Abstract
It has been suggested that neutrophil tissue repopulation following bone marrow transplantation (BMT) serves as an earlier and more relevant marker of susceptibility to infection than circulating neutrophil counts. In a previous study using an oral rinse protocol, we found that oral neutrophil recovery always preceded blood neutrophil engraftment and that the day of oral neutrophil detection served as a predictor of patient susceptibility to infection after BMT. Consequently, we have developed and validated a mouse BMT model which uses bone marrow transplants containing enhanced green fluorescent protein-expressing neutrophils to follow neutrophil tissue repopulation after BMT. Using this in vivo cell migration model, we assessed the significance of neutrophil tissue recruitment kinetics with neutrophil functionality and in vivo bacterial killing after BMT. Using the animal model, we have demonstrated that protection against bacterial infection is conferred at the time of neutrophil tissue delivery, which always occurs before neutrophils are detected in the blood. We therefore conclude that neutrophil tissue recovery is an early measure of the restoration of cellular innate immune function after BMT. This model will help us better understand the factors regulating neutrophil recruitment to the tissues.
Introduction
In the current bone marrow transplantation setting, engraftment is defined as the first of 3 consecutive days the patient presents with an absolute neutrophil count (ANC) of 0.5 × 109/L or more.1-4 Although myeloid engraftment should mark the beginning of hematologic recovery and resolution of neutropenia, some bone marrow transplantation (BMT) patients who have obtained ANCs that suggest engraftment continue to experience life-threatening, prolonged, or recurrent infection, whereas others who present with an ANC less than 0.5 × 109/L do not develop any fever related to infection.2 Evidently, ANC does not provide complete information on neutrophil functionality or capacity to reach peripheral sites of microbial challenge in the crucial days following BMT.3 To address this, in a previous study using an oral rinse protocol, we confirmed in a pediatric patient population that neutrophils reappear and return to stable levels earlier in the mouth than in the blood subsequent to intense conditioning therapy followed by BMT.4 Moreover, the time span between oral neutrophil recovery and blood neutrophil recovery after BMT was inversely related to the number of infection-related fever episodes after BMT and therefore served as an indicator of patient susceptibility to infection.4 These findings raised important questions regarding the biology of neutrophil tissue delivery and how it relates to susceptibility to infection.
Given the obvious difficulties in studying neutrophil dynamics in human patients in vivo, animal models have been extensively used as experimental model systems. However, most of those studies have focused primarily on circulating neutrophil kinetics and overall neutrophil production.5,6 Our goal was to develop a murine BMT model system that would allow us to determine whether the timing of neutrophil tissue repopulation following transplantation is a better predictor of innate immune function recovery than the timing of neutrophil reappearance in the blood. We demonstrate here the importance of early neutrophil tissue repopulation after BMT in conferring protection from bacterial infection.
Materials and methods
Murine bone marrow transplantation
Six- to 8-week-old SV129/black recipient mice were maintained under specific, pathogen-free conditions at the University of Toronto Animal Facility. Two to 3 hours prior to transplantation, they were irradiated with 9 Gy (900 rad) of lethal, whole-body γ-irradiation (cesium 137).7 Recipient mice were reconstituted by intravenous tail vein injection with 3 to 6 × 106 bone marrow cells collected from mice with E-GFP (enhanced green fluorescent protein)-expressing granulocytes.8-10 E-GFP is a fluorescent marker that has been widely used to identify donor-derived fluorescence-positive neutrophils in recipient mice. Our donor mice were genotyped by polymerase chain reaction (PCR) to make sure they expressed the E-GFP gene downstream of the Lys-Cre promoter.8 In all of our reconstitution experiments, the number of E-GFP-positive neutrophils in the blood and the tissues was determined by using a fluorescence-activated Beckman Coulter Epics Altra flow cytometer using 488 nm and 515 nm for excitation and emission wavelengths, respectively (Beckman Coulter, Mississauga, ON, Canada). Neutrophils were identified based on cell size and granularity. Preliminary testing using fluorescence microscopy (to identify cells based on characteristic nuclear structures) and fluorescence-activated flow cytometry revealed that mice that received a transplant maintained consistently high levels of E-GFP-expressing donor neutrophils in their circulation and tissues for well more than 2 months after transplantation, confirming that our BMT protocol leads to successful donor engraftment in the recipient (data not shown).
Our Animal Use Protocol was approved by the University of Toronto Animal Care Committee and in accordance with the Guide for the Humane Use and Care of Laboratory Animals.11
Images were visualized using a Nikon Eclipse E100 (Nikon, Melville, NY), a Nikon 40×/0.95 numeric aperture Plan Apo objective, and a Hamamatsu digital camera, model C4742-80 (Hamamatsu, Bridgewater, NJ). Images were collected using Compix Simple PCI software version 5.3 (Compix, Mississauga, ON, Canada).
Blood collection and tissue harvesting to determine BE and TE
Circulating neutrophil counts of mice that received a transplant were monitored daily after BMT to determine when blood engraftment (BE) occurred. Neutrophil blood count data were obtained from 3 to 12 recipient mice for each day following BMT, up to day 10 after BMT. Blood neutrophil engraftment was defined by the day on which donor E-GFP neutrophil counts began their steady rise and recovery in the blood of the recipient after BMT. Single peripheral blood samples were collected from the saphenous vein of mice that received a transplant, and circulating neutrophils were quantified by a HEMAVET multispecies hematology analyzer (Drew Scientific, Oxford, CT).12
On the basis of these findings, mice were killed by cervical dislocation on days prior to and following BE to assess neutrophil repopulation of the tissues or neutrophil tissue engraftment (TE). Neutrophil tissue count data were obtained from 3 to 12 recipient mice for each day following BMT, up to day 8 after BMT. Tissue neutrophil engraftment is defined by the day on which donor E-GFP neutrophil counts begin their steady rise and recovery in the tissues of the recipient after BMT. The tissues that were examined were the lungs and the spleen. The lungs were digested into single-cell suspensions with 150 U/mL Type III Collagenase (Sigma-Aldrich, Oakville, ON, Canada) at 37°C for 3 hours with gentle agitation.13 Splenic cells were prepared by gently pressing the spleen between the frosted ends of 2 glass slides with PBS.14 To identify the proportion of E-GFP, engrafted neutrophils in the lungs and spleen of recipient mice, we used florescence-activated flow cytometry.
G-CSF treatment and neutrophil functional assays
To examine the effects of granulocyte colony-stimulating factor (G-CSF) treatment on neutrophil recovery and function after BMT in mice, we injected a group of mice that received a transplant with 5 μg G-CSF (Neupogen Filgastrim; Amgen, Thousand Oaks, CA) subcutaneously once daily after BMT until they were killed for analysis.5 Blood and tissue neutrophil engraftment were determined in this group of mice as previously described. Neutrophil blood and tissue count data were obtained from 3 to 12 recipient mice for each day following BMT, up to day 10 after BMT.
Furthermore, the effect of G-CSF treatment on neutrophil chemotactic response was assessed by inducing experimental peritonitis through the intraperitoneal injection of 1 mL of 5 mM sodium periodate (Sigma-Aldrich) in PBS. The mice (3-8 mice for each day following BMT, up to day 10 after BMT) were killed 3 hours later, and the peritoneal exudates were collected by lavage with chilled PBS (20 mL/mouse). Neutrophils were counted by a Coulter Z2 particle counter (Beckman Coulter).9
Reconstitution of innate immune function
Pseudomonas aeruginosa strain PAOI was used to induce bacterial pneumonia in mice before and after TE and BE. This allowed us to assess the relation between neutrophil blood and tissue recovery following BMT and innate immune function recovery. PAOI was cultured overnight, and, on the day of the experiment, the material was diluted to a concentration of 108. The mice were anesthetized with a combination of Rompun and Ketamine injected intraperitoneally, and approximately 108 CFU PAOI was implanted in the lungs by intratracheal instillation. Following a 4-hour period of infection, bronchoalveolar lavage (BAL) was conducted by instilling five 1-mL aliquots of PBS via a cannula placed into the trachea and secured with ligatures. The concentration of neutrophils present in BAL fluid was determined using differential cytology (counting a total of 200 cells per slide). BAL fluid and lungs were also analyzed for bacterial persistence. Lungs were collected and homogenized in 2 mL PBS by using a tissue homogenizer (Brinkman Instruments, Mississauga, ON, Canada). Serial 10-fold dilutions of lung homogenates and BAL fluid were plated on LB agar plates. The number of viable CFUs of PAOI was determined after a 24-hour incubation at 37°C.15-17 Three to 10 mice were tested for days 1 through 5 after BMT and for 1 month after BMT.
Neutrophil tissue and blood half-life
To determine neutrophil blood and tissue half-life, E-GFP mice were lethally irradiated as previously described without being treated with a bone marrow transplant. Three to 15 mice were then killed per day on days 1 through 7 (most mice in this set of experiments did not survive past 7 days) following irradiation. Neutrophil blood and tissue content was determined on those days as previously described to track neutrophil blood and tissue pool depletion.
To study blood neutrophil half-life (t1/2) more precisely, donor mice with E-GFP-expressing neutrophils were killed by cervical dislocation, their femurs and tibias were removed, and bone marrow was isolated and layered onto discontinuous Percoll (Sigma-Aldrich) gradient of 82%/65%/55%.9 Mature, E-GFP-expressing neutrophils were recovered at the 82%/65% interface and counted using a hemacytometer. Subsequently, 15 × 106 mature E-GFP-expressing neutrophils were injected intravenously into untreated SV129/black mice. Blood was collected from the recipient mice (6-12 mice for each time point) immediately following transfusion and then every 2 hours, up to 8 hours after injection. A blood sample 24 hours after transfusion was also collected from this group of mice. The survival of transfused, E-GFP neutrophils was assessed at each of these time points by using fluorescence-activated flow cytometry.
Statistical analysis
Student t tests were used to examine the effect of G-CSF on neutrophil function and recovery. Analysis of variance and post hoc tests were performed to compare the effect of BE day, TE day, and the number of days elapsed after BMT on innate immune function recovery as defined by neutrophil recruitment to the lungs and the amounts of viable bacteria persistent in the lungs and lung lavages following a 4-hour infection period. Values are expressed as mean ± SEM or SD except as otherwise noted. Differences were considered significant at P values of .05 or less. Data that did not fall within a 95% confidence interval was removed prior to statistical analysis.
Results
Blood and tissue neutrophil recovery in the mouse BMT model
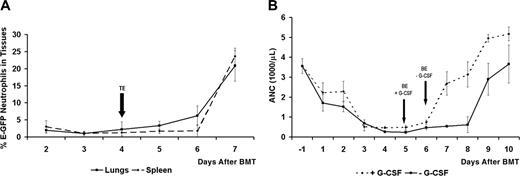
Using a standard murine bone marrow ablation protocol, all mice became severely neutropenic following radiation treatment (Figure 1).7 Following bone marrow transplantation by intravenous tail vein injection of 3 to 6 × 106 bone marrow cells collected from mice with E-GFP-expressing granulocytes, we were able to easily identify the delivery of donor-derived, E-GFP-positive neutrophils in the tissues (lungs and spleen) of animals that received a transplant during the recovery phase.8-10 Neutrophil tissue repopulation (TE) of the spleen and lungs preceded the recovery of neutrophils in the circulation on average by 2 days after BMT (Figure 2A).
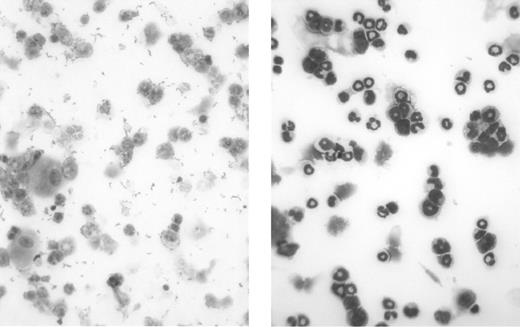
Myeloablative effectiveness of the irradiation protocol before BMT. These are light micrographs of bone marrow cytospin preparations. Mouse bone marrow was isolated and layered onto discontinuous Percoll (Sigma-Aldrich) gradient of 82%/65%/55%. Neutrophils were recovered at the 82%/65% interface, and cytospin preparations were subsequently prepared and stained using the Wright-Giemsa method. The left panel shows mouse bone marrow neutrophils 1 week after irradiation without BMT rescue, and the right panel shows mouse bone marrow neutrophils from a healthy control mouse. These micrographs demonstrate the effectiveness of our radiation protocol in depleting the recipient's immune system in preparation for donor BMT. Original magnification, × 40.
Myeloablative effectiveness of the irradiation protocol before BMT. These are light micrographs of bone marrow cytospin preparations. Mouse bone marrow was isolated and layered onto discontinuous Percoll (Sigma-Aldrich) gradient of 82%/65%/55%. Neutrophils were recovered at the 82%/65% interface, and cytospin preparations were subsequently prepared and stained using the Wright-Giemsa method. The left panel shows mouse bone marrow neutrophils 1 week after irradiation without BMT rescue, and the right panel shows mouse bone marrow neutrophils from a healthy control mouse. These micrographs demonstrate the effectiveness of our radiation protocol in depleting the recipient's immune system in preparation for donor BMT. Original magnification, × 40.
Effect of G-CSF treatment on neutrophil recovery and function after BMT
G-CSF treatment accelerated the recovery of neutrophil levels in the blood on average by 1 day (Figure 2B). To assess the in vivo effect of G-CSF treatment on neutrophil function after BMT, we studied neutrophil chemotactic recruitment to the peritoneum following the induction of experimental peritonitis on days following BMT, with or without G-CSF treatment.5,9 As shown in Figure 3, G-CSF treatment resulted in an increased recruitment of neutrophils to the peritoneum to reach average normal levels (recruited in untreated, control mice) quicker on days following BMT compared with the control group where no G-CSF treatment was administered. The t tests revealed that G-CSF treatment resulted in a significant increase in neutrophil chemotactic recruitment for day 3, day 6, and day 7 after BMT.
Neutrophil recovery in the tissues and in the circulation after BMT. (A) Neutrophil recovery in the tissues after BMT. This plot shows the mean percentage of E-GFP (percentage of total cells) donor neutrophils recovered in the spleen and the lungs of SV129/black recipient mice after BMT. Flow cytometry was used to determine the percentage of E-GFP neutrophils present in single-cell suspensions of the lungs and spleen of mice that received a transplant. Tissue neutrophil engraftment is defined by the day from which the numeric values of donor E-GFP neutrophil counts begin to rise steadily, reflecting the beginning of neutrophil recovery in the tissues after BMT. In this study, tissue engraftment (TE) in the lungs and spleen occurred, on average, on day 4 after BMT, on average 2 days earlier than in the blood circulation of mice that received a transplant not treated with G-CSF (see panel B). Data are shown as mean ± SD (3-12 mice per day after BMT). (B) Neutrophil recovery in the circulation, with and without G-CSF treatment after BMT. This plot shows the mean blood neutrophil counts over time (absolute neutrophil counts, ANCs) in SV129/black recipient mice with and without G-CSF treatment after BMT. Blood neutrophils were quantified by a HEMAVET multispecies hematology analyzer (Drew Scientific). Blood neutrophil engraftment is defined by the day from which the numeric values of donor E-GFP neutrophil counts begin to rise steadily, reflecting the beginning of neutrophil recovery in the blood after BMT. Day of blood engraftment (BE) for the +G-CSF and -G-CSF treatment groups occurs, on average, on day 5 and day 6 after BMT, respectively. G-CSF accelerates blood neutrophil engraftment on average by 1 day, and neutrophil levels reach pretreatment levels much faster in the +G-CSF treatment group than in the -G-CSF group. Data are shown as mean ± SD (3-12 mice per day after BMT).
Neutrophil recovery in the tissues and in the circulation after BMT. (A) Neutrophil recovery in the tissues after BMT. This plot shows the mean percentage of E-GFP (percentage of total cells) donor neutrophils recovered in the spleen and the lungs of SV129/black recipient mice after BMT. Flow cytometry was used to determine the percentage of E-GFP neutrophils present in single-cell suspensions of the lungs and spleen of mice that received a transplant. Tissue neutrophil engraftment is defined by the day from which the numeric values of donor E-GFP neutrophil counts begin to rise steadily, reflecting the beginning of neutrophil recovery in the tissues after BMT. In this study, tissue engraftment (TE) in the lungs and spleen occurred, on average, on day 4 after BMT, on average 2 days earlier than in the blood circulation of mice that received a transplant not treated with G-CSF (see panel B). Data are shown as mean ± SD (3-12 mice per day after BMT). (B) Neutrophil recovery in the circulation, with and without G-CSF treatment after BMT. This plot shows the mean blood neutrophil counts over time (absolute neutrophil counts, ANCs) in SV129/black recipient mice with and without G-CSF treatment after BMT. Blood neutrophils were quantified by a HEMAVET multispecies hematology analyzer (Drew Scientific). Blood neutrophil engraftment is defined by the day from which the numeric values of donor E-GFP neutrophil counts begin to rise steadily, reflecting the beginning of neutrophil recovery in the blood after BMT. Day of blood engraftment (BE) for the +G-CSF and -G-CSF treatment groups occurs, on average, on day 5 and day 6 after BMT, respectively. G-CSF accelerates blood neutrophil engraftment on average by 1 day, and neutrophil levels reach pretreatment levels much faster in the +G-CSF treatment group than in the -G-CSF group. Data are shown as mean ± SD (3-12 mice per day after BMT).
Neutrophil tissue delivery and restoration of innate immune function
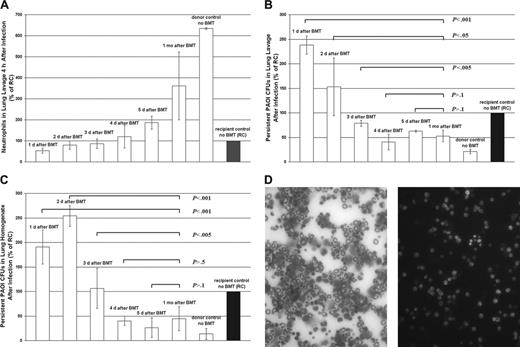
To assess the relation between neutrophil tissue delivery after BMT and innate immune function recovery, P aeruginosa strain PAOI, a Gram-negative bacterium, was used in an acute pulmonary inflammation model.15-17 We compared the inflammatory response in mice 1 month after blood confirmed neutrophil engraftment (on average, BE = day 6 after BMT; see Figure 2B) with that of mice infected on days before and after neutrophil tissue delivery (on average, TE = day 4 after BMT; see Figure 2A), but before BE. Days 1, 2, and 3 after BMT are before TE and BE, day 4 after BMT is TE, day 5 after BMT is after TE and before BE, and 1 month after BMT is long after BE. Neutrophil delivery to the acutely infected lungs increased significantly from day to day during and following bone marrow engraftment (Figure 4A). To determine the extent of in vivo neutrophil function, bacterial persistence following infection in all 6 groups of mice was assessed by plating lung lavages and lung homogenates on LB agar plates. We found that lung lavages taken from mice infected 1 month after BMT exhibited a significantly greater bacterial killing compared with mice infected on day 1, day 2, and day 3 after BMT. However, mice similarly infected on day 4 (TE day) or day 5 (post-TE day and pre-BE day) after BMT had similar levels of bacterial killing as mice infected 1 month after BMT. Additionally, the lung lavages taken from mice infected on day 4 after BMT (TE day) exhibited a significantly lesser amount of bacterial persistence than mice infected on day 1, day 2, and day 3 after BMT (Figure 4B). These results were confirmed by using the lung homogenates of the infected mice (Figure 4C). Figure 4D (right) illustrates E-GFP-expressing, engrafted neutrophils found in the lung lavage of a mouse infected 1 month after BMT. Neutrophils in the lung lavage of this same mouse were also easily identified using differential cytology (Figure 4D, left).
Effect of G-CSF treatment on neutrophil chemotaxis function recovery after BMT. This chart shows the mean number of neutrophils recruited to the peritoneal cavity following the introduction of NaOI in SV129/black mice with and without G-CSF treatment after BMT. Neutrophils were counted by a Coulter Z2 particle counter (Beckman Coulter Canada). The mean number of neutrophils recruited in untreated recipient control mice is represented by the dotted line (8.00 × 105 cells/mL). G-CSF treatment resulted in the increased recruitment of neutrophils to the peritoneum to reach normal levels (8.00 × 105 cells/mL) faster on days following BMT than when no G-CSF treatment was administered. The t tests revealed that G-CSF treatment resulted in a 3-fold significant increase in neutrophil chemotactic recruitment for day 3 (P < .005) and day 6 (P < .005) and a 2-fold increase for day 7 (P < .05) after BMT. Data are shown as mean ± SEM (3-8 mice per day after BMT).
Effect of G-CSF treatment on neutrophil chemotaxis function recovery after BMT. This chart shows the mean number of neutrophils recruited to the peritoneal cavity following the introduction of NaOI in SV129/black mice with and without G-CSF treatment after BMT. Neutrophils were counted by a Coulter Z2 particle counter (Beckman Coulter Canada). The mean number of neutrophils recruited in untreated recipient control mice is represented by the dotted line (8.00 × 105 cells/mL). G-CSF treatment resulted in the increased recruitment of neutrophils to the peritoneum to reach normal levels (8.00 × 105 cells/mL) faster on days following BMT than when no G-CSF treatment was administered. The t tests revealed that G-CSF treatment resulted in a 3-fold significant increase in neutrophil chemotactic recruitment for day 3 (P < .005) and day 6 (P < .005) and a 2-fold increase for day 7 (P < .05) after BMT. Data are shown as mean ± SEM (3-8 mice per day after BMT).
Recovery of innate immune protection from acute infection after BMT and how it relates to TE and BE. (A) This plot shows the mean number of E-GFP neutrophils (expressed as percentage of recipient control) in lung lavage samples from mice infected on days before and after tissue (TE) and blood (BE) engraftment and after BMT. Following a 4-hour period of acute infection, BAL fluid is collected, and the concentration of neutrophils present in BAL fluid samples is determined by using differential cytology (counting a total of 200 cells per slide). Mice infected 1 month after BMT experienced a significantly greater pulmonary recruitment of neutrophils following infection than all other groups of mice, including day 1 (P < .001), day 2 (P < .001), day 3 (P < .001), day 4 (P < .01), and day 5 (P < .005) after BMT. These results demonstrate that neutrophil levels recruited to a site of infection increase significantly from day to day as mice recover from BMT to resemble more and more the values found in their healthy donors (donor control, no BMT) than their own control values (recipient control, no BMT), before BMT treatment. Three to 10 mice were tested for days 1 through 5 after BMT and for 1 month after BMT. Mean ± SD was calculated. (B) This chart shows the mean bacterial survival (expressed as percentage of recipient control) in lung lavage samples from mice infected on days before and after tissue (TE) and blood (BE) engraftment and after BMT. Following a 4-hour period of acute infection, BAL fluid is analyzed for bacterial persistence. Serial 10-fold dilutions of BAL fluid are plated on LB agar plates. The number of viable CFUs of PAOI is determined after 24 hours of incubation at 37°C. Lung lavages taken from mice infected 1 month after BMT exhibited significantly greater bacterial killing than mice infected on day 1 (P < .001), day 2 (P < .05), and day 3 (P < .005) after BMT. However, mice similarly infected on day 4 or TE day (P > .1) and day 5 or after TE and before BE day (P > .1) had similar levels of bacterial killing as did mice infected 1 month after BMT. Additionally, lung lavages taken from mice infected on day 4 (TE day) exhibited significantly greater bacterial killing than mice infected on day 1(P < .001), day 2 (P < .05), and day 3 (P < .01). Three to 10 mice were tested for days 1 through 5 after BMT and for 1 month after BMT. Mean ± SD was calculated. (C) This plot shows the mean bacterial survival (expressed as percentage of recipient control) in lung homogenate samples from mice infected on different days before and after tissue (TE) and blood (BE) engraftment and after BMT. Following a 4-hour period of acute infection, lungs are collected and homogenized in 2 mL PBS using a tissue homogenizer (Brinkman Instruments). Serial 10-fold dilutions of lung homogenates are plated on LB agar plates. The number of viable CFUs of PAOI is determined after a 24-hour incubation at 37°C. The lavage results were confirmed by using the lung homogenates of the infected mice. No significant differences were observed between the amount of bacterial survival in the lungs of mice infected on day 4 (P > .5) or day 5 (P > .1) after BMT when compared with those of mice infected 1 month after BMT and long after blood confirmed engraftment. However, a significant decrease in bacterial persistence was observed in mice infected 1 month after BMT when compared with mice infectedonday1(P < .001), day2(P < .001), and day 3 (P < .005) after BMT. Three to 10 mice were tested for days 1 through 5 after BMT and for 1 month after BMT. Mean ± SD was calculated. (D) Light micrographs of lung lavage fluid cytospin preparations. These preparations were stained using the Wright-Giemsa method showing (left) neutrophils found in BAL fluid of a mouse infected 1 month after BMT and (right) a fluorescent micrograph of neutrophils found in BAL fluid of the same mouse. Original magnification, × 40.
Recovery of innate immune protection from acute infection after BMT and how it relates to TE and BE. (A) This plot shows the mean number of E-GFP neutrophils (expressed as percentage of recipient control) in lung lavage samples from mice infected on days before and after tissue (TE) and blood (BE) engraftment and after BMT. Following a 4-hour period of acute infection, BAL fluid is collected, and the concentration of neutrophils present in BAL fluid samples is determined by using differential cytology (counting a total of 200 cells per slide). Mice infected 1 month after BMT experienced a significantly greater pulmonary recruitment of neutrophils following infection than all other groups of mice, including day 1 (P < .001), day 2 (P < .001), day 3 (P < .001), day 4 (P < .01), and day 5 (P < .005) after BMT. These results demonstrate that neutrophil levels recruited to a site of infection increase significantly from day to day as mice recover from BMT to resemble more and more the values found in their healthy donors (donor control, no BMT) than their own control values (recipient control, no BMT), before BMT treatment. Three to 10 mice were tested for days 1 through 5 after BMT and for 1 month after BMT. Mean ± SD was calculated. (B) This chart shows the mean bacterial survival (expressed as percentage of recipient control) in lung lavage samples from mice infected on days before and after tissue (TE) and blood (BE) engraftment and after BMT. Following a 4-hour period of acute infection, BAL fluid is analyzed for bacterial persistence. Serial 10-fold dilutions of BAL fluid are plated on LB agar plates. The number of viable CFUs of PAOI is determined after 24 hours of incubation at 37°C. Lung lavages taken from mice infected 1 month after BMT exhibited significantly greater bacterial killing than mice infected on day 1 (P < .001), day 2 (P < .05), and day 3 (P < .005) after BMT. However, mice similarly infected on day 4 or TE day (P > .1) and day 5 or after TE and before BE day (P > .1) had similar levels of bacterial killing as did mice infected 1 month after BMT. Additionally, lung lavages taken from mice infected on day 4 (TE day) exhibited significantly greater bacterial killing than mice infected on day 1(P < .001), day 2 (P < .05), and day 3 (P < .01). Three to 10 mice were tested for days 1 through 5 after BMT and for 1 month after BMT. Mean ± SD was calculated. (C) This plot shows the mean bacterial survival (expressed as percentage of recipient control) in lung homogenate samples from mice infected on different days before and after tissue (TE) and blood (BE) engraftment and after BMT. Following a 4-hour period of acute infection, lungs are collected and homogenized in 2 mL PBS using a tissue homogenizer (Brinkman Instruments). Serial 10-fold dilutions of lung homogenates are plated on LB agar plates. The number of viable CFUs of PAOI is determined after a 24-hour incubation at 37°C. The lavage results were confirmed by using the lung homogenates of the infected mice. No significant differences were observed between the amount of bacterial survival in the lungs of mice infected on day 4 (P > .5) or day 5 (P > .1) after BMT when compared with those of mice infected 1 month after BMT and long after blood confirmed engraftment. However, a significant decrease in bacterial persistence was observed in mice infected 1 month after BMT when compared with mice infectedonday1(P < .001), day2(P < .001), and day 3 (P < .005) after BMT. Three to 10 mice were tested for days 1 through 5 after BMT and for 1 month after BMT. Mean ± SD was calculated. (D) Light micrographs of lung lavage fluid cytospin preparations. These preparations were stained using the Wright-Giemsa method showing (left) neutrophils found in BAL fluid of a mouse infected 1 month after BMT and (right) a fluorescent micrograph of neutrophils found in BAL fluid of the same mouse. Original magnification, × 40.
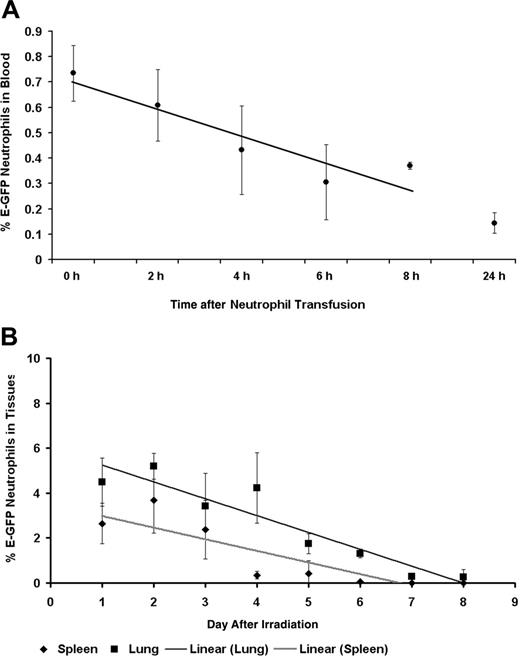
Neutrophil circulating and tissue half-life. (A) Neutrophil circulating half-life. This graph shows the mean percentage of E-GFP (percentage of total cells) neutrophils that remain in the circulation following their intravenous transfusion into SV129/black untreated, recipient mice. Flow cytometry was used to determine the percentage of E-GFP neutrophils present in the blood. From these findings we were able to calculate that the average half-life of a neutrophil is 8 hours long. Neutrophil t1/2 was calculated as previously described.16,17 Data are shown as mean ± SD (6-12 mice for each time point following transfusion). (B) Neutrophil tissue half-life. This graph shows the mean percentage of E-GFP (percentage of total cells) neutrophils that occupy the spleen and lung tissue compartments of E-GFP mice on days after irradiation and no BMT. Flow cytometry was used to determine the percentage of E-GFP neutrophils present in single-cell suspensions of the lungs, spleen, and oral cavity. Neutrophil tissue half-life was calculated as previously described and found to be, on average, 2 days and 6 days long for the spleen and lungs, respectively.16,17 Data are shown as mean ± SD (3-15 mice for each day after irradiation).
Neutrophil circulating and tissue half-life. (A) Neutrophil circulating half-life. This graph shows the mean percentage of E-GFP (percentage of total cells) neutrophils that remain in the circulation following their intravenous transfusion into SV129/black untreated, recipient mice. Flow cytometry was used to determine the percentage of E-GFP neutrophils present in the blood. From these findings we were able to calculate that the average half-life of a neutrophil is 8 hours long. Neutrophil t1/2 was calculated as previously described.16,17 Data are shown as mean ± SD (6-12 mice for each time point following transfusion). (B) Neutrophil tissue half-life. This graph shows the mean percentage of E-GFP (percentage of total cells) neutrophils that occupy the spleen and lung tissue compartments of E-GFP mice on days after irradiation and no BMT. Flow cytometry was used to determine the percentage of E-GFP neutrophils present in single-cell suspensions of the lungs, spleen, and oral cavity. Neutrophil tissue half-life was calculated as previously described and found to be, on average, 2 days and 6 days long for the spleen and lungs, respectively.16,17 Data are shown as mean ± SD (3-15 mice for each day after irradiation).
Neutrophil blood and tissue half-life
Having established that neutrophils survived less than 1 day in the circulation following lethal irradiation treatment with no immune reconstitution through BMT (data not shown), we infused untreated SV129/black mice with E-GFP-expressing mature bone marrow neutrophils to assess their circulating half-life. Transfused E-GFP-positive neutrophils were found to circulate with an average half-life (t1/2) of 8 hours (Figure 5A). Neutrophil tissue compartment depletion was then examined in lethally irradiated E-GFP mice that had not received BMT rescue. Mean tissue neutrophil half-disappearance time was found to be 2 and 6 days for the spleen and lungs, respectively (Figure 5B).
Discussion
In the crucial days following transplantation, circulating neutrophil levels are routinely monitored to determine the advent of engraftment and eventual resolution of neutropenia. Often important treatment decisions, such as when patients can be removed from isolation and the cessation of prophylactic antibiotics, are partly based on circulating white blood cell counts. However, circulating neutrophil levels only indicate that neutrophils are present in the blood. Because their primary role is to fight infection outside the vasculature, assessing neutrophil penetration into the tissues is likely to be a better indicator of innate immune function recovery following chemotherapy or transplantation. We describe here, as we have shown in human patients, a mouse BMT model in which neutrophils accumulate in the tissues prior to detection in the blood after BMT and that this early return is an early predictor of the restoration of innate immune function.4
Neutrophil kinetics following transplantation
We demonstrate here that monitoring neutrophil tissue delivery following BMT serves as an early predictor of blood neutrophil recovery. The murine BMT model that we developed has allowed us to assess the kinetics of neutrophil recovery, tissue delivery, and functionality following transplantation. Our results show that, just as in humans, blood neutrophil counts do not reflect the delivery of neutrophils to the tissues where the primary defense functions of these white blood cells are carried out. In this case where the neutrophil source in the recipient is eliminated through chemotherapy, it is expected that engrafted donor marrow will supply neutrophils that rapidly pass from the marrow to the blood and accumulate in the tissues where these cells are most needed to fend off infections (Figure 2). Only following tissue repopulation will neutrophils begin to accumulate in the circulation.
G-CSF treatment and neutrophil chemotactic function recovery
In the current clinical BMT setting, G-CSF is administered to accelerate neutrophil recovery.18,19 However, there are contradictory reports in the literature with regard to the effect of G-CSF on neutrophil function. The murine BMT model system allowed for a direct, in vivo assessment of G-CSF's effect on neutrophil chemotaxis function after BMT. We observed that G-CSF provides a benefit through improving neutrophil chemotactic function following BMT, therefore potentially contributing to preventing infections in the post-BMT setting (Figure 3).20,21
Neutrophil tissue delivery and recovery of innate immune protection
To study the relevance of neutrophil tissue repopulation following transplantation, we used mice that received a new transplant in an in vivo pulmonary chemotaxis model system. Our data showed that the earlier neutrophil return to the tissues confers the same level of protection from bacterial infection that is expected to take place after blood confirmed neutrophil engraftment. Even though the number of neutrophils recruited to the infected lungs of mice increases with time following BMT (Figure 4A), it seems that only a basal level is required in the tissues to confer protection from infection. On the basis of our findings summarized in Figure 4B-C, the basal level of neutrophils needed to provide protection against infections is recovered on tissue engraftment day (TE day). What is more, this early protection against infection is not reflected in the circulating neutrophil values that are typically used to assess susceptibility to infection following transplantation. These findings strongly suggest that neutrophil tissue repopulation may be used as an earlier and therefore more accurate indicator of innate immune function recovery during the process of transplantation.
Many patients after receiving a BMT who experience a single febrile episode before engraftment continue to receive broad-spectrum antibiotics and remain in protective isolation until blood-confirmed engraftment, despite being subsequently afebrile and well.21 Knowing that neutrophil tissue engraftment has occurred in these patients, using our previously validated oral rinse assay (which measures neutrophil tissue recovery) may allow for a safe reduction of antibiotic therapy and G-CSF administration after tissue neutrophil engraftment but before blood engraftment.4 This may lead to a significant reduction of drug side effects and costs related to prolonged hospital stays, thereby potentially improving patient management and well-being following transplantation.
Conclusion
Our findings confirm that neutrophil tissue recovery after BMT is an earlier, reliable indicator of engraftment and protection from infection compared with the standard measures of circulating neutrophil levels. These results have added to the validity of our clinical data, which suggest that neutrophil tissue delivery is the key predictor of innate immune restoration.4 This novel in vivo cell migration model will help us to better understand the molecular mechanisms of neutrophil mobilization to the tissues.
Prepublished online as Blood First Edition Paper, June 27, 2006; DOI 10.1182/blood-2006-04-018184.
Supported by a CIHR New Investigator Award and the B. Rosenstadt Fund at the University of Toronto (M.G.) and by a CIHR Clinical Research Initiatives Fellowship and the CIHR Strategic Training Program, Cell Signaling in Mucosal Inflammation and Pain (STP-52877) (C.C.). M.G. is a CIHR New Investigator.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Rebecca Schneider and Fei Zhu for their assistance.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal