Abstract
Upon local delivery, adenovirus (Ad) serotype 5 viruses use the coxsackie and Ad receptor (CAR) for cell binding and αv integrins for internalization. When administered systemically, however, their role in liver tropism is limited because CAR-permissive and mutated viruses show similar biodistribution, a finding recently attributed to blood coagulation factor (F) IX or complement protein C4BP binding to the adenovirus fiber and “bridging” to either low-density lipoprotein receptor-related protein or heparan sulfate proteoglycans. Here, we show that hepatocyte transduction in vitro can be enhanced by the vitamin K-dependent factors FX, protein C, and FVII in addition to FIX but not by prothrombin (FII), FXI, and FXII. This phenomenon was not dependent on proteolytic activation or cell signaling activity and for FX was mediated by direct virus-factor binding. Human FX substantially enhanced hepatocyte transduction by CAR-permissive and mutated viruses in an ex vivo liver perfusion model. In vivo, global down-regulation of vitamin K-dependent zymogens by warfarin significantly diminished liver uptake of CAR-deleted Ads; however, this phenomenon was fully rescued by acute infusion of human FX. Our results indicate a common and pivotal role for distinct vitamin K-dependent coagulation factors in mediating hepatocyte transduction by adenoviruses in vitro and in vivo.
Introduction
Adenoviral-based vectors (most commonly serotype 5) show broad appeal for gene delivery to a host of cells and organs and thus have been used extensively for preclinical studies. The varied complexities of these protocols necessitate a detailed knowledge of adenovirus (Ad) biology and pathology and the processes that govern infectivity and toxicity in different settings, particularly in the setting of intravascular gene delivery where the Ad vector intimately contacts blood proteins, blood cells, the vascular endothelium, and cellular components of selected organs.
Unraveling the complex interplay between primary and coreceptors and the adenovirus capsid structures as well as interactions with blood-derived cells and proteins is fundamentally important if systemic adenoviral gene therapy is to reach routine clinical medicine. The interaction of the primary tethering receptor (coxsackie and adenovirus receptor [CAR]) with the adenovirus serotype 5 fiber was described in 19971,2 and, together with αvβ3 and αvβ5 integrin-mediated virus internalization via penton-base activation,3 is fundamentally important for virus infection in vitro. Involvement of CAR in liver transduction following intravascular delivery of vectors is less clear. This has been driven by conflicting data relating to the loss of liver-targeting capacity of adenovirus type 5 vectors mutated in their ability to bind CAR.4-6 As such, vectors with mutations that ablate CAR binding fail to retarget efficiently to alternate sites systemically when targeting peptides are inserted in the fiber (reviewed by Nicklin et al7 ) even though retargeting in vitro or locally to defined tissues is efficacious.8,9 A recent report described the importance of circulating factors (FIX and complement factor C4BP) in supporting adenovirus type 5 and type 35 delivery to the liver.10 It was demonstrated that FIX (but not FX) and C4BP bound directly to the fiber and “bridged” the virus to alternate receptors, including heparan sulfate proteoglycans (HSPGs) and/or low-density lipoprotein receptor-related protein (LRP).10 Essentially, the presence of FIX and/or C4BP binding independently compensated for the lack of CAR binding, thus explaining why CAR-binding-deleted viruses do not have a reduced liver tropism when delivered systemically.
Here we investigated the involvement of blood coagulation factors in adenovirus-mediated gene delivery to hepatocytes and define a common role for the vitamin K-dependent proteins FVII, FIX, FX, and PC. Our studies initially focus on the ability of FIX concentrate used clinically for hemophilia B treatment to enhance adenoviral uptake into hepatocytes and then on human coagulation factors in vitro, ex vivo, and in vivo.
Materials and methods
Materials
Human FIX plasma concentrate (HT-DEFIX) is an impure FIX-rich product with other factors present (factor X, factor II, and antithrombin [AT]: each vial contains more than 360 IU factor IX, less than 200 IU factor II, less than 200 IU factor X, and less than 5 IU AT) and was obtained from the Scottish National Blood Transfusion Service ([SNBTS] Edinburgh, United Kingdom). Purified human blood coagulation factors FII, FIX, FX, FXa, FXa-EGR, FX-GD (Gla-domainless FX), FXI, FXII, and PC were purchased from Haematologic Technologies (Essex Junction, VT). Recombinant FVII was produced as previously described11 ; recombinant tick anticoagulant protein (TAP) was kindly provided by Dr George Vlasuk (Corvas International, San Diego, CA); and protease-activated receptor (PAR) 1-4 peptide agonists (PAR 1/2-SFLLRN, PAR 2-SLIGRL, PAR 3-TFRGAP, and PAR 4-GYPGQV) were synthesized by the Peptide Synthesis Service (MRC Clinical Sciences Centre, London, United Kingdom) and verified by mass spectroscopy. Arixtra solution containing the indirect FXa inhibitor fondaparinux was from GlaxoSmithKline (Harlow, United Kingdom).
Cell lines
Human HepG2 cells (American Type Culture Collection, Manassas, VA) were cultured in Dulbecco modified Eagle medium (DMEM; BioWhittaker, Wokingham, United Kingdom) supplemented with 2 mM l-glutamine (Invitrogen, Paisley, United Kingdom) and 10% fetal calf serum (FCS; PAA Laboratories, Yeovil, United Kingdom). CHO-K1 and CHO-pgsA745 cells12 were cultured in phenol red-free DMEM supplemented with 2 mM l-glutamine, 10% FCS, and antibiotics. Cells were subcultured using trypsin/EDTA solution (Invitrogen).
Adenoviruses
Adenoviral vectors used in this study were based on human Ad serotype 5 dl327 and encode a Rous sarcoma virus (RSV)-promoted nuclear localized lacZ cDNA in an E1-/E3-deleted genome and have been described previously.13-15 Genomes were measured by real-time polymerase chain reaction (PCR). Virus particle (VP) number was determined using micro-bicinchoninic acid and the established formula 1 μg protein = 4 × 109 VP.16
In vitro analysis of coagulation factor-induced transduction
Cells were plated out into 96-well plates 24 hours prior to infection. For infection, cells were washed twice with phosphate-buffered saline (PBS), and 100 μL serum-free medium containing the relevant coagulation factors (plus 0 to 100 μg/mL lactoferrin17 [Sigma-Aldrich, Gillingham, United Kingdom] for some experiments) was added per well. Unless specified, all coagulation factors were used at physiologic concentration (1 IU/mL). Adenovirus was diluted in PBS to 1000 VP per cell, and then virus diluent was added per well and incubated for 3 hours at 37°C. Medium was subsequently removed from the cells, and the cells washed briefly with PBS. Medium was replaced and the cells maintained at 37°C until harvesting 72 hours after infection. In experiments involving TAP, a molar ratio of 5.78:1 of TAP/FX or FXa was used, which has previously been shown to be sufficient for FXa inhibition.18 Arixtra (fondaparinux) solution (where indicated) was diluted into serumfree media to a final working concentration of 2 μg/mL fondaparinux in the presence of AT.
Analysis of transgene expression
β-Galactosidase from in vitro experiments was quantified using Tropix Galacto-Light Plus (Applied Biosystems, Foster City, CA). β-galactosidase activity was quantified by plate assay using a Wallac VICTOR2 (PerkinElmer Life and Analytical Sciences, Boston, MA) and recombinant β-galactosidase as standard. Protein concentrations were measured by bicinchoninic acid assay (Perbio Science, Cramlington, United Kingdom). All data are expressed as relative light units (RLU) per milligram of protein. For visualization of β-galactosidase-expressing cells, cells were fixed in 2% paraformaldehyde for 15 minutes at 37°C, stained using 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) stain overnight at 37°C, and then washed briefly with PBS. Cells were subsequently imaged in PBS using an Olympus BX40 microscope (London, United Kingdom) and Olympus PLAN 20 ×/0.40 numeric aperture objective. Representative areas were photographed using a Hitachi HV-C20A color camera controlled by Image-Pro Hitachi 5.1 computer software. For visual assessment of β-galactosidase from in vivo experiments, liver tissue was fixed for 4 hours in 100% ethanol and then incubated overnight in X-Gal stain and fixed in formalin for photography and quantified by commercial enzyme-linked immunosorbent assay (ELISA) (Roche Diagnostics, Mannheim, Germany) using a colorimetric spectrophotometer and expressed per milligram of protein.
Surface plasmon resonance analysis
Surface plasmon resonance experiments were carried out using a Biacore X instrument (Biacore, Stevenage, United Kingdom). Human coagulation FX and FXI were covalently immobilized onto separate flow cells of a CM5 biosensor chip by amine coupling according to the manufacturer's instructions. Virus in 50 mM Tris (pH 7.4), 150 mM NaCl, 5 mM CaCl2, and 0.005% Tween 20 was passed over the chip at a flow rate of 20 μL/min. Sensor chips were regenerated between virus injections by injection of 10 mM HEPES (pH 7.4), 150 mM NaCl, 3 mM EDTA, and 0.005% Tween 20.
In situ perfusion model using FX
In situ perfusion of C57BL/6 mouse livers in the presence or absence of FX was performed as described previously.10 Briefly, the vena porta and vena cava inferior were cannulated, and blood was flushed from the liver through the portal vein with 20 mL PBS using a low-speed peristaltic pump. After 10 minutes of continuous flushing, the liver color changed from dark brown to light yellow-brown, demonstrating that most of the blood was efficiently removed. Virus infection studies were started when the liver flow through (collected from the vena cava inferior) was free of blood. Next, virus (2.5 × 1010 VP/mL, with or without 8 μg/mL FX) in 8 mL PBS was infused through the portal vein, and the circulation between the vena porta and the vena cava was closed, allowing asanguineous, isolated liver perfusion (at 37°C). Thirty minutes after virus application, hepatocytes were isolated by collagenase perfusion. Reporter gene expression in plated hepatocytes was analyzed 48 hours after infection.
Effect of warfarin and factor X rescue
Mice (MF1 outbred mice, 6 to 12 weeks of age, approximately 30 g) were administered subcutaneously with 133 μg warfarin (Sigma-Aldrich, Dorset, United Kingdom) (dissolved in peanut oil) 3 days and 1 day prior to Ad injection. AdKO1, 4 × 1011 VP per mouse, was subsequently injected intravenously, and the levels of β-galactosidase activity were quantified 48 hours after injection either by X-Gal staining of liver segments for β-galactosidase-expressing cells or by ELISA to detect and quantify β-galactosidase expression. To assess the effect of warfarin on clotting times (CTs), 80-μL samples of citrated plasma taken immediately after virus administration and human placental thromboplastin/calcium chloride reagent (Thromborel S; Dade Behring, Milton Keynes, United Kingdom) were mixed in glass clotting tubes at 37°C and CTs determined visually. Normal murine pooled plasma derived from ICR mice was used as a normal control (Harlan Sera-Lab, Loughborough, United Kingdom) and gave CTs of 9 to 11 seconds. The effect of warfarinization can be quantified as the ratio of the test CT to normal pool CT. Plasma samples with a CT above 300 seconds are essentially incoagulable. A 1-stage assay for murine FIX clotting activity (mFIX:C) was performed essentially as described previously19 using human FIX-depleted substrate plasma and a normal human plasma reference: Results are given in human units per milliliter. All murine citrated plasma samples were diluted 100-fold before assay to remove any nonspecific influence of murine coagulation factors. To assess the ability of FX to rescue liver transduction in warfarin-treated mice, animals were given intravenous injections of 33 μg FX to selectively restore physiologic FX levels prior to injection with 4 × 1011 VP of AdKO1.
Statistical analysis
In vitro experiments were performed in triplicate on at least 3 independent occasions. In vivo experiments were performed with at least 4 animals per group. Analysis was by unpaired Student t test with statistical significance accepted at P below .05.
Results
Hepatocyte transduction in vitro mediated by Ad is enhanced by FIX concentrate
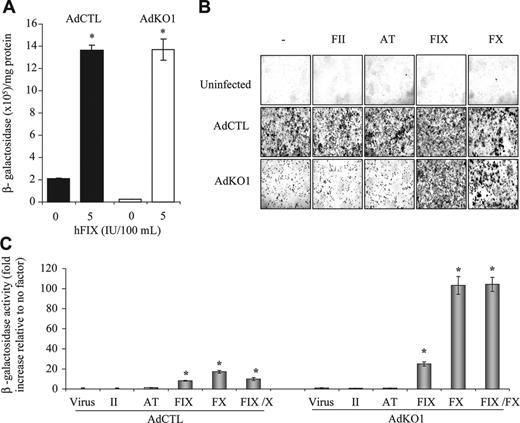
We initially confirmed10 that a FIX-rich concentrate was effective in enhancing transduction of adenoviral vectors10 in human HepG2 hepatoma cells in vitro. As predicted, transduction was enhanced in the presence of physiologically relevant levels of FIX-rich concentrate for both AdCTL (nonmodified fiber) and AdKO1 (CAR-binding ablated fiber) (Figure 1A). Of note, AdKO1 transduction levels in the absence of FIX-rich concentrate were, as expected, far lower than those mediated by AdCTL; however, following exposure to FIX-rich concentrate, transduction levels mediated by AdCTL and AdKO1 were both significantly higher and equivalent. This confirms previous data10 showing the effect of FIX in mediating enhanced hepatocyte transduction mediated by Ad type 5 vectors.
Coagulation FX also promotes hepatocyte transduction
Upon injection of either AdCTL or AdKO1 into FIX-knockout mice (F9-/-), no differences in liver transduction rates were observed between AdCTL and AdKO1 (data not shown), suggesting compensation for the deficiency in FIX by additional pathways. Moreover, because plasma-derived FIX concentrate also contains other coagulation factors (FII and FX as well as AT) we hypothesized that one or more factors could also enhance hepatocyte transduction. Using human proteins we assessed the ability of each component individually to enhance Ad-mediated gene transfer in HepG2 cells (Figure 1B-C). Neither FII nor AT modified Ad-mediated gene delivery; however, FX was highly effective in mediating enhanced transduction of hepatocytes (Figure 1B-C). No additive effect by FIX and FX coaddition on infectivity enhancement was observed (Figure 1C).
Rapid infectivity enhancement mediated by FX
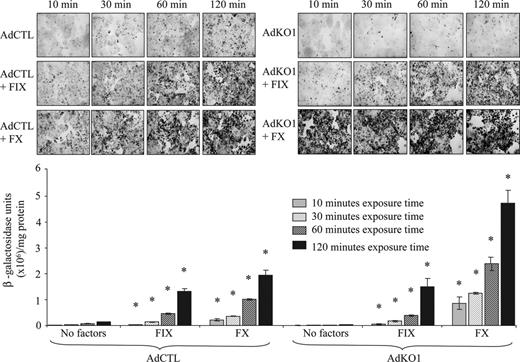
We assessed the effect of different exposure times of FX or FIX and virus to HepG2cells on the transduction level. We exposed cells to virus and FX or FIX for 10, 30, 60, and 120 minutes, washed cells, and assessed transduction at 72 hours using physiologic concentrations of FIX and FX. For both AdCTL and AdKO1, a time-dependent increase in transduction was observed (Figure 2). Notably, a substantial difference was observed between the effect of FIX and FX on AdKO1 virus even at short exposure times, with FX being far more effective (Figure 2).
Transduction enhancement by FX is not dependent on zymogen activation
Activated serine proteases (SPs) have been previously implicated in virus tropism. For example, protease FXa is a tropism determinant for paramyxovirus,20 and proteases have now been shown to be highly influential in severe acute respiratory syndrome (SARS) infectivity in the lung.21 We therefore tested whether the effect of FX was also observed with active SP FXa and whether the effect of coagulation factors was blocked in the presence of protease inhibitors. Transduction of HepG2 cells in the presence of FXa (8 μg/mL) was similar to that achieved with the respective zymogen (Figure 3A). Active site-inhibited FXa (FXa-EGR) at identical concentration to FX also mediated the phenomenon for both AdCTL and AdKO1 (Figure 3B), suggesting proteolytic activity of FXa was not required. To confirm this, we performed 2 experiments. First, we incubated FX in the presence of TAP, a direct FXa inhibitor, prior to addition to AdCTL or AdKO1 and HepG2 cells (Figure 3C). Second, incubation in the presence of the indirect FXa inhibitor fondaparinux (in the presence of AT) was performed (Figure 3D). Neither TAP nor fondaparinux blocked FX-mediated transduction. Furthermore, peptide agonists (100 μM) of the coagulation factor-activated receptors PAR1-4 did not mimic the FX enhancement of transduction when incubated with HepG2 cells in the presence of AdCTL or AdKO1 (Figure 3E). To confirm this in vivo, we pretreated mice with 10 mg/kg fondaparinux intraperitoneally 30 minutes prior to injection of AdKO1. No difference in liver transduction levels were observed after harvesting at 48 hours after injection (Figure 3F).
Effect of FIX concentrate HT-DEFIX and its individual component proteins on transduction of HepG2 hepatocytes by AdCTL and AdKO1. (A) HepG2 cells were infected with AdCTL or AdKO1 in the absence and presence of HT-DEFIX (shown as levels of human FIX within the HT-DEFIX purification) for 3 hours in serum-free medium. Following extensive washing, cells were cultured in serum containing medium and harvested at 72 hours after transduction. *P < .05 versus cells without addition. (B) Human proteins (FII, AT, FIX, FX, all at physiologic levels) were used as for panel A but in place of HT-DEFIX. Representative X-Gal-stained images are shown. (C) Quantification of β-galactosidase levels 72 hours after infection in the presence of FII, AT, FIX, or FX alone or FIX and FX in combination. *P < .05 versus virus alone.
Effect of FIX concentrate HT-DEFIX and its individual component proteins on transduction of HepG2 hepatocytes by AdCTL and AdKO1. (A) HepG2 cells were infected with AdCTL or AdKO1 in the absence and presence of HT-DEFIX (shown as levels of human FIX within the HT-DEFIX purification) for 3 hours in serum-free medium. Following extensive washing, cells were cultured in serum containing medium and harvested at 72 hours after transduction. *P < .05 versus cells without addition. (B) Human proteins (FII, AT, FIX, FX, all at physiologic levels) were used as for panel A but in place of HT-DEFIX. Representative X-Gal-stained images are shown. (C) Quantification of β-galactosidase levels 72 hours after infection in the presence of FII, AT, FIX, or FX alone or FIX and FX in combination. *P < .05 versus virus alone.
The effect of virus-factor exposure time to HepG2 hepatocytes on transduction. HepG2 cells were infected with AdCTL or AdKO1 in the presence of FIX or FX for varying times (indicated) in serumfree media. Following extensive washing, cells were cultured in serum containing media and harvested at 72 hours after transduction and levels of β-galactosidase determined by X-Gal staining and quantitative analysis. *P < .05 versus virus alone.
The effect of virus-factor exposure time to HepG2 hepatocytes on transduction. HepG2 cells were infected with AdCTL or AdKO1 in the presence of FIX or FX for varying times (indicated) in serumfree media. Following extensive washing, cells were cultured in serum containing media and harvested at 72 hours after transduction and levels of β-galactosidase determined by X-Gal staining and quantitative analysis. *P < .05 versus virus alone.
A common role for homologous coagulation factors in mediating hepatocyte transduction
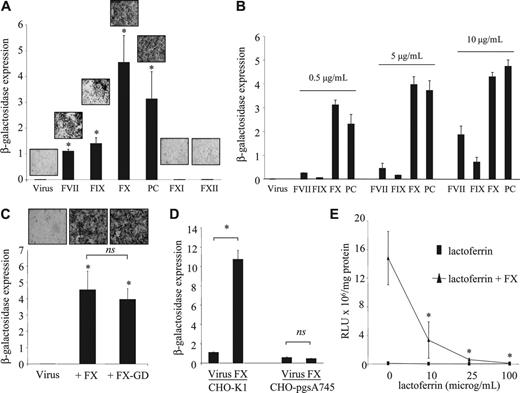
Because FIX and FX share identical domain structures (comprising a γ-carboxylated glutamic acid [Gla] domain, an epidermal growth factor-like (EGF1) domain, an EGF2 domain, and an SP domain) with FVII and PC, we tested these coagulation factors in comparison with FXI and FXII, which do not share this domain structure. Transduction of HepG2 cells with AdKO1 was markedly enhanced by physiologic concentrations of both FVII and PC while FXI and FXII showed no effect (Figure 4A). This suggests a common mechanism for the Gla-EGF1-EGF2-SP zymogens (FVII, FIX, FX, and PC) in mediating hepatocyte transduction. This also suggested that FX and PC were more effective than FIX or FVII at physiologic levels of each factor. Because each factor is present in blood at different concentrations, we assessed transduction enhancement at equal concentrations for each factor (Figure 4B). All factors at all concentrations evaluated significantly enhanced virus transduction, although the extent of the enhancement was clearly very different, with FX and PC evoking significantly higher levels of transduction than FIX or FVII at all concentrations tested. We next determined whether the Gla domains were integral to this mechanism by infecting HepG2 cells in the presence of Gla-domainless FX (FX-GD) and compared this with the FX-mediated effect on HepG2 transduction. Infection with an equimolar concentration of FX-GD led to a near identical enhancement in transduction of AdKO1 (Figure 4C), suggesting that the Gla domain is not involved in this mechanism. In earlier studies it was shown that FIX-mediated Ad infection occurs through HSPGs and LRP.10 To clarify whether FX-mediated Ad cell infection occurs via a pathway similar to FIX, we infected either control CHO cells (expressing HSPGs) or CHO cells deficient in HSPG expression (CHO-pgsA74512 ) with AdKO1 in the presence of each individual zymogen at physiologic concentrations. While control CHO-K1 cells showed enhanced transduction by FX, transduction of CHO-pgs745 cells showed no enhancement (Figure 4D). FX-mediated enhancement of HepG2 cell transduction by AdKO1 was blocked by coincubation with lactoferrin, which uses HSPGs and LRP as natural receptors10 (Figure 4E).
Effect of FXa and FXa inhibitors on transduction of HepG2 cells in vitro and liver transduction in vivo. HepG2 cells were transduced with AdCTL or AdKO1 (1000 VP/cell) in the presence of 1 IU/mL FX or FXa (A), 1 IU/mL active site-blocked Xa (FXa-EGR) (B), coincubated with 1 IU/mL FX and TAP at a molar ratio of 5.78:1 (TAP/FX) (C) or 2 μg/mL fondaparinux (D) for 3 hours, washed, and analyzed for β-galactosidase expression at 72 hours after infection. (E) Assessment of 100 μM PAR agonists on transduction by AdKO1. (F) Mice were given injections of AdKO1 in the absence or presence of pretreatment with fondaparinux (10 mg/kg intraperitoneally) and transgene expression in the liver assessed at 48 hours after infection. X-Gal-stained images are shown.
Effect of FXa and FXa inhibitors on transduction of HepG2 cells in vitro and liver transduction in vivo. HepG2 cells were transduced with AdCTL or AdKO1 (1000 VP/cell) in the presence of 1 IU/mL FX or FXa (A), 1 IU/mL active site-blocked Xa (FXa-EGR) (B), coincubated with 1 IU/mL FX and TAP at a molar ratio of 5.78:1 (TAP/FX) (C) or 2 μg/mL fondaparinux (D) for 3 hours, washed, and analyzed for β-galactosidase expression at 72 hours after infection. (E) Assessment of 100 μM PAR agonists on transduction by AdKO1. (F) Mice were given injections of AdKO1 in the absence or presence of pretreatment with fondaparinux (10 mg/kg intraperitoneally) and transgene expression in the liver assessed at 48 hours after infection. X-Gal-stained images are shown.
Effect of homologous and related but structurally divergent coagulation factors on adenoviral infectivity of HepG2 cells. (A-D) β-Galactosidase expression is expressed as RLU × 107/mg protein. (A) HepG2 cells were incubated with 1 IU/mL human FVII, FIX, FX, PC, FXI, or FXII in the presence of AdKO1 for 3 hours, cells washed, and transgene quantified at 72 hours after infection. Representative X-Gal-stained images are shown for each condition. *P < .05 versus virus alone. (B) HepG2 cells were incubated with varying concentrations (as indicated) of human FVII, FIX, FX, PC, FXI, or FXII in the presence of AdKO1 for 3 hours, cells washed, and transgene quantified at 72 hours after infection. (C) HepG2 cells were exposed to 1 IU/mL FX or an equimolar concentration of FX-GD in the presence of AdKO1 for 3 hours, cells washed, and transgene quantified at 72 hours after infection. *P < .05 versus virus alone. (D) CHO-K1 or CHO-pgsA745 cells were incubated with 1000 VP-per-cell AdKO1 in the absence or presence of 1 IU/mL FX for 3 hours, washed, and transgene quantified at 72 hours after infection. *P < .05 versus virus alone. (E) HepG2 cells were exposed to 1 IU/mL FX in the presence or absence of increasing doses of lactoferrin and transgene quantified at 72 hours after infection. *P < .05 versus incubation in the absence of lactoferrin.
Effect of homologous and related but structurally divergent coagulation factors on adenoviral infectivity of HepG2 cells. (A-D) β-Galactosidase expression is expressed as RLU × 107/mg protein. (A) HepG2 cells were incubated with 1 IU/mL human FVII, FIX, FX, PC, FXI, or FXII in the presence of AdKO1 for 3 hours, cells washed, and transgene quantified at 72 hours after infection. Representative X-Gal-stained images are shown for each condition. *P < .05 versus virus alone. (B) HepG2 cells were incubated with varying concentrations (as indicated) of human FVII, FIX, FX, PC, FXI, or FXII in the presence of AdKO1 for 3 hours, cells washed, and transgene quantified at 72 hours after infection. (C) HepG2 cells were exposed to 1 IU/mL FX or an equimolar concentration of FX-GD in the presence of AdKO1 for 3 hours, cells washed, and transgene quantified at 72 hours after infection. *P < .05 versus virus alone. (D) CHO-K1 or CHO-pgsA745 cells were incubated with 1000 VP-per-cell AdKO1 in the absence or presence of 1 IU/mL FX for 3 hours, washed, and transgene quantified at 72 hours after infection. *P < .05 versus virus alone. (E) HepG2 cells were exposed to 1 IU/mL FX in the presence or absence of increasing doses of lactoferrin and transgene quantified at 72 hours after infection. *P < .05 versus incubation in the absence of lactoferrin.
FX directly binds AdCTL and AdKO1
Because FX activation to FXa was not required for enhanced Ad transduction to occur, we next assessed whether FX could directly interact with AdCTL and AdKO1 using surface plasmon resonance analysis. FX (test) and FXI (negative control) were coupled to separate flow cells of a biosensor chip and various dilutions of either AdCTL or AdKO1 injected (Figure 5). Strong association of each virus to FX but not FXI was observed in a dose-dependent manner, and dissociation of virus after injection was undetectable. However, virus could readily be dissociated upon the addition of 3 mM EDTA. Hence, Ad binds to FX directly in a calcium-dependent manner. AdCTL displayed a markedly reduced binding to FX in comparison with AdKO1. Analysis indicated that the AdCTL and AdKO1 bound to FX with apparent affinity constants of 1.9 × 105 and 2.7 × 105 VP/mL, respectively.
FX enhances transduction by AdCTL and fully restores liver transduction by AdKO1 in a bloodfree ex vivo liver perfusion model
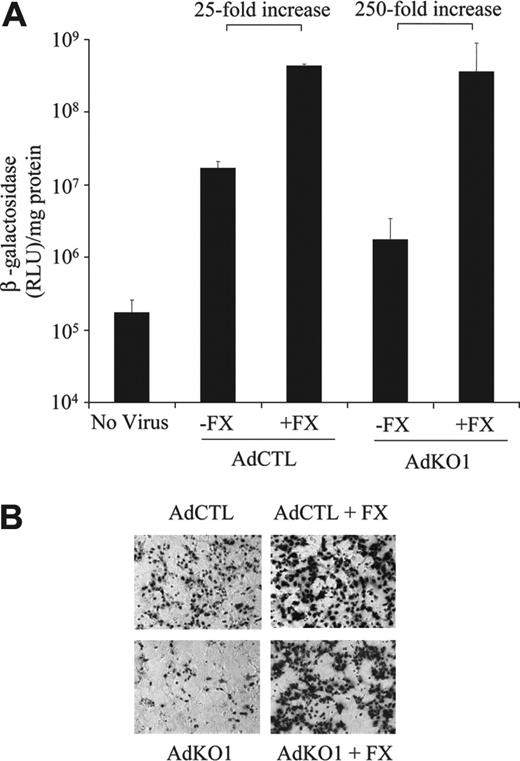
To directly assess whether FX possessed the ability to (a) enhance AdCTL infectivity to hepatocytes and (b) to fully restore transduction mediated by AdKO1 to that seen with AdCTL, we used an established ex vivo liver perfusion technique in the absence of blood10 (see “Materials and methods”). Following injection of viruses in the presence or absence of human FX in perfusates, transduction of hepatocytes was assessed from resulting primary cultures. Transduction levels by AdCTL were approximately 10-fold higher than AdKO1 in the presence of perfusate lacking FX (Figure 6). In the presence of human FX in the perfusate (1 U/mL), levels of transduction by AdCTL and AdKO1 were both substantially higher and essentially identical (Figure 6). Levels of transduction for AdCTL were 25-fold higher in the presence of FX while those from AdKO1 were 250-fold higher, and levels of transduction mediated by AdCTL and AdKO1 in the presence of FX were identical (Figure 6).
Surface plasmon resonance analysis of FX-Ad interactions. AdCTL or AdKO1 was perfused over FX and FXI immobilized onto a CM5 sensor chip in 50 mM Tris (pH 7.4), 150 mM NaCl, 5 mM CaCl2, and 0.005% Tween 20 at a flow rate of 20 μL/min at 25°C. Depicted are typical sensorgrams following injection of AdCTL (A) and AdKO1 (B) showing association with FX but not FXI, with undetectable dissociation of virus from FX following the end of the injection but ready dissociation upon injection of 3 mM EDTA. The differential sensorgrams, FXI signal subtracted from the FX signal (FX - FXI), showing the association of AdCTL (C) and AdKO1 (D) with FX at various concentrations (× 1011 VP/mL) were superimposed, demonstrating the concentration dependence. The steady-state change in RU (δRU) was plotted against virus concentration (E), and affinity constants were determined by performing nonlinear regression fitting of the data (R2 values: AdCTL = 0.99 and AdKO1 = 0.99).
Surface plasmon resonance analysis of FX-Ad interactions. AdCTL or AdKO1 was perfused over FX and FXI immobilized onto a CM5 sensor chip in 50 mM Tris (pH 7.4), 150 mM NaCl, 5 mM CaCl2, and 0.005% Tween 20 at a flow rate of 20 μL/min at 25°C. Depicted are typical sensorgrams following injection of AdCTL (A) and AdKO1 (B) showing association with FX but not FXI, with undetectable dissociation of virus from FX following the end of the injection but ready dissociation upon injection of 3 mM EDTA. The differential sensorgrams, FXI signal subtracted from the FX signal (FX - FXI), showing the association of AdCTL (C) and AdKO1 (D) with FX at various concentrations (× 1011 VP/mL) were superimposed, demonstrating the concentration dependence. The steady-state change in RU (δRU) was plotted against virus concentration (E), and affinity constants were determined by performing nonlinear regression fitting of the data (R2 values: AdCTL = 0.99 and AdKO1 = 0.99).
In vivo transduction of liver following systemic AdKO1 injection is reduced by pretreatment of mice with warfarin and rescued by human FX injection
Because FIX-deficient mice showed no difference in liver transduction mediated by AdCTL or AdKO1 following systemic injection (not shown and Shayakhmetov et al10 ), we assessed the effect of warfarin pretreatment on liver transduction mediated by AdKO1. Warfarin inhibits the vitamin K-dependent γ carboxylation of glutamic acid residues in the N-termini of FII, FVII, FIX, FX, and PC and results in reduced circulating levels of these blood coagulation factors as well as abolition of γ carboxylation of circulating protein. The effect of warfarin treatment was confirmed by coagulation tests (Table 1). Citrated plasma from mice pretreated with warfarin showed a profound prolongation of prothrombin time, and FIX:C levels were reduced below detectable limits (less than 0.03 U/mL) while plasma from control mice showed normal prothrombin times and healthy FIX:C levels. Lower FIX:C values in control mice compared with a normal murine citrated plasma pool may reflect the strain difference (ICR versus C57BL/6) (Table 1). Mice pretreated with warfarin showed a substantial reduction in liver transduction 48 hours after systemic AdKO1 administration assessed by X-Gal staining and ELISA (Figure 7). Finally, mice pretreated with warfarin and given injections of human FX intravenously (33 μg per mouse) 30 minutes prior to AdKO1 injection showed a 100% restoration in liver transduction to levels observed in control nonwarfarinized mice (Figure 7).
Coagulation parameters in warfarinized and control mice
. | Prothrombin time, s . | FIX:C, human U/mL . |
|---|---|---|
| Mouse no. | ||
| 1 | > 300 | < 0.03 |
| 2 | > 300 | < 0.03 |
| 3 | > 300 | < 0.03 |
| 4 | > 300 | < 0.03 |
| 5 | > 300 | < 0.03 |
| 6 | 11.2 | ND |
| 7 | 10.4 | 0.55 |
| 8 | 11.0 | 0.52 |
| 9 | 10.6 | 0.47 |
| 10 | 9.8 | 0.57 |
| Normal mouse pool | 10.6 | 1.05 |
. | Prothrombin time, s . | FIX:C, human U/mL . |
|---|---|---|
| Mouse no. | ||
| 1 | > 300 | < 0.03 |
| 2 | > 300 | < 0.03 |
| 3 | > 300 | < 0.03 |
| 4 | > 300 | < 0.03 |
| 5 | > 300 | < 0.03 |
| 6 | 11.2 | ND |
| 7 | 10.4 | 0.55 |
| 8 | 11.0 | 0.52 |
| 9 | 10.6 | 0.47 |
| 10 | 9.8 | 0.57 |
| Normal mouse pool | 10.6 | 1.05 |
Animals 1 to 5 were warfarinized, and 6 to 10 received no warfarin. Citrated plasma samples taken 1 hour after virus administration were assessed by prothrombin time and 1-stage FIX:C assay (results given in human U/mL FIX)
ND indicates not determined
In situ liver perfusion to assess FX involvement in liver hepatocyte transduction. (A) Mouse livers were perfused with PBS to create a blood-free environment, and then 2 × 1011 VP was infused in the presence or absence of human FX at 1 IU/mL. Hepatocytes were then isolated and cultured and reporter gene quantified 48 hours later. (B) Representative images showing β-Gal-expressing cells from the same experiments.
In situ liver perfusion to assess FX involvement in liver hepatocyte transduction. (A) Mouse livers were perfused with PBS to create a blood-free environment, and then 2 × 1011 VP was infused in the presence or absence of human FX at 1 IU/mL. Hepatocytes were then isolated and cultured and reporter gene quantified 48 hours later. (B) Representative images showing β-Gal-expressing cells from the same experiments.
Effect of warfarin pretreatment on liver transduction after systemic injection and tropism rescue by exogenous FX. (A) Mice were pretreated with 133 μg warfarin 72 and 24 hours prior to injection of AdKO1 virus (4 × 10 VP per mouse) in normal mice versus mice pretreated with warfarin or with warfarin plus 33 μg human FX per mouse and transgene quantified by ELISA. *P < .001. (B) X-Gal staining of liver sections; n = 5 animals per group.
Effect of warfarin pretreatment on liver transduction after systemic injection and tropism rescue by exogenous FX. (A) Mice were pretreated with 133 μg warfarin 72 and 24 hours prior to injection of AdKO1 virus (4 × 10 VP per mouse) in normal mice versus mice pretreated with warfarin or with warfarin plus 33 μg human FX per mouse and transgene quantified by ELISA. *P < .001. (B) X-Gal staining of liver sections; n = 5 animals per group.
Discussion
A combination of in vitro, ex vivo, and in vivo analyses have been used in this study to define a common role for the vitamin K-dependent blood coagulation factors FVII, FIX, FX, and PC in Ad serotype 5 transduction of hepatocytes. This appears specific for vitamin K-dependent coagulation factors that share a common domain structure comprising a Gla-EGF1-EGF2-SP domain structure,22 because other coagulation factors that do not share this domain structure (FXI and FXII) show no transduction enhancement. Interestingly, prothrombin, which is also a zymogen of a vitamin K-dependent SP but has 2 “kringle” (KR) domains in place of the EGF domains (Gla-KR1-KR2-SP), showed no transduction enhancement. Mechanistically, our data strongly suggest that increased Ad transduction occurs by direct binding of FX to the virus and that this can be mediated through HSPGs. Because no additive effect through combining factors was observed, we envisage that binding of the factor likely occurs at the same locale in the virus capsid. Because AdCTL and AdKO1 liver transduction levels in FIX-deficient mice were not different (this study and Shayakhmetov et al10 ), compensatory mechanisms by other factors are likely responsible for this effect. Here we identify FX, FVII, and PC as the candidate compensatory proteins. Because FX and PC homozygous gene deletion is incompatible with life,23,24 we used warfarin pretreatment to reduce circulating levels of all 4 factors through blockade of γ carboxylation. This had a striking effect on liver transduction levels mediated by AdKO1. Notably, warfarin does not reduce circulating complement factor C4BP levels,25 thus suggesting that C4BP cannot directly compensate for liver infectivity in the presence of reduced vitamin K-dependent coagulation factors. We further showed that FX injection just prior to AdKO1 infusion into warfarin-treated mice fully restored virus infectivity toward hepatic cells in vivo. Taken together with the ex vivo liver perfusion experiments and in vitro data, this suggests that FVII, FIX, FX, and PC are a unique group of plasma proteins that can bind Ad and mediate efficient gene delivery to hepatocytes.
We demonstrate clearly and using multiple reagents and protocols that conversion of proteolytic activity of FXa is not required to elicit the tropism-enhancing effect (Figure 3). However, a recent study26 showed that administration of TAP to mice reduced resulting Ad 5-mediated liver transduction using a single low dose of vector. TAP is an FXa-selective inhibitor, thus suggesting that Ad infusion induced cleavage of zymogen to active FXa, which in turn mediated liver transduction in a TAP-sensitive pathway. In addition to our extensive in vitro analyses, we tested the selective indirect FXa inhibitor fondaparinux in vivo and failed to show any modification of liver tropism by AdKO1. Taken together, our data suggest that conversion of FX to FXa is not required for liver tropism of Ad 5 in vitro or in vivo. In addition, we show that the transduction-enhancing effect is not mediated through the Gla domain because FX, which lacks a Gla domain (FX-GD), showed near identical transduction-enhancing effects in vitro. It will be critical for future experiments to identify the site(s) on these coagulation proteins for Ad 5 binding.
Surface plasmon resonance experiments clearly showed that Ads (both AdCTL and AdKO1) bound specifically to FX but not FXI. This was both dose dependent and calcium dependent. The virus-FX interaction was rapid and strong, because undetectable dissociation occurred following cessation of virus injection (Figure 5). However, the interaction between the virus and FX was readily dissociated upon the addition of EDTA. We used intact virus for these experiments, thus showing direct association of FX with one or more components of the Ad capsid protein(s). Because the fiber structure is the main determinant of virus tropism, it is likely that FX interacts directly with the fiber protein, but this requires confirmation. Previous studies have shown a direct interaction of the fiber with FIX,10 thus suggesting that FX will also bind directly to the fiber protein. Importantly, we show no association with FXI in parallel with FXI transduction experiments (Figure 4).
Liver targeting by Ads following systemic injection is rapid27-29 and occurs in a nonlinear fashion. Adenovirus is rapidly sequestered to Kupffer cells, a pathway that does not lead to cellular transduction but rather a host-mediated virus clearance mechanism. Following sequestration, Kupffer cells become “saturated,” leading to enhanced uptake by hepatocytes, a phenomenon that can be reproduced experimentally to increase hepatocyte transduction using gadolinium chloride or an Ad “preinjection.”30,31 The precise mechanism(s) that leads to Kupffer cell and hepatocyte uptake has not been fully detailed, but the role of coagulation factors in each of these processes will be important to define during the course of future experiments. Defining such pathways and mechanisms has important bearings on understanding Ad tropism and toxicity in vivo. Moreover, this may aid our development of Ad use clinically for human gene therapy. A particularly pertinent point for clinical translation is the relevance of coagulation factor-Ad interactions in different species. We already know that striking differences between species occur for host responses to Ad injection relating to injected dose, inflammatory responses after injection, and infectivity profiles of CAR-binding mutants.15,32,33 It will therefore be necessary to assess the importance of coagulation factors FVII, FIX, FX, and PC on Ad liver targeting in multiple species.
In recent years intense efforts to ablate liver tropism of Ad 5 and retarget the virus to alternate tissues have been made, largely without success (reviewed by Nicklin et al7 ). Although remaining controversial, a number of studies have failed to show any difference in liver uptake by CAR binding-deleted viruses, although differences in the precise mutants and model systems used varied.4,5,15,34,35 Interestingly, one CAR-binding ablation study using the K420A mutation in the β sheet produced a 10-fold reduction in liver transduction after systemic delivery.6 It will be important to define whether this mutation also disrupts coagulation factor binding. An Ad 5 vector mutated at the putative HSPG-binding site KKTK (so-called S* mutant) also shows reduced liver tropism in mice, rats, and nonhuman primates, which is particularly marked when combined with the CAR-binding mutation KO1.13-15 Because we show that the coagulation factor FX works through HSPGs, it will be intriguing to define whether S* mutants are sensitive to the coagulation factor pathway. Alternate fibers pseudotyped onto Ad 5 capsids have also been proposed as agents able to enhance transduction of target tissues and some, but not all of those tested, show reduced liver infectivity compared with Ad 5-based vectors. For example, Ad19p-based vectors show strikingly reduced liver targeting after systemic injection,36 but longer-shafted Ad35 viruses remain liver targeted.10 It will be important to correlate infectivity of liver tissue with alternate pseudotyped Ads with direct fiber/capsid binding of coagulation factors. This will aid in the understanding of basic principles dictating the in vivo infectivity of Ad and Ad mutants.
In conclusion, we define a common role for the blood coagulation factors FVII, FIX, FX, and PC in mediating hepatocyte transduction of adenoviral vectors using a series of model systems. This has fundamental implications for Ad biology and their use as vectors for human gene therapy as well as assigning an important role for defined coagulation factors in Ad biology in vivo.
Prepublished online as Blood First Edition Paper, June 20, 2006; DOI 10.1182/blood-2006-04-008532.
Supported by the European Commission (A.H.B.) and Biotechnology and Biophysical Research Council (A.H.B.). Haemostasis and Thrombosis are supported by the Medical Research Council. S.N.W. is a recipient of the Philip Gray Memorial Fellowship, Katharine Dormandy Trust.
S.N.W., G.K.-C., and J.H.M. designed and performed experiments; A.L.P., C.G.N., D.M.S., S.M.B., L.D., and S.N. performed experiments; A.L. and S.A.N. supervised the study; and A.H.B. supervised the study and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Nicola Britton and Gregor Aitchison for technical assistance and Dr John McClure for statistical advice.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal