure 2A-B) and therefore by their prestudy response to weeks 1 to 3 of induction chemotherapy (INDI): 78% of patients in the alternating cohort but only 56% in the parallel treatment cohort were in CR after INDI (Figure 2A-B). In the latter cohort, 43% (19/44) of patients were nonresponders or had achieved only a PR at the time of enrollment (Figure 2B). Thus, a higher proportion of patients in the concurrent than in the alternating treatment cohort displayed up-front resistance to chemotherapy alone (P = .06).
Patient characteristics at study entry
Characteristics . | Alternating . | Concurrent . |
|---|---|---|
| Median age, y (range) | 46 (21-65) | 41 (19-63) |
| Sex, no. (%) | ||
| Male | 25 (53) | 23 (51) |
| Female | 22 (47) | 22 (49) |
| Subtype of ALL, no. (%) | ||
| c-ALL | 33 (70) | 28 (62) |
| Pre-B-ALL | 10 (21) | 14 (31) |
| NA | 0 (0) | 3 (7) |
| Lymphoid blast crisis | 4 (9) | 0 (0) |
| BCR-ABL transcripts, no. (%) | ||
| P210BCR-ABL | 20 (43) | 12 (27) |
| P190BCR-ABL | 26 (55) | 31 (69) |
| NA | 1 (2) | 2 (4) |
| Disease status at study start, no. (%)* | ||
| CR1 | 44 (94) | 24 (54) |
| PR | 3 (6) | 10 (22) |
| No response | 0 (0) | 9 (20) |
| NA | 0 (0) | 2 (4) |
Characteristics . | Alternating . | Concurrent . |
|---|---|---|
| Median age, y (range) | 46 (21-65) | 41 (19-63) |
| Sex, no. (%) | ||
| Male | 25 (53) | 23 (51) |
| Female | 22 (47) | 22 (49) |
| Subtype of ALL, no. (%) | ||
| c-ALL | 33 (70) | 28 (62) |
| Pre-B-ALL | 10 (21) | 14 (31) |
| NA | 0 (0) | 3 (7) |
| Lymphoid blast crisis | 4 (9) | 0 (0) |
| BCR-ABL transcripts, no. (%) | ||
| P210BCR-ABL | 20 (43) | 12 (27) |
| P190BCR-ABL | 26 (55) | 31 (69) |
| NA | 1 (2) | 2 (4) |
| Disease status at study start, no. (%)* | ||
| CR1 | 44 (94) | 24 (54) |
| PR | 3 (6) | 10 (22) |
| No response | 0 (0) | 9 (20) |
| NA | 0 (0) | 2 (4) |
For patients on the alternating schedule, n = 47; for patients on the concurrent schedule, n = 45.
NA indicates not available; CR1, first complete remission; and PR, partial remission
With the alternating schedule, patients were enrolled after INDII if they had achieved a remission in response to induction chemotherapy; with the concurrent administration schedule, patients already entered the study after INDI, irrespective of their response to chemotherapy
Alternating schedule: feasibility and outcome
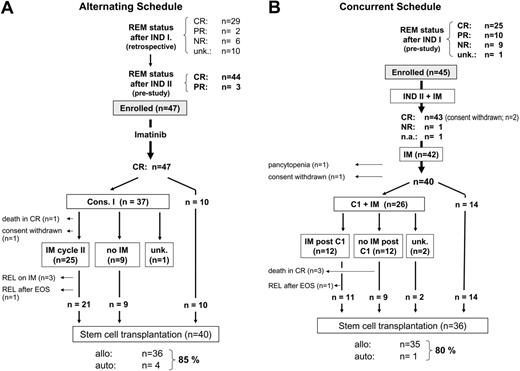
Given the requirement for recovery of PB values before study entry, imatinib was started a median of 19 days (range, 5-52 days) after INDII, and was administered for a median of 28 days (range, 14-64 days; 25% and 75% percentile 28 days and 28.5 days, respectively). Median ANCs and platelet counts during the 4-week postinduction imatinib cycle decreased by 22.6% (from 3.1 × 109/L to 2.5 × 109/L) and 33.7% (from 264 × 109/L to 175 × 109/L), respectively. No grade III or IV hematologic toxicities or infectious or bleeding complications were recorded during this imatinib cycle. Five patients received fewer than the scheduled 28 days of imatinib: 1 patient discontinued imatinib after 2 weeks subsequent to withdrawing consent for all antileukemic therapy; in 4 patients, interruption or early termination of imatinib because of grade 3 myalgia (n = 1), grade 2 facial edema (n = 1), and grade 3 nausea resulted in treatment durations of 25 days and 27 days in 2 patients each. When dose reductions and days of imatinib actually delivered were considered together, 36 (78%) of 46 evaluable patients received at least 100% of the scheduled dose, and 85% of patients received 90% or more of the planned cumulative dose of imatinib.
As only patients already in CR were eligible for study entry in this cohort, efficacy was assessed primarily at the molecular level by analysis of BCR-ABL transcript levels. Nevertheless, all 3 patients who entered the study with a PR achieved a CR after the first 28-day cycle of imatinib. Moreover, no patient relapsed during the postinduction imatinib cycle.
The treatment course is depicted in Figure 2A. Thirty-seven patients (79%) received consolidation (C1), with a median interval of 5 days (range, 1-16 days) from stopping imatinib; 10 patients were transferred to SCT by center decision instead of receiving C1. There was one septic death during cytopenia after C1; the other patients recovered without unexpected toxicity. Twenty-five (69%) of the 36 patients who completed C1 received a second imatinib cycle, with a median duration of 41 days (range, 10-68 days).
Flow chart of the disease status and treatment until transfer to stem cell transplantation in patients treated within the 2 study cohorts. (A) Alternating schedule. (B) Concurrent schedule. REM status indicates remission status; INDI, induction phase 1; INDII, induction phase 2; CR, complete remission; PR, partial remission, NR, nonresponder; unk, unknown; na, not available; C1, consolidation phase 1; IM, imatinib; REL, relapse; EOS, end of study; allo, allogeneic; and auto, autologous.
Flow chart of the disease status and treatment until transfer to stem cell transplantation in patients treated within the 2 study cohorts. (A) Alternating schedule. (B) Concurrent schedule. REM status indicates remission status; INDI, induction phase 1; INDII, induction phase 2; CR, complete remission; PR, partial remission, NR, nonresponder; unk, unknown; na, not available; C1, consolidation phase 1; IM, imatinib; REL, relapse; EOS, end of study; allo, allogeneic; and auto, autologous.
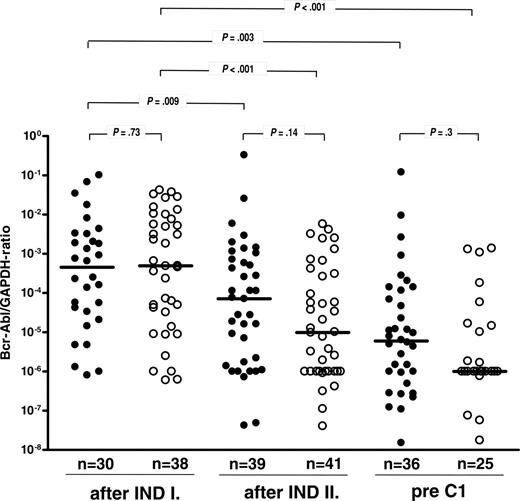
BCR-ABL transcript levels in bone marrow samples determined by quantitative RT-PCR and given as ratio of BCR-ABL to GAPDH signal. Samples were collected at predefined time points during the study (ie, prior to and after INDII and prior to C1 as shown in Figure 1). Values are depicted on a logarithmic scale for patients treated within the alternating (•) or concurrent (○) schedule, with median values represented by solid lines. Negative RT-PCR results are shown at the level of the sensitivity of the PCR reaction.
BCR-ABL transcript levels in bone marrow samples determined by quantitative RT-PCR and given as ratio of BCR-ABL to GAPDH signal. Samples were collected at predefined time points during the study (ie, prior to and after INDII and prior to C1 as shown in Figure 1). Values are depicted on a logarithmic scale for patients treated within the alternating (•) or concurrent (○) schedule, with median values represented by solid lines. Negative RT-PCR results are shown at the level of the sensitivity of the PCR reaction.
Overall, 40 of the 45 patients from this cohort who were considered eligible for SCT underwent allogeneic (n = 36) or autologous (n = 4) SCT in CR1, for an overall transplantation frequency of 85%. The median time from diagnosis to SCT was 165 days (range, 103-287 days). Three patients relapsed while receiving imatinib after C1 (ie, prior to planned SCT) (Figure 2A), with a remission duration of 4.9 to 6.2 months. Two elderly patients who were ineligible for SCT relapsed 7.6 and 12 months after imatinib was discontinued; the remission duration was 8.6 and 14.4 months. One patient withdrew consent after C1 and relapsed 6.4 months after imatinib was discontinued.
Concurrent schedule: hematologic response
As described in “patients,” 43% of patients in the concurrent cohort had not achieved a CR after INDI (Figure 2B). All 10 PR patients and 8 of 9 nonresponders after INDI subsequently achieved a CR with concurrent administration of INDII and imatinib. One patient with primary refractory leukemia after INDI did not respond to coadministration of imatinib and INDII and was taken off study; a subsequent salvage attempt with C1-based chemotherapy plus imatinib was unsuccessful. All 25 CR patients maintained their response after INDII, resulting in an overall CR rate of 95.6% (43 of 45 patients; not evaluable for response: n = 1) (Figure 2B). Prolonged pancytopenia developed after INDII in one patient, leading to removal from the study. Three patients withdrew consent before receiving post-IND imatinib (n = 2) and prior to C1 (n = 1).
None of the 40 patients who remained on study relapsed or died prior to C1 (n = 26) or SCT that was performed instead of C1 (n = 14) (Figure 2B). Twenty-six patients (55.6%) received C1 in conjunction with imatinib, 12 of whom continued imatinib after C1 until allogeneic SCT. One patient relapsed before SCT could be performed, 9 days after discontinuation of imatinib.
Eighty percent (36/45) of patients were transferred to SCT in CR1. This was not significantly different from the transplantation frequency (85%) in the alternating treatment cohort (Figure 2A-B). Median time from diagnosis to SCT was also similar (136 days; range, 90 to 193 days).
Concurrent schedule: feasibility and tolerability
Median duration of imatinib treatment from start of INDII to C1 was 78 days (range, 8-138 days; evaluable, n = 35). Imatinib was initiated at 600 mg once daily. Dosing was interrupted because of grade III or IV hematologic toxicity in 87% of patients (39 of 45) and because of nonhematologic toxicity in 53% of patients (19 of 36); the latter was due primarily to transient hepatic toxicity in form of transaminase elevation and hyperbilirubinemia. Imatinib was dose-reduced and/or interrupted in 32 patients (71%). The duration of grade III or IV thrombocytopenia and neutropenia was 12 days (range, 3-57 days) and 16 days (range, 3-47 days), respectively. The median duration of INDII plus imatinib was 22 days (range, 12-59 days). This was not significantly longer than the duration of INDII alone in the alternating cohort (21 days; range, 14-49 days). There were 3 septicemia-related deaths during the cytopenia that followed C1, all in patients who had not received imatinib after C1.
Molecular response by treatment schedule
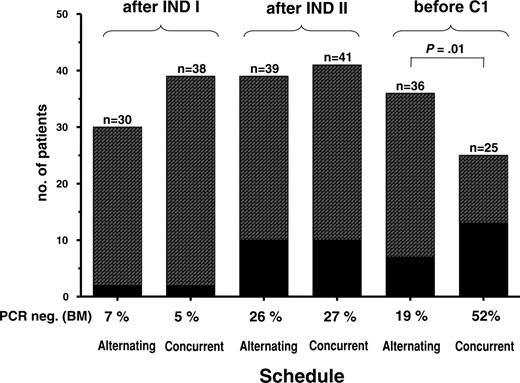
BCR-ABL transcript levels after INDI did not differ significantly in the 2 study cohorts (median BCR-ABL/GAPDH ratios 4.5 × 10-4 vs 4.9 × 10-4) (Figure 3). At this time point, 6% of all patients were PCR negative (4 of 68 evaluable patients) (Figure 4). During INDII, MRD levels decreased by a median of 0.9 log with the alternating schedule (ie, with chemotherapy alone; P = .009) and by 1.6 log (from 4.9 × 10-4 to 9.8 × 10-6) with simultaneous imatinib and chemotherapy (P < .001). BCR-ABL/GAPDH ratios at the end of induction were 1.1 log lower in patients who had received INDII concurrently with imatinib rather than INDII alone, although this difference was not statistically significant (P = .3) (Figure 3). Similarly, the proportion of patients who were PCR negative at the end of induction therapy did not differ significantly between alternating and concurrent treatment cohorts, reaching 26% and 27%, respectively (Figure 4).
With both schedules, the 4-week postinduction imatinib cycle was associated with an additional, approximately 1-log decrease of bcr/abl transcript levels assessed prior to C1 (P = .3) (Figure 3). Notably, the proportion of patients in whom BCR-ABL transcripts became undetectable prior to C1 increased to 52% in the concurrent treatment cohort but did not increase further in the alternating treatment group (19%) (Figure 4). This difference in PCR negativity achieved with the 2 treatment schedules is significant (P = .01).
Proportion of patients with PCR negativity in BM after INDI and INDII and prior to C1 in patients treated within the alternating or concurrent schedule.
Proportion of patients with PCR negativity in BM after INDI and INDII and prior to C1 in patients treated within the alternating or concurrent schedule.
Diagram showing absolute BCR-ABL transcript levels in bone marrow samples after INDI and INDII and prior to C1 by remission status after INDI. The medians are represented by solid lines, and negative PCR results are shown at the level of the sensitivity of the PCR reaction. CR indicates complete remission; PR, partial remission; and NR, nonresponder.
Diagram showing absolute BCR-ABL transcript levels in bone marrow samples after INDI and INDII and prior to C1 by remission status after INDI. The medians are represented by solid lines, and negative PCR results are shown at the level of the sensitivity of the PCR reaction. CR indicates complete remission; PR, partial remission; and NR, nonresponder.
Molecular response in relation to hematologic response after INDI
Whereas early phase 1 and 2 studies showed that single-agent imatinib induced a CR in approximately 20% of Ph+ALL patients who failed chemotherapy, a CR rate exceeding 90% has been reported in response to imatinib in patients with newly diagnosed Ph+ALL.27,28 We therefore examined whether the quality of the molecular response to concurrent imatinib and INDII differed by the hematologic response to weeks 1 to 3 of INDI. As expected, prestudy BCR-ABL transcript levels were higher in patients with a poor response to INDI (PR and nonresponse, n = 15) than in patients who had achieved a CR (n = 21) (median: 7.6 × 10-3 vs 5 × 10-5; P = .001) (Figure 5). Following coadministration of imatinib and INDII, MRD levels decreased by a median of 1.1 log in the prior good responders and by 2.2 log in the poor responders. When patients with a poor and good response to INDI were compared regarding their molecular response after INDII with or without imatinib, neither median MRD levels (5.3 × 10-5 vs 3.8 × 10-6; P = .16) nor the proportion of patients who were PCR negative differed significantly (27% vs 29%; P = NS). The probability of subsequent relapse was not significantly different in the 2 groups of patients (33% versus 19%; P = NS). These data indicate that in newly diagnosed Ph+ALL, a poor hematologic response to the initial phase of remission induction chemotherapy is largely compensated by the subsequent administration of imatinib in combination with chemotherapy.
Treatment outcome by schedule
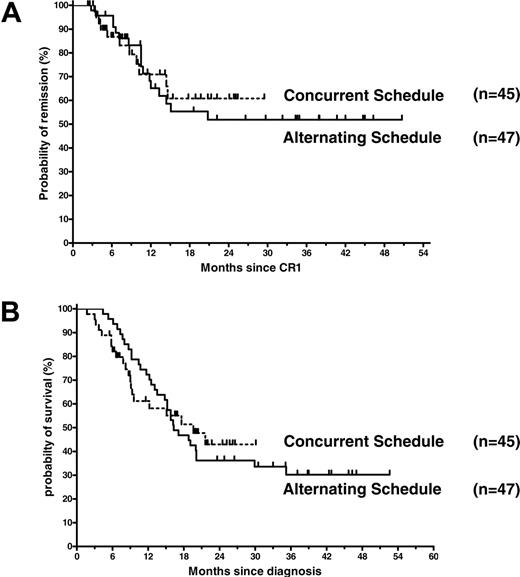
To determine whether the imatinib schedule had an impact on treatment outcome, we compared remission duration and overall survival of the 2 cohorts by Kaplan-Meier analysis (Figure 6A-B). In both patient cohorts, median remission duration is as yet undefined. The estimated probability of remission 12 months and 24 months after first documented CR was 65% ± 8% and 52% ± 9% with the alternating schedule and 71% ± 8.5% and 61% ± 10% with the concurrent schedule (P = .83).
Median survival of patients treated according to the alternating and concurrent schedules was 16.3 months and 19.6 months, respectively. By Kaplan-Meier analysis, the estimated probability of survival 12 months and 24 months after diagnosis was 72% ± 6.5% and 36.2% ± 7%, respectively, with the alternating schedule and 61% ± 8% and 43% ± 9%, respectively, with the concurrent schedule (P = .97).
Discussion
Recent clinical trials using different schedules of chemotherapy and imatinib in patients with newly diagnosed Ph+ALL have demonstrated higher CR rates than in historical cohorts treated with induction chemotherapy alone.23-26 However, it has not been established how to best schedule imatinib in relation to chemotherapy during first-line treatment of Ph+ALL. We therefore prospectively investigated the tolerability and antileukemic efficacy of 2 treatment strategies in which imatinib was combined with remission induction and consolidation chemotherapy using either an alternating or a concurrent application schedule. Since safety data addressing the potential of imatinib to aggravate chemotherapy-related toxicity were lacking when the clinical trial was first initiated, the first patient cohort to be investigated received chemotherapy and imatinib sequentially. We demonstrate that administration of imatinib for 28 days following remission induction chemotherapy is feasible and safe, with essentially no severe imatinib-related hematologic or nonhematologic toxicity. Of importance, subsequent intensive consolidation chemotherapy could be administered without aggravating toxicity after only a short (median, 5 days) washout period for imatinib. Molecular analysis of MRD demonstrated a moderate 1.1-log reduction of BCR-ABL transcript levels during this 4-week cycle of postinduction imatinib, but the proportion of patients achieving PCR negativity, confirmed by nested RT-PCR, did not increase further when compared with the results after induction chemotherapy (19% versus 26%). These results are consistent with data recently published by Lee et al,31 who found a 0.83-log decrease of the median BCR-ABL/abl ratio but no molecular CR in patients who received a 4-week imatinib cycle after induction chemotherapy. Surprisingly, we also found that the molecular response to first-line imatinib in this cohort of chemosensitive patients was not superior to the 1.37-log reduction of the BCR-ABL/GAPDH ratio that we previously observed in 56 patients with relapsed or refractory Ph+ALL after 4 weeks of imatinib given at the same dose levels (400-600 mg).32 A possible explanation for this lower than expected molecular response is a lower sensitivity to imatinib in the subpopulation of leukemic cells that persists after chemotherapy. Alternatively, the administration of cytotoxic agents prior to imatinib may induce imatinib resistance (eg, by up-regulation of cellular drug efflux pumps such as MDR1/ABCB133,34 or [BCRP]/ABCG235 ). Conceivably, a better molecular response could have been achieved by extending the duration of imatinib beyond 4 weeks, as we previously showed that the median time to best BM response in advanced Ph+ALL was 39 days.32 Nevertheless, the addition of imatinib between chemotherapy cycles had a favorable clinical impact evident from the low incidence of relapse on study: no patient had recurrent leukemia prior to consolidation therapy and only 3 of 47 enrolled patients relapsed on study prior to SCT.
Probabilities of remission and overall survival. (A) Kaplan-Meier estimates of remission duration for patients in cohort 1 (n = 47) treated according to the alternating schedule and patients in cohort 2 (n = 45) receiving imatinib simultaneously with induction 2 chemotherapy (relative risk in the alternating treatment schedule, 1.086; 95% confidence interval, 0.5094 to 2.317; P = .83 by the log-rank test) and (B) of overall survival by treatment schedule (relative risk in the alternating treatment schedule, 1.009; 95% confidence interval, 0.5773 to 1.766; P = .97 by the log-rank test).
Probabilities of remission and overall survival. (A) Kaplan-Meier estimates of remission duration for patients in cohort 1 (n = 47) treated according to the alternating schedule and patients in cohort 2 (n = 45) receiving imatinib simultaneously with induction 2 chemotherapy (relative risk in the alternating treatment schedule, 1.086; 95% confidence interval, 0.5094 to 2.317; P = .83 by the log-rank test) and (B) of overall survival by treatment schedule (relative risk in the alternating treatment schedule, 1.009; 95% confidence interval, 0.5773 to 1.766; P = .97 by the log-rank test).
After demonstrating good tolerability but a suboptimal molecular response with the alternating schedule, we examined whether the antileukemic efficacy of imatinib-based induction therapy was more pronounced when imatinib was administered concurrently with the same induction and consolidation chemotherapy as in the first cohort. Patients were enrolled after the first 3 weeks of induction chemotherapy rather than immediately after diagnosis to avoid delaying treatment while awaiting clarification of the patients' BCR-ABL status in this multicenter setting. In contrast to the cohort receiving the alternating schedule, patients were also eligible if they had not achieved a CR after INDI. Due to this change of eligibility criteria, a higher proportion of patients with an unfavorable prognosis was treated according to the concurrent schedule; this imbalance was in our view justified by a less than 20% probability that nonresponders to INDI would achieve a CR after completing induction chemotherapy alone.
Our study clearly demonstrates that the simultaneous administration of imatinib and induction and consolidation chemotherapy is highly effective first-line treatment for adult Ph+ALL, with a CR rate of 95%. PCR negativity was achieved in 50% of patients. Overall, these data are consistent with the hematologic and molecular remission rates reported in 2 smaller studies that examined the use of imatinib and chemotherapy in first-line therapy of adult Ph+ALL. Thomas et al23 reported a CR rate of 100% in 15 patients with active leukemia, 11 with de novo disease and 4 primary failures, who were treated with hyper-CVAD (cyclophosphamide, vincristine, adriamycin, and dexamethasone) given concurrently with 14-day cycles of 400 mg imatinib. This study also reported complete molecular remissions, confirmed by nested PCR, in 5 of the 15 patients with de novo leukemia, first documented between 1 and 9 months of therapy (ie, after a variable number of imatinib/hyper-CVAD consolidation cycles). In another clinical trial reported by the Japan Adult Leukemia Study Group (JALSG), induction chemotherapy was followed by imatinib (600 mg/d) until day 63, with 2 vincristine doses on days 15 and 22 given simultaneously with imatinib. In an interim analysis of 24 patients, the CR rate attained after a single induction cycle was 96%. PCR negativity, though not confirmed by nested PCR, was achieved in 50% of patients on day 63, prior to first consolidation. These results correspond well with the 52% incidence of PCR negativity that we observed in the 45 patients receiving concurrent imatinib and chemotherapy, whereas sequential treatment with chemotherapy and imatinib (alternating cohort, n = 47) resulted in a molecular remission rate prior to consolidation of only 19%. These differences between the JALSG and our study may be due to the more stringent criteria for PCR negativity in our study, which required confirmation by nested PCR. Differences in the chemotherapy regimens also need consideration, although the GMALL induction regimen is more intensive, which is difficult to reconcile with an inferior response unless the cytotoxic drugs reduce the sensitivity to imatinib, as discussed previously.
Remarkably, hematologic as well as molecular responses in the concurrent cohort of our study were independent of the remission status after the first 3 weeks of chemotherapy. Patients who were refractory and those in CR after INDI did not differ significantly in terms of their probability of achieving either a hematologic or a molecular remission. In view of the 17% to 30% CR rates with imatinib monotherapy in Ph+ALL patients failing chemotherapy in the initial phase 1 and 2 studies,17-19 these results are most likely attributable to synergy between imatinib and the cytotoxic agents used in our induction regimen rather than to the difference in cumulative dose of imatinib, although this cannot be unequivocally proven given the design of our study. In agreement with our data, Thomas et al23 reported that 4 patients with refractory Ph+ALL achieved a CR after receiving imatinib in combination with one cycle of chemotherapy according to the hyper-CVAD regimen. These observations are also consistent with preclinical data showing additive or even synergistic effects when imatinib is combined with cytotoxic agents.20-22
Further clinical support for the superior antileukemic efficacy of the concurrent as opposed to the alternating schedule stems from our data showing that a significantly higher percentage of patients converted to PCR negativity, despite a higher proportion of patients with adverse clinical risk features in this cohort. Also, no patient in the concurrent treatment cohort relapsed on study, in contrast to 3 patients receiving the alternating schedule, although this difference was not statistically significant. Of interest, the greatest differential effect of schedule on the molecular response became apparent only after the 4-week imatinib cycle that was administered between INDII and C1, with PCR negativity developing in 52% and 19% of patients treated according to the concurrent and alternating schedule, respectively. These response kinetics highlight the importance of timing when collecting BM samples to evaluate treatment efficacy at the molecular level, particularly for comparison of different regimens.
The toxicity of treatment associated with coadministration of imatinib and INDII chemotherapy of the GMALL regimen was manageable but significant, with grade III/IV cytopenias and transient hepatotoxicity necessitating treatment interruptions in the majority of patients. Notably, the duration of INDII was essentially identical in patients treated according to the concurrent and the alternating schedules; this indicates that the severe adverse events were attributable primarily to chemotherapy and/or that imatinib-related toxicity was transient. The incidence of nonhematologic toxicities in the JALSG study with a primarily alternating schedule of chemotherapy and imatinib and the MDACC study with a parallel schedule were both acceptable, with infections and febrile neutropenia being the predominant grade 3 or 4 toxicities.23,24 Conspicuously, we observed in our study a significantly higher rate of grade 3 and 4 transaminase elevations and hyperbilirubinemia during the coadministration of imatinib and chemotherapy. This may be related to the use of pegylated asparaginase during INDI, which is frequently associated with delayed hepatotoxicity, and/or to 6-mercaptopurine, an agent used during INDII in the GMALL protocol but not in the JALSG or hyper-CVAD regimens.23,24 Although hepatotoxicity was transient and not associated with severe liver dysfunction, this highlights the need to consider possible drug-drug interactions when combining imatinib or other kinase inhibitors with conventional cytotoxic agents.
Currently, allogeneic SCT is thought to be the only curative therapy for Ph+ALL in adults, with transplantation in CR1 yielding the, by far, best results.6-11 With both of our treatment schedules, overall transplantation frequencies in CR1 (80%-85%) and the proportion of patients undergoing allogeneic SCT (77%) were comparable. This allogeneic transplantation rate is considerably higher than the approximately 50% previously reported by the LALA and GMALL multicenter cooperative group trials, which did not incorporate imatinib as an element of first-line treatment (Dombret et al36 and data not shown). It can be attributed to a low relapse rate and acceptable toxicity in each of the 2 treatment regimens examined. Similarly high transplantation frequencies were recently reported by Lee et al31 from a single-center study using a similar schedule of imatinib and chemotherapy as in our study, and in the study from the JALSG, in which 15 (63%) of 24 patients underwent alloSCT in CR1.24 In children, the level of residual disease prior to SCT has been shown to correlate with long-term outcome.14-15 Similarly, in an analysis of patients treated in the LALA-94 trial who were eligible for SCT, Dombret et al4 reported superior treatment outcome in the subgroup who had achieved BCR-ABL negativity after 2 chemotherapy cycles when compared with patients who remained BCR-ABL positive by PCR. While the percentage of patients undergoing SCT in our study was uniformly high, the proportion of patients achieving PCR negativity clearly favored the concurrent treatment schedule. We are not able to show a difference in either remission duration or overall survival when comparing outcome of patients treated according to either the concurrent or the alternating imatinib schedule, however. It should be noted that our study was neither designed nor powered to detect such a difference, particularly in view of the overriding impact of subsequent stem cell transplantation in 80% to 85% of enrolled patients. Conspicuously, our results are inferior to those reported by Towatari et al24 and by Lee et al,31 even though both of these groups used a strategy of combined imatinib and chemotherapy that was similar to ours, with transplantation rates in CR1 of 63% and 82%. One potentially crucial difference between our and their studies is patient age, with a rather high median age of 41 years and 46 years in our treatment cohorts compared with 36 years in the study reported by Lee et al.31 Additional differences may be donor selection, degree of HLA matching, and center effects when comparing results from single or few transplant centers with those obtained in a multicenter setting such as in our patients. While we did not compare treatment outcome in our study with the results of a historical control group, the probability of survival in Ph+ALL patients treated in 2 previous German Multicenter Trials of Adult ALL including stem cell transplantation was 15% at 3 years.3 In the French LALA-94 trial, estimated survival of 103 patients who were eligible for SCT and had a donor was 37%; the estimated incidence of relapse was 50% at 3 years and of death in CR 24% at 2 years.4 Taken together, available data do not suggest that administration of imatinib as part of front-line treatment has a detrimental effect on overall or posttransplantation treatment outcome.
Meanwhile, our study strongly suggests that schedules based on the simultaneous administration of imatinib and cytotoxic agents should form the basis for prospective, comparative studies aimed at improving the pretransplantation molecular response during first-line treatment of Ph+ALL.
Prepublished online as Blood First Edition Paper, April 25, 2006; DOI 10.1182/blood-2005-11-4386.
Supported by grants from the BMBF Competence Network “Acute Leukemias” (grant no. 01GS0441); the German Genome Research Network (NGFN); the Wilhelm Sander Stiftung and the Adolf-Messer Foundation, Germany; and Novartis Pharma, Nuremberg, Germany.
O.G.O., B.W., and D.H. designed and implemented the study, treated patients, analyzed data, and wrote the paper; H.P. and P.B. performed research (MRD analysis); N.G. and R.R. analyzed data; D.W.B., J.B., M. Stelljes, M.B., A.R., J.P., R.H., A.G., M. Schmid, L.K., G.L., and M.K. enrolled and treated patients; A.B. coordinated the study and managed and analyzed data; H.G. contributed to study design and gave logistical support; and S.S. performed diagnostic laboratory assessment.
B.W. and H.P. contributed equally to this paper.
Presented in part at the 43rd annual meeting of the American Society of Hematology, San Diego, CA, December 7, 2004.26
One of the authors (H.G.) is employed by a company (Novartis) whose product was studied in the present work. O.G.O. and D.H. received research support from Novartis and served as consultants.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Brigitte Gehrke, Tamara Hirdes, Doreen Badowski, and Sandra Markovic for their excellent technical assistance.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal