Abstract
Cytokine secretion profiles of activated T cells are critical for maintaining the immunologic balance between protection and tolerance. In mice, several cytokines have been reported to exhibit monoallelic expression. Previously, we found that the human interleukin-1 alpha (IL1A) gene exhibits a stable allele-specific expression pattern in CD4+ T-cell clones. We investigated whether DNA methylation is involved in the allele-specific expression of IL-1α. Here, we show that differential methylation of CpGs in the proximal promoter region is associated with allele-specific expression of IL-1α in CD4+ T cells. The differential methylation pattern is already observed in naive T cells. In keratinocytes, which constitutively produce IL-1α, the proximal promoter is hypomethylated. CpGs located further upstream and in intron 4 were almost all methylated, irrespective of expression. Treatment of nonexpressing cells and of T-cell clones with 5-aza-2′deoxycytidine induced IL-1α expression in the nonexpressing cells and induced expression of the formerly silent allele in T-cell clones. In addition, electrophoretic mobility shift assays showed that methylation of CpGs in the proximal promoter resulted in direct inhibition of binding of nuclear factor(s). Taken together, these results suggest that allele-specific expression of IL-1α in CD4+ cells is achieved, at least in part, by differential methylation of the promoter.
Introduction
Cytokines are proteins that are produced by immunocompetent cells, controlling immune activity within the cell or at a distance. Cytokines mediate immunologic cross-talk between and within immune cells, and play a major part in inflammatory responses.
The interleukin 1 (IL-1) gene family consists of IL1A, IL1B, and IL18, and 6 additional putative ligand members have been reported.1 The IL-1 family also includes a naturally occurring competitive IL-1 receptor antagonist (IL-1RA).2 IL-1α and IL-1β have similar biologic activities and are generally known as highly inflammatory cytokines.2 They are produced by various immune cells and IL-1α is constitutively expressed by epithelial, endothelial, fat, and microglia cells.3 Also, keratinocytes constitutively produce large amounts of precursor IL-1α in healthy human skin.4 Monocytes, macrophages, and CD4+ T cells are known to produce IL-1α only after stimulation by various exogenous and endogenous stimuli.5,6
Both IL-1α and IL-1β are synthesized as precursors without leader sequences, and processing of the precursors to “mature” proteins requires specific cellular proteases. The IL-1α proform is bioactive in contrast to the IL-1β proform2 and is not commonly found in the circulation or in body fluids, except during severe disease.6
IL-1 is the dominant cartilage-destructive cytokine in animal models7 in which a deficiency of IL-1RA relative to IL-1 leads to more severe autoimmune disease and to the spontaneous development of arthritis.8 These studies suggest that IL-1 expression needs to be tightly regulated.
Recently, there have been various reports on monoallelic expression of murine and human cytokines such as IL-2,9 IL-4,10 and GMCSF,10 and also other proteins such as p120 catenin,11 TLR4,12 Pax5,13 and Perforin.14 Previously we, and others,15 have shown stable nonimprinted (random), imbalanced allelic expression of IL-1α in human CD4+ T-cell clones (TCCs) in which either one of the alleles is strongly favored (> 8- to 16-fold).16 This indicates that allelic differences in gene expression (allelic imbalances) could provide an additional level of regulation of gene expression. These observations are supported by larger scale screenings of allelic expression, which show that many genes do not express both alleles of autosomal genes at equal levels.15
Here, we investigated whether the allele-specific activation or silencing is established by an epigenetic mechanism. Methylation of CpG dinucleotides is an epigenetic mechanism used for silencing of long-term gene expression and serves to recruit histone deacetylases, histone methyltransferase, and chromatin remodeling complexes that promote a closed chromatin structure.17 Hypermethylation of DNA has been negatively correlated with gene expression in many cases, and is thought to exert its repressive effects on transcription by inhibiting the binding of transcription factors to their cis-element and also indirectly via the recruitment of methyl-DNA–binding proteins.18
Mammals exhibit several epigenetic phenomena that prevent simultaneous gene expression from both alleles of a given locus such as nonimprinted (random) X chromosome inactivation in females, nonrandom parental imprinting of selected autosomal genes, allelic exclusion of antigen receptors in lymphocytes, and odorant receptor gene clusters in olfactory sensory neurons.9 IL-1α, like other cytokine genes, does not reveal any of the features such as recombinase-activation gene (RAG)–mediated cleavage established for loci known to be allelically excluded.19 The IL1A gene is not a known target of parental imprinting. Recently, it has been shown that transcription of NK cell receptors (KIR genes), which are present in a cluster of genes, precisely correlates with allele-specific DNA methylation.20,21
IL1A is localized on human chromosome 2 and is encoded by a single gene. Little is known about the regulation of human IL-1α expression. It has been reported that several regions are involved in inducible gene expression, including a GC-rich element, containing CpGs just upstream of the transcription start site.6,22,23 The IL-1α 5′ flanking region contains multiple CpG nucleotides that could render this locus susceptible to epigenetic regulation by DNA methylation. It is thus an attractive hypothesis that allele-specific expression of IL-1α is associated with allele-specific methylation.
Materials and methods
Cells
Human T-cell clones were prepared and cultured as described before.16 CD45RA+CD45RO– naive CD4+ Th cells were isolated from peripheral blood mononuclear cells (PBMCs) from healthy individuals by magnetic-activated cell sorter (MACS) isolation as described before24 or fluorescence-activated cell sorter (FACS) flow sorted. Keratinocytes were isolated from foreskin as described before.25 The IL-1α–nonexpressing cell line used in one of the experiments is the Namalwa cell line, a human lymphoblastoid cell line established from a Burkitt lymphoma patient.
Drug treatment
For treatment, restimulated T-cell clones or Namalwa cells were seeded at a density of 1 × 106 cells/mL and treated with 0.1 μM, 1 μM, or 2 μM 5-aza-CdR for 24 hours. Subsequently, the medium was changed and cells were cultured for 48 hours. The T-cell clones cells were stimulated for 6 hours with PMA (5 ng/mL) and ionomycin (250 ng/mL).
RNA and DNA extraction
Total RNA isolation including a DNase treatment was performed using the RNeasy kit (Qiagen, Leusden, the Netherlands) according to the manufacturer's instructions. Genomic DNA was isolated with the DNeasy kit (Qiagen). Polymerase chain reaction (PCR) products were purified from gel with QIAEX II agarose gel extraction kit (Qiagen) or Qiaquick column purification (Qiagen).
5′ RACE
The 5′ end of IL-1α mRNAwas determined using the RLM-RACE kit (Ambion, Austin, TX) according to the manufacturer's instructions. The sequences of gene-specific primers were as follows: IL-1α (TSS1) outer 5′-ACA GAT TGA TCC ATG CAG CCT TCA-3′; IL-1α (TSS1) inner 5′-GGA GTG GGC CAT AGC TTA CAT GAT-3′; IL-1α (TSS2) outer 5′-CCC TGT TAC AGT AAA GTA GCC CTC TA-3′; and IL-1α (TSS2) inner 5′-GAG AAG CCT GGT CTT CTG TAG GA-3′. The IL-1α (TSS1) inner 5′ RLM-RACE PCR product was cut out from a 2% agarose gel, purified using the Gel Extraction Kit (Qiagen), and sequenced with a nested gene-specific primer 5′-GTC TTC TTC ATT TTC ACT GTAACA GTT C-3′.
cDNA synthesis, real-time RT-PCR, RT-PCR, and ASTQ analysis
First-strand cDNA was synthesized from total RNA using a cDNA synthesis kit (RevertAid H minus First-strand cDNA synthesis kit; MBI Fermentas, St Leon Roth, Germany). Real-time reverse-transcriptase (RT)–PCR for IL-1α and GAPDH was performed on an ABI 7900HT sequence detection system (Applied Biosystems, Foster City, CA) using SYBR Green Master Mix (Applied Biosystems) according to the manufacturer's guidelines. The endogenous reference gene used was GAPDH. Primer information is available upon request. Allele-specific transcript quantification (ASTQ) was performed as described before.26 In brief, this method starts with RT-PCR with a final-cycle labeling to allow visualization of de novo homoduplex PCR fragments. Hereby, we prevent the induction of heteroduplex PCR fragments in our analysis due to mismatch annealing. Subsequently, PCR products are digested to discriminate between the +4845G/T alleles.
Detection of DNA methylation
The bisulfite conversion of genomic DNA was adapted from Frommer et al27 with some modifications. DNA (1-3.5 μg) in a volume of 60 μL was treated with bisulfite and purified on a P30 column (Biorad, Veenendaal, the Netherlands). We have also used the EZ DNA Methylation kit (ZYMO research, Leiden, the Netherlands), obtaining similar results. After modification with bisulfite, 40 to 150 ng bisulfite-treated DNA was used to amplify specific regions by PCR: a 2-minute denaturation step was followed by 5 cycles of 1 minute at 94°C, 2 minutes at 50°C, and 3 minutes at 72°C, and 25 cycles of 30 seconds at 94°C, 2 minutes at 50°C, and 1.5 minutes at 72°C. The PCR products were amplified using a nested set of primers with the same amplification program. Primers specific for bisulfite-converted DNA were designed, and used as follows: DNA molecules of the promoter region were analyzed in 3 parts, namely containing CpG site –13 and –12; containing CpG sites –11, –10, –9, and –8; and containing CpG sites –8 or –7 through –1 or +1; CpG sites in intron IV were analyzed in a fragment containing CpG sites +17 through +29. After amplification of bisulfite-converted DNA, methylation levels were measured on several CpGs (–8, –7, –2, or +1) either by methylation-sensitive single nucleotide primer extension (Ms-SNuPE)28 or by sequencing of cloned PCR products. To this aim, PCR products were purified using the QIAEXII Agarose gel extraction kit (Qiagen) and cloned using pGEM-T Easy Cloning kits (Promega, Leiden, the Netherlands) or TOPO TA cloning kit (Invitrogen, Breda, the Netherlands). More than 9 cloned fragments were sequenced for each amplified region of each clone or cell type. Sequences were determined using the BigDye Terminator v1.1 Cycle Sequencing Kit on an ABI 3100 sequencer and analyzed with ABI prism 310 collection software (all products from Applied Biosystems). Sequences for primers for bisulfite-PCR and Ms-SNuPE can be obtained upon request.
Labeling of probes and electrophoretic mobility shift assay (EMSA)
An EMSA was performed as described before,22 with some minor modifications. Briefly, 400 ng ssDNA was end labeled with T4 polynucleotide kinase (NEB, Ipswich, MA) using 3 μL γ32P-ATP (220 TBq/mM) according to the manufacturer's instructions in a volume of 50 μL and purified using the Quick Spin Columns (Roche Diagnostics, Indianapolis, IN). After precipitation, the pellet was dissolved and annealed in 2 μL10 × annealing buffer (10 mM Tris-HCl, pH 7.5, 50 mM NaCl, 10 mM MgCl2, 1 mM EDTA, and 1 mM DTT), 14 μLdH2O, and 600 ng opposing-strand oligonucleotide. The binding reaction with HaCaT or Namalwa nuclear extracts (prepared as described before29 ) was incubated on ice for 30 minutes. Unlabeled competitor ds-oligonucleotides were added to the reaction mix prior to the protein extract. The wild-type ds-oligonucleotide used was –68 through –42 relative to the transcription start site: 5′-ACT TGT AGC CAC GTA GCC ACG CCT ACT-(–42)-3′.22 For the methylated oligonucleotide, the methylation was introduced at the cytosines (underlined) in the CpG dinucleotides at both strands (Operon, Huntsville, AL). The Sp1 ds-oligonucleotide was 5′-ATT CGA TCG GGG CGG GGC GAG C-3′. The whole sample was loaded on a 6% polyacrylamide gel in 0.5 × TBE.
Results
Transcription start site of IL-1α in lymphocytes
The genome database contains several IL-1α mRNA transcripts, suggesting that there are 2 or more transcription start sites (TSSs). GenBank (NM_000575) is a transcript with a long 5′ UTR of about 960 nucleotides (n) (TSS2).
Another transcript with a much shorter 5′ UTR of 59 bp was also reported, which was determined by primer extension (TSS1).30 It was demonstrated that the region upstream of TSS1 acts as a promoter able to control the expression of a reporter gene.22 To determine which of the 2 TSSs is used in stimulated lymphocytes, we performed a 5′-rapid amplification of cDNA ends (RACE) analysis using transcript-specific primers (Figure 1). A PCR product was detected with the TSS1 gene–specific primers, but not with the TSS2 gene–specific primers. The product was subcloned and sequenced with a nested gene-specific primer, which showed that the 5′ end of the mRNA was 59-bp upstream of the ATG. This transcription start site (TSS1) was confirmed by RT-PCR on RNA from stimulated CD4+ T cells from different donors (data not shown). We therefore conclude that the core-promoter directing transcription in lymphocytes starts 59-bp upstream of the ATG.
IL-1α promoter methylation in constitutively IL-1α–expressing keratinocytes is restricted to a confined region
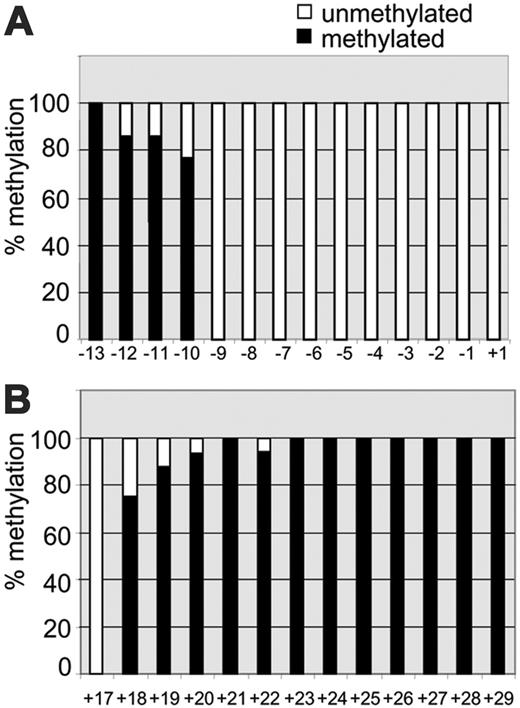
We first determined the methylation status of CpG dinucleotides in the IL-1α proximal promoter in keratinocytes that constitutively produce IL-1α.4 We used bisulfite genomic sequencing to examine the methylation status of between –1-kb upstream and +77-bp downstream of the transcription start site. This region contains 14 CpG sites (labeled –13 through +1 as shown in Figure 1). This cluster of 14 CpG sites in the IL-1α promoter region –1 kb through +76 bp is not identified as a CpG island, as calculated by the CpG Island Searcher.31 The region was examined by 3 PCRs that produced partially overlapping sequences. This revealed that the CpGs –7 through +1 are mostly unmethylated, while CpG –8 through –12 showed partial methylation. CpG –13 was always fully methylated (Figure 2A). A similar pattern was observed in the keratinocyte cell line HaCaT32 (data not shown). Subsequently, we analyzed the methylation of 13 CpGs (+17 through +29) in intron IV, which showed that these intronic CpGs were all methylated, except for the most 5′ CpG (CpG +17, Figure 2B). In conclusion, this shows that in cells constitutively expressing IL-1α, the CpGs within 130 bp from the transcription start site are unmethylated, whereas other CpGs are mostly methylated. Further studies were therefore directed to the proximal promoter region containing CpGs –7 through +1.
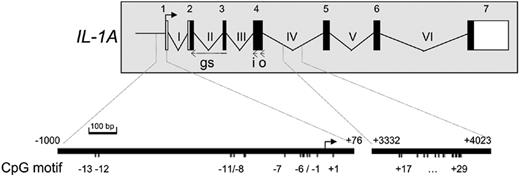
Schematic representation of the humanIL1Agene and 5′ RACE. Exons are depicted as rectangles, white for untranslated and black for translated region. The transcription start site (TSS1) of IL-1α as investigated by 5′RACE is indicated by an arrow. Positions of cDNA-specific primers are indicated by i (inner primer), o (outer primer), and gs (gene specific). The CpG sites in the promoter (numbered –13 through +1) and intron IV (numbered +17 through +29) of the IL1A gene are numbered relative to the transcription start site and are given in an enlarged representation at bottom. Nucleotide numbers of the enlarged regions are given relative to the transcription start site in the genomic DNA and are indicated above the fragments.
Schematic representation of the humanIL1Agene and 5′ RACE. Exons are depicted as rectangles, white for untranslated and black for translated region. The transcription start site (TSS1) of IL-1α as investigated by 5′RACE is indicated by an arrow. Positions of cDNA-specific primers are indicated by i (inner primer), o (outer primer), and gs (gene specific). The CpG sites in the promoter (numbered –13 through +1) and intron IV (numbered +17 through +29) of the IL1A gene are numbered relative to the transcription start site and are given in an enlarged representation at bottom. Nucleotide numbers of the enlarged regions are given relative to the transcription start site in the genomic DNA and are indicated above the fragments.
Methylation pattern of the IL-1α promoter and intron IV in IL-1α–producing keratinocytes as determined by bisulfite sequencing. The level of methylation is expressed as the percentage of methylated CpGs (black bars) and unmethylated CpGs (white bars). (A) Methylation pattern of 14 CpGs (–13 through +1) located in the proximal promoter and 5′ UTR was analyzed by 3 separate PCR fragments as described in “Materials and methods,” based on at least 10 cloned fragments. (B) Methylation pattern of 13 CpGs in intron IV (+17 through +29), based on at least 10 cloned fragments.
Methylation pattern of the IL-1α promoter and intron IV in IL-1α–producing keratinocytes as determined by bisulfite sequencing. The level of methylation is expressed as the percentage of methylated CpGs (black bars) and unmethylated CpGs (white bars). (A) Methylation pattern of 14 CpGs (–13 through +1) located in the proximal promoter and 5′ UTR was analyzed by 3 separate PCR fragments as described in “Materials and methods,” based on at least 10 cloned fragments. (B) Methylation pattern of 13 CpGs in intron IV (+17 through +29), based on at least 10 cloned fragments.
Differential methylation of IL-1α in allele-specific IL-1α–expressing T-cell clones
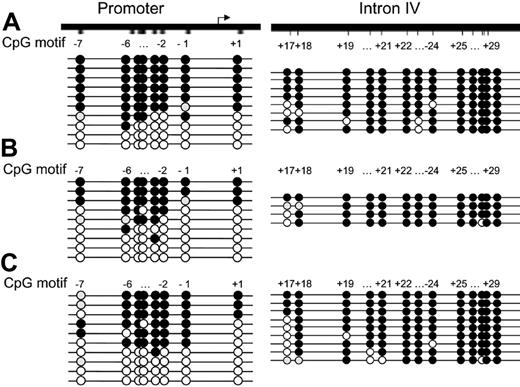
We next analyzed the methylation status of the CpGs in the IL-1α proximal promoter region in allele-specific–expressing T-cell clones. This revealed that the CpGs –7 through +1 were either almost all unmethylated or all methylated in 3 representative T-cell clones (Figure 3A-C).
As expected, bisulfite sequencing of the 13 CpG sites (+17 through +29) in intron IV revealed that CpGs +18 through +29 were almost all found to be methylated on both alleles as was observed in DNA isolated from keratinocytes. Only CpG +17 showed differential methylation (Figure 3A-C). Similar results were obtained for the intron IV CpGs with other T-cell clones exhibiting allele-specific expression. Taken together, this shows that differential methylation of only CpGs –7 through +1 in the proximal promoter region is associated with allele-specific gene expression.
Treatment with 5-aza-CdR induces IL-1α mRNA expression in nonexpressing cells
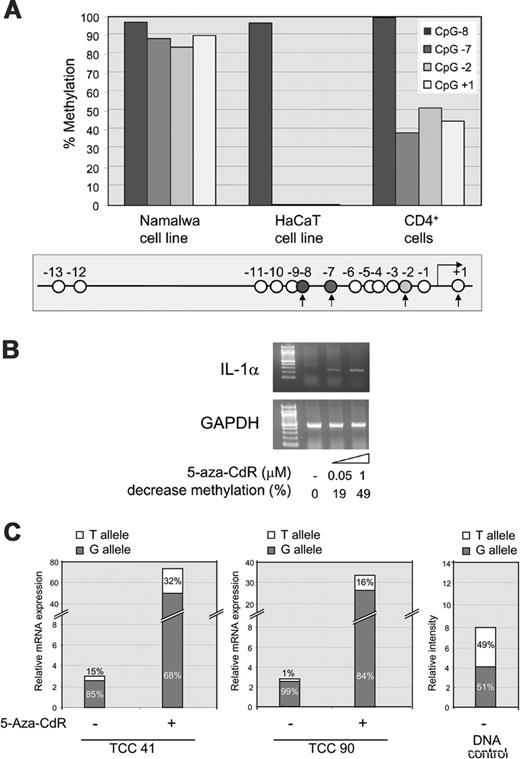
We next analyzed the methylation status of the lymphoblastoid Namalwa cell line, which does not express IL-1α. The methylation of CpGs +1, –2, and –7, which were differentially methylated in the proximal promoter of the T-cell clones, was measured by methylation-sensitive single nucleotide primer extension (Ms-SNuPE) analysis. As a control, we included a CpG that was shown to be highly methylated in keratinocytes (CpG –8). This revealed that the CpG at –8 was highly methylated in all cells (Figure 4A). In contrast, the 3 CpGs found to be differentially methylated in the T-cell clones were highly methylated in the nonexpressing Namalwa cells and were unmethylated in IL-1α–expressing HaCaT cells. With this assay, these CpGs in CD4+ cells showed a differential methylation pattern of 50%. Cloning and sequencing of the bisulfite-treated DNA confirmed that the CpGs on one allele were either methylated or unmethylated (data not shown).
Differential methylation of the proximal IL-1α promoter region in predominantly allele-specific–expressing CD4+T-cell clones. DNA from T-cell clone 41 (A), 90 (B), and 36 (C) was treated with bisulfite, and the promoter region and intron IV were amplified by PCR and cloned. Of each region, multiple clones were sequenced. Each circle represents a CpG. In this and all subsequent figures, filled black circles represent a methylated CpG, white circles represent an unmethylated CpG, and gray circles represent a CpG that could not be analyzed in the sequence. Eight CpGs were analyzed in the 5′ regulatory region (–7 through +1). Thirteen CpGs were analyzed (+17 through +29) in intron IV.
Differential methylation of the proximal IL-1α promoter region in predominantly allele-specific–expressing CD4+T-cell clones. DNA from T-cell clone 41 (A), 90 (B), and 36 (C) was treated with bisulfite, and the promoter region and intron IV were amplified by PCR and cloned. Of each region, multiple clones were sequenced. Each circle represents a CpG. In this and all subsequent figures, filled black circles represent a methylated CpG, white circles represent an unmethylated CpG, and gray circles represent a CpG that could not be analyzed in the sequence. Eight CpGs were analyzed in the 5′ regulatory region (–7 through +1). Thirteen CpGs were analyzed (+17 through +29) in intron IV.
Treatment with 5-aza-CdR changes allele-specific IL-1α mRNA expression
To examine whether the methylated promoter is indeed inactive, cells were treated with the methylation inhibitor 5-aza-CdR. The Namalwa cell line was treated with increasing concentrations of 5-aza-CdR (0.05 μM and 1 μM) after which IL-1α was measured by RT-PCR. This treatment indeed induced IL-1α mRNA expression (Figure 4B). The methylation of CpGs +1, –2, and –7 in the 5-aza-CdR–treated samples was analyzed by Ms-SNuPE, which showed an average decrease of methylation of 19% and 49%, respectively, in cells treated with 0.05 μM and 1 μM 5-aza-CdR. These transcripts were initiated at TSS1 and not at TSS2 (data not shown).
To examine the effect of 5-aza-CdR on IL-1α expression in T-cell clones expressing IL-1α allele specifically, cells were restimulated and incubated in the presence of 2 μM 5-aza-CdR for 24 hours. Cells were stimulated with PMA and ionomycin for 6 hours and IL-1α expression was measured. IL-1α expression was measured by quantitative real-time RT-PCR analysis, which revealed a strong up-regulation of total IL-1α mRNA compared with the untreated cells. Two representative clones, 41 and 90, are shown in Figure 4C.
To see if 5-aza-CdR leads to the activation of the silent allele, we used allele-specific transcript quantification (ASTQ), a quantitative method to discriminate between the alleles. As a positive control, we used genomic DNA that showed an expected G-allele–T-allele ratio of almost 1 (51%:49%, Figure 4C). In the 5-Aza-CdR untreated but stimulated cells, allele-specific expression was found, as expected. In the case of clone 41, the G-allele–T-allele expression ratio was 5.7 (85%:15%), and for clone 90 the G-allele–T-allele expression ratio was 99 (99%:1%, Figure 4C). After incubation with 5-aza-CdR, the expression ratios changed to 2.1 (68%: 32%) and 5.2 (84%:16%) for clones 41 and 90, respectively, indicative of a relative increased expression of the silent allele (Figure 4C). From these results, we conclude that 5-aza-CdR treatment induces IL-1α mRNA expression in nonexpressing cells and that it affects the relative allelic transcript production in T-cell clones with an allelic-imbalanced IL-1α expression pattern.
IL-1α promoter methylation in naive CD4+ T cells
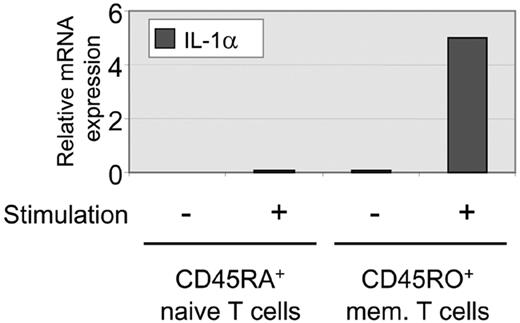
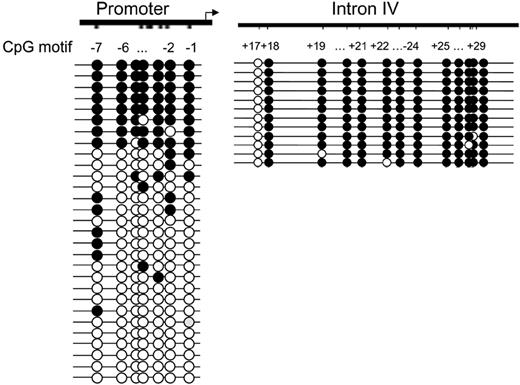
To address the question of whether differentially methylated IL-1α alleles in T-cell clones are predetermined or induced during T-cell differentiation, we investigated the methylation pattern in undifferentiated naive and memory T cells. RT-PCR on sorted CD4+CD45RA+ naive T cells and CD4+CD45RO+ differentiated T cells shows that unstimulated and stimulated naive T cells do not produce detectable levels of IL-1α mRNA (Figure 5, and data not shown). Only stimulated memory cells produce low amounts of IL-1α mRNA (Figure 5) as previously reported. To determine the methylation pattern of the proximal promoter region and intron IV, bisulfite sequencing was performed on DNA isolated from naive T cells. The methylation pattern in naive T cells was analyzed for CpGs –7 through –1 (Figure 6). Of interest, the bisulfite sequencing revealed a similar methylation pattern as observed in the differentiated T-cell clones. The proximal promoter CpGs (CpG –7 through –1) were either completely methylated or unmethylated. Also, the CpG sites +18 through +29 in intron IV from both alleles were highly methylated (Figure 6), as we have seen in keratinocytes and T-cell clones. These methylation patterns were also found in cells from a second donor (data not shown). This analysis shows that both naive and differentiated T cells contain methylated and unmethylated IL-1 promoter alleles, suggesting that differential methylation of the alleles is already established before the formation of naive T cells.
Up-regulation of IL-1α mRNA expression after treatment with 5-aza-CdR. (A) Ms-SNuPE analysis of 4 representative CpGs (CpGs +1, –2, –7, and –8) in the IL-1α promoter in nonexpressing lymphoblastoid cell line Namalwa, the constitutive IL-1α–expressing cell line HaCaT, and CD4+ T cells. CpG –8 was always highly methylated and the other 3 CpGs were differentially methylated in the T-cell clones. The analyzed CpGs are indicated by the filled circles and black arrows in the schematic representation of the promoter region. The open circles indicate the CpGs not analyzed. (B) Expression of IL-1α in nonexpressing cell line Namalwa after treatment with 0.05 or 1 μM 5-aza-CdR. mRNA expression for IL-1α (35 cycles) and GAPDH (20 cycles) was examined by RT-PCR. The decrease in methylation indicated below each of the 5-aza-CdR concentrations used was quantitated by Ms-SnuPE and given as a percentage of the untreated sample. (C) Relative expression of the G and T allele in the predominantly G allele–expressing T-cell clones 41 and 90 that have been treated with (+) or without (–) 2 μM 5-aza-CdR and after a 6-hour stimulation with PMA and ionomycin. IL-1α mRNA levels were determined by real-time PCR and the contribution of each allele quantified by ASTQ. The contribution of each allele is represented as a percentage of the total with the G allele in gray and the T allele in white. Genomic DNA was analyzed by ASTQ as a control with an expected equal contribution of each allele.
Up-regulation of IL-1α mRNA expression after treatment with 5-aza-CdR. (A) Ms-SNuPE analysis of 4 representative CpGs (CpGs +1, –2, –7, and –8) in the IL-1α promoter in nonexpressing lymphoblastoid cell line Namalwa, the constitutive IL-1α–expressing cell line HaCaT, and CD4+ T cells. CpG –8 was always highly methylated and the other 3 CpGs were differentially methylated in the T-cell clones. The analyzed CpGs are indicated by the filled circles and black arrows in the schematic representation of the promoter region. The open circles indicate the CpGs not analyzed. (B) Expression of IL-1α in nonexpressing cell line Namalwa after treatment with 0.05 or 1 μM 5-aza-CdR. mRNA expression for IL-1α (35 cycles) and GAPDH (20 cycles) was examined by RT-PCR. The decrease in methylation indicated below each of the 5-aza-CdR concentrations used was quantitated by Ms-SnuPE and given as a percentage of the untreated sample. (C) Relative expression of the G and T allele in the predominantly G allele–expressing T-cell clones 41 and 90 that have been treated with (+) or without (–) 2 μM 5-aza-CdR and after a 6-hour stimulation with PMA and ionomycin. IL-1α mRNA levels were determined by real-time PCR and the contribution of each allele quantified by ASTQ. The contribution of each allele is represented as a percentage of the total with the G allele in gray and the T allele in white. Genomic DNA was analyzed by ASTQ as a control with an expected equal contribution of each allele.
DNA/protein binding at the proximal promoter region is significantly reduced by methylation of CpGs –2 and –3
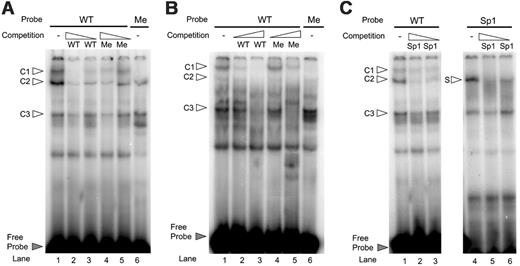
EMSA was performed to compare the binding characteristics of the unmethylated versus the methylated ds-oligonucleotide containing CpGs –2 and –3. This sequence contains a specific region that was shown to be protected by DNase I footprinting and showed transcription factor binding in EMSA.22,23 In addition, nucleotide substitutions in this sequence abrogated transcription activity dramatically in a reporter gene assay.22 Nuclear protein extracts from HaCaT cells, which express IL-1α, were incubated with labeled wild-type (WT) or methylated (Me) ds-oligonucleotides. Three complexes were formed with the WT probe (c1, c2, and c3, Figure 7A lane 1), whereas only 2 were formed with the methylated probe (c2 and c3, lane 6), in which 2 CpGs were methylated on both strands. The formation of c1, c2, and c3 complexes that were formed with the WT probe was dose-dependently inhibited by competition with a 200- and 20-fold molar excess of unlabeled WT probe (Figure 7A, lanes 2-3). As anticipated, competition with a 200- or 20-fold molar excess of unlabeled methylated probe was less efficient in competing for c1 formation (Figure 7A, lanes 4-5). These data suggest that formation of complex 1 is methylation sensitive.
We next investigated whether the WT ds-oligonucleotides also formed the methylation-sensitive complex with nuclear proteins isolated from IL-1α–nonexpressing cells. Therefore, nuclear protein from Namalwa cells was incubated with end-labeled WT and methylated probes. This experiment revealed the formation of 3 complexes that run with the same mobility, as the complexes formed with HaCaT nuclear extracts. The nuclear protein extracts from Namalwa cells were able to form complex c3, the methylation-sensitive complex c1, and also complex c2, but to a lesser extent (Figure 7B lane 1). As was also seen with the HaCaT nuclear proteins, the formation of the methylation-sensitive complex c1 was completely abrogated with the methylated probe (Figure 7B, lane 6). Accordingly, c1 formation is dose-dependently inhibited by competition with a 20- or 200-fold molar excess of unlabeled WT probe, whereas the methylated unlabeled probe is much less efficient to compete for binding (Figure 7B, compare c1 lanes 2 and 3 with 4 and 5, respectively).
Expression levels of IL-1α in naive and memory T cells. mRNA was isolated from CD4+RA+ naive T cells and CD4+RO+ memory T cells, and analyzed for IL-1α expression by real-time PCR. Cells were either left unstimulated (–) or were stimulated with PMA and ionomycin for 6 hours (+).
Expression levels of IL-1α in naive and memory T cells. mRNA was isolated from CD4+RA+ naive T cells and CD4+RO+ memory T cells, and analyzed for IL-1α expression by real-time PCR. Cells were either left unstimulated (–) or were stimulated with PMA and ionomycin for 6 hours (+).
Methylation pattern of the proximal IL-1α promoter and of intron IV in naive T cells. DNA from naive T cells was treated with bisulfite, and different parts of the promoter (CpGs –7 through –1, left) and intron IV (CpGs +17 through +29, right) were amplified by PCR and cloned. From each region, multiple clones were sequenced. Each circle represents a CpG and is numbered relative to the transcription start site.
Methylation pattern of the proximal IL-1α promoter and of intron IV in naive T cells. DNA from naive T cells was treated with bisulfite, and different parts of the promoter (CpGs –7 through –1, left) and intron IV (CpGs +17 through +29, right) were amplified by PCR and cloned. From each region, multiple clones were sequenced. Each circle represents a CpG and is numbered relative to the transcription start site.
These findings indicate that nonexpressing cells, which have a fully methylated IL-1α promoter (Figure 4A), contain transcription factors that are able to bind to an unmethylated promoter but that binding is inhibited by CpG methylation.
Previously, it has been shown that Sp1 binds sequences within the region examined.23 We therefore performed an EMSA with a WT probe and Sp1 probe as a control. Incubation with the WT probe resulted in the formation of the 3 complexes c1 to c3 (Figure 7C, lane 1) of which only complex 2 was dose-dependently inhibited by the competition with a 200- and 20-fold molar excess unlabeled specific Sp1 oligo (lanes 2-3). The control Sp1 probe showed the formation of one specific complex (S) that could be competed for with unlabeled Sp1 oligo (Figure 7C, lanes 4-6). This result shows that the methylation-sensitive complex does not contain Sp1 protein.
Discussion
Here, we investigated DNA methylation in the proximal promoter region of the IL1A gene to see whether DNA methylation might be involved in the allele-specific expression of IL-1α in CD4+ T cells. We showed that the CpGs –7 through +1 relative to the transcription start site within a region of 130 bp are differentially methylated. The patterns found correlate with the expression of IL-1α in the cell types examined. In expressing keratinocytes, the CpGs of both alleles are hypomethylated, in nonexpressing cells the CpGs are hypermethylated, and in predominant allele-specific–expressing CD4+ T cells, alleles were either hypermethylated or hypomethylated. This differential methylation appears to be confined to the promoter region as no differences were found in a cluster of 14 CpGs in intron IV, which were hypermethylated in all cells examined, irrespective of IL-1α expression. Thus the distinct pattern of methylated and unmethylated CpGs appears to be confined to the promoter region where transcription is regulated.
Electrophoretic mobility shift assay showing inhibition of binding by DNA methylation. (A) Unmethylated wild-type probe (WT) from –68 bp through –42 bp relative to the transcription start site containing 2 CpGs formed 3 specific DNA-protein complexes with nuclear extract from HaCaT cells (c1-c3 as indicated by white arrows, lane 1) that could be specifically competed for by the excess from high to low (× 200 and × 20) of the unlabeled WT ds-oligonucleotide (lanes 2-3), but less by the excess (× 200 and × 20) of the unlabeled methylated (Me) probe (lanes 4-5). Methylation of the 2 CpGs (lane 6) in the probe sequence affected the formation of the DNA-protein complexes. (B) Unmethylated wild-type probe also formed specific DNA-protein complexes with nuclear extract from nonexpressing Namalwa cells (lane 1) that could be specifically competed for by the excess from low to high (× 20 and × 200) of the unlabeled WT probe (lanes 2-3). The upper complex, which runs with the same mobility as c1 formed with HaCaT nuclear extracts, was not or to a lesser extent competed for by the excess (× 20 and × 200) of the unlabeled but methylated (Me) probe (lanes 4-5). Binding to the methylated probe (lane 6) abrogated the formation of DNA-protein complex c1. (C) The binding activities were competed for with excess from high to low (× 200 and × 20) unlabeled Sp1 probe (lanes 2-3). As a control, the labeled Sp1 oligo was used to show that by competition with excess unlabeled oligo, the specific Sp1 complex (S) was competed away (lanes 5-6).
Electrophoretic mobility shift assay showing inhibition of binding by DNA methylation. (A) Unmethylated wild-type probe (WT) from –68 bp through –42 bp relative to the transcription start site containing 2 CpGs formed 3 specific DNA-protein complexes with nuclear extract from HaCaT cells (c1-c3 as indicated by white arrows, lane 1) that could be specifically competed for by the excess from high to low (× 200 and × 20) of the unlabeled WT ds-oligonucleotide (lanes 2-3), but less by the excess (× 200 and × 20) of the unlabeled methylated (Me) probe (lanes 4-5). Methylation of the 2 CpGs (lane 6) in the probe sequence affected the formation of the DNA-protein complexes. (B) Unmethylated wild-type probe also formed specific DNA-protein complexes with nuclear extract from nonexpressing Namalwa cells (lane 1) that could be specifically competed for by the excess from low to high (× 20 and × 200) of the unlabeled WT probe (lanes 2-3). The upper complex, which runs with the same mobility as c1 formed with HaCaT nuclear extracts, was not or to a lesser extent competed for by the excess (× 20 and × 200) of the unlabeled but methylated (Me) probe (lanes 4-5). Binding to the methylated probe (lane 6) abrogated the formation of DNA-protein complex c1. (C) The binding activities were competed for with excess from high to low (× 200 and × 20) unlabeled Sp1 probe (lanes 2-3). As a control, the labeled Sp1 oligo was used to show that by competition with excess unlabeled oligo, the specific Sp1 complex (S) was competed away (lanes 5-6).
The differential methylation pattern prompted us to analyze the effect of the DNA demethylating agent 5-aza-CdR on IL-1α expression. The treatment of nonexpressing cells with a fully methylated promoter was effective in inducing the IL-1α mRNA expression, indicating a role for DNA methylation in gene expression. Most interestingly, in allele-specific–expressing T-cell clones with a differentially methylated promoter, 5-aza-CdR indeed restored the expression of the previous silent allele, to some extent. Also, the total IL-1α mRNA levels increased. The latter observation probably reflects indirect effects of 5-aza-CdR, suggesting that there are also other mechanisms operating in the regulation of IL-1α. The first observation indicates that demethylation of a single allele releases expression of the silenced allele and changes the allele expression ratio in favor of the previous silent allele. It should be noted that the expression ratio of both alleles was not completely restored to a 1:1 ratio. An explanation for this could be that treatment with 5-aza-CdR will result maximally in a demethylation of one new strand (ie, 50%) per cell division.33 Therefore in our experimental setup, restoration of allele expression to a 1:1 ratio in these cell clones was not expected, because part of the cells still has one methylated allele. Longer treatment with 5-aza-CdR severely affected the cells, which would hamper further analysis of allelic expression. It is clear, however, from these data that 5-aza-CdR does induce expression of the silent allele.
Little is known about the regulation of IL-1α expression, but it is interesting to note that a specific region in the proximal promoter containing the differentially methylated CpGs we have studied has been shown to be important for transcriptional regulation of IL-1α. Zaldivar et al22 analyzed the –224 to +5 proximal promoter region, which drives transcription from TSS1 and contains the differentially methylated CpGs. Several investigators have confirmed the importance of this region for promoter activity by showing that deleting the region between –63 to –49 significantly affects the expression of IL-1α in a variety of cell lines.34,35 By DNase I footprint analysis, it was shown that several regions are protected.22,23 Mutations within a triple GCC repeat that was shown to bind transcription factors at the –65 to –41 protected area reduced promoter activity by 90% and also affected the protein binding properties to this GCC element.22 Recently it was shown that a binding site for Sp1 is present within this –52 to +64 region,23 which we could confirm in this study by EMSA. We have shown that methylation of these CpGs resulted in prevention of the binding of another nuclear factor, present both in cells that express the gene and have the intrinsic proximal promoter unmethylated, as well as in cells that do not express the gene and have the intrinsic proximal promoter methylated. The latter observation supports a role for methylation in the regulation of IL-1α, because the nonexpressing cells that have a methylated promoter do contain transcription factor(s) that can bind only to the unmethylated promoter sequence (Figure 7A-B). Taken together, these results clearly show that within this part of the promoter, which is important for transcription activation, methylation of the binding element prevents the binding of transcription factor(s) and, concomitantly, the methylation inhibits transcription activity of IL-1α. These observations would imply that regulation of IL-1α by differential DNA methylation ultimately results in an adjustment of IL-1α production.
It is known that epigenetic mechanisms operate during differentiation of naive T cells into Th1 and Th2 cells to result in the precise expression or silencing of cytokines. For IL-2, it has been shown that the immediate proximal promoter region containing 8 CpGs is demethylated in activated T lymphocytes. These epigenetic changes are necessary and sufficient for transcription.36 We show that methylation of 2 CpGs in the IL-1α proximal promoter, which were differentially methylated in the CD4+ T cells, resulted in direct inhibition of binding of nuclear factor(s), which consequently would affect transcription activity. In naive T cells, IL-4 is hypermethylated in the 5′ regulatory region of the promoter, and this region is specifically demethylated in Th2 cells.37 Also, a distinct region of the IFNγ promoter, containing a similar number of CpGs within 300 bp of the TSS as the IL-1α promoter, is hypomethylated in differentiated T lymphocytes.38 Many of these cytokines have been reported to exhibit allele-specific expression.9,10 In a recent study by Allen et al,39 it is shown that known monoallelically expressed genes are flanked by high concentrations of long interspersed nuclear elements (LINEs) that distinguish them from other genes. Using these observations, they could identify candidate human monoallelic genes. Of interest, this prediction included several other IL-1 family members such as IL1F5, IL1F8, and IL1F10. Further research is necessary to investigate if this is indeed the case and if these LINE-1 elements play a role in the formation of nonequivalent epigenetic modifications including DNA methylation of the alleles and if they are involved in, for example, asynchronous replication, another epigenetic mark of monoallelically expressed sequences.19
Here, we show that the allele-specific expression of the human IL1A gene is associated with epigenetically different alleles. Our data suggest that the unmethylated strands are derived from the expressed allele, although further experiments will be required to demonstrate this link. Furthermore, the differential methylation pattern in the proximal promoter region, as observed in the CD4+ T cells, is already present in the naive T-cell population, suggesting that allele-specific expression of IL-1α occurs earlier during T-cell differentiation and does not involve an active methylation or demethylation step upon T-cell maturation as opposed to other cytokines.
Prepublished online as Blood First Edition Paper, June 20, 2006; DOI 10.1182/blood-2006-01-021147.
Supported by the Research Council for Earth and Life Sciences (ALW) with financial aid from the Netherlands Organization for Scientific Research (NWO) (J.G.I.R.), and National Institutes of Health grant CA82422 (P.A.J.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We kindly thank Dr C. L. Lebre and R. Westland for providing materials. We thank Drs G. Egger, T. Miranda, G. Liang, and C. Cortez for critically reading the paper and for helpful discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal