Abstract
Umbilical cord blood (UCB) is increasingly used as an alternative source of hematopoietic stem cells for transplantation for patients who lack a suitable sibling donor. Despite concerns about a possible increased risk of Epstein-Barr virus (EBV) posttransplantation lymphoproliferative disorder (PTLD) after UCB transplantation, early reports documented rates of PTLD comparable to those reported after HLA-matched unrelated marrow myeloablative (MA) transplantations. To further investigate the incidence of EBV PTLD after UCB transplantation and potential risk factors, we evaluated the incidence of EBV-related complications in 335 patients undergoing UCB transplantation with an MA or nonmyeloablative (NMA) preparative regimen. The incidence of EBV-related complications was a 4.5% overall, 3.3% for MA transplantations, and 7% for NMA transplantations. However, the incidence of EBV-related complications was significantly higher in a subset of patients treated with an NMA preparative regimen that included antithymocyte globulin (ATG) versus those that did not (21% vs 2%; P < .01). Nine of 11 patients who developed EBV PTLD were treated with rituximab (anti-CD20 antibody), with the 5 responders being alive and disease free at a median of 26 months. Use of ATG in recipients of an NMA preparative regimen warrants close monitoring for evidence of EBV reactivation and potentially preemptive therapy with rituximab.
Introduction
Umbilical cord blood (UCB) transplantation has become a valuable alternative for patients who require hematopoietic stem cell transplantation (HSCT) but who lack an HLA-matched sibling donor.1 Compared with grafts from unrelated adult donors, UCB is readily available,2 has a low risk of infection transmission, and has lower than expected incidence of graft-versus-host disease (GVHD), considering the degree of HLA mismatch.3
Epstein Barr virus (EBV) viremia4-12 and posttransplantation lymphoproliferative disorder (PTLD)12-23 are well-recognized complications of allogeneic HSCT. These complications have been associated with unrelated donor transplants, HLA mismatch, antithymocyte globulin (ATG) administration, and ex vivo or in vivo T-cell depletion.4,6,10-20,23 Despite concerns regarding immune reconstitution24 and case reports of EBV PTLD25,26 following UCB transplantation (UCBT), a retrospective analysis at 2 institutions found the incidence of EBV PTLD after a myeloablative (MA) preparative therapy and UCBT to be low.22 A recent analysis found no significant difference in the risk of serious viral infections, including PTLD, in recipients of unrelated donor UCB or unmanipulated marrow.27 However, an increased number of cases of EBV PTLD has been observed recently at our center, leading to a new analysis of EBV-related complications in our patient population that received UCB transplants, with the aim of assessing incidence and identifying potential risk factors.
Patients, materials, and methods
Patients and UCB grafts
Three hundred thirty-five consecutive patients who underwent UCBT at the University of Minnesota Medical Center-Fairview and University of Minnesota Children's Hospital-Fairview between July 1994 and March 2005 were included in this analysis. Median age was 16 years (range, 0.2-69 years), median weight was 53.7 kg (range, 3.8-134.0 kg), and median follow-up was 1.2 years (range, 77 days-9.2 years). Patients were stratified according to type of preparative therapy. Compared with recipients of nonmyeloablative (NMA) regimen, patients treated with an MA preparative regimen were significantly younger (median 8 years vs 50 years; P < .01), had lower weight (median 30 kg vs 78 kg; P < .01), and had longer median follow-up (1.5 years vs 1.2 years; P = .02). Grafts were 4 to 6 of 6 HLA matched (HLA A, B [intermediate resolution], and DRB1 [high resolution]) to the recipient, except one with 3 of 6 HLA-matched grafts. One hundred twenty-six (38%) patients received 2 UCB units; 240 (72%) received an MA preparative regimen; 250 received transplants for a malignant disease. Of the 85 patients who received transplants for a nonmalignant disease, 83 received an MA preparative regimen. The median infused total nucleated cell dose (TNC) was significantly higher among recipients of an MA preparative regimen (4.1 × 107/kg vs 3.6 × 107/kg; P < .01). Median CD34 cell dose was 4.4 × 105 cells/kg (range, 0.4 × 105 cells/kg to 96.7 × 105 cells/kg) and was similar for recipients of MA and NMA preparative regimens. All transplantation protocols were approved by the University of Minnesota Institutional Review Board. All patients or their legal guardians provided written informed consent for the transplantation procedure.
Preparative regimen
The MA preparative regimen included cyclophosphamide regimen with either busulfan or total body irradiation (TBI) and equine ATG (ATGAM; Pharmacia, Kalamazoo, MI) in 174 patients (73%). ATG was administered at 15 mg/kg every 12 hours for 6 doses on days -5 through -3. The NMA preparative regimen consisted of cyclophosphamide, fludarabine, and TBI 200 cGy as detailed elsewhere.28 After April 2002, 30 patients (32%) who had not received multi-agent chemotherapy in the preceding 3 months (excluding those with prior autologous transplantation) received ATG as additional pretransplantation immune suppression. ATG was incorporated into the NMA therapy for these patients without recent chemotherapy because of a higher incidence of graft failure.29 ATG was initially administered at 15 mg/kg every 12 hours for 6 doses on days -3 through -1, and administration was moved to days -6 through -4 in November 2004.
Posttransplantation immunosuppression and GVHD therapy
All patients received posttransplantation immunosuppression with either cyclosporine A (CsA)/mycophenolate mofetil (MMF; 50%), CsA/methotrexate (1%), or CsA/methylprednisolone (49%). CsA and MMF were administered in the same dose and schedule for the MA and NMA settings. CsA was administered twice daily with a target trough level 200 to 400 ng/mL, measured by high-performance liquid chromatography (HPLC) whole-blood mass spectroscopy. MMF was administered at 1 g twice daily between days -3 and +30, with no taper. Methotrexate was administered in the MA regimen at 15 mg/m2 on day 1 and 10 mg/m2 on days 3, 6, and 11. Methylprednisolone was administered in the MA regimen at 1 mg/kg every 12 hours between days +5 and +19, with subsequent taper. Grades II to IV acute GVHD were treated with CsA, target levels as described, and prednisone 60 mg/m2/d for 7 days, followed by a rapid 8-week taper. Extensive chronic GVHD was treated with CsA (target level as described), methylprednisolone 15 mg/kg as a bolus intravenous injection weekly for 8 weeks, and prednisone 0.5 mg/kg on alternate days for 12 months, followed by a slow taper. During GVHD therapy, patients received antimicrobial, antiviral, antifungal, and Pneumocystis prophylaxis as described in “Antiviral prophylaxis/supportive care after UCBT.”
Antiviral prophylaxis/supportive care after UCBT
Patients who were cytomegalovirus (CMV) IgG antibody seropositive prior to transplantation received antiviral prophylaxis with high-dose acyclovir (800 mg [18 mg/kg for children] orally 5 times daily or 10 mg/kg intravenously 3 times daily). Antifungal and antibacterial prophylaxis was provided using fluconazole and penicillin or levofloxacin, respectively. Selected patients at high risk for the development of filamentous fungal infection, such as those with an underlying condition of myelodysplastic syndrome, aplastic anemia, heavily pretreated acute leukemia, or those with fungal infection prior to transplantation, received antifungal prophylaxis with voriconazole in place of fluconazole. All patients received G-CSF from the day of transplantation until they had a neutrophil count of 2.5 × 109/L (2500/μL). Irradiated filtered blood products and parenteral nutrition were administered according to institutional guidelines. Pneumocystis prophylaxis was initiated following engraftment.
EBV assay
Through 2003, blood for quantitative EBV polymerase chain reaction (PCR) testing was performed off-site, using primers for the Ebna1 gene (Eastern Virginia Medical School, Norfolk). The EBV PCR assay included positive and negative control samples. The lower limit of detection of the assay was 100 copies of viral DNA per 100 000 cells. At the beginning of 2004, most testing was performed on-site at the University of Minnesota, using real-time TaqMan PCR. The amplicon was a 71-bp portion of the Ebna1 gene. Quantitative EBV data were expressed as viral copies per milliliter. The limit of detection of the assay was 10 viral copies/reaction.30
EBV-related disease and rituximab therapy
EBV viremia was defined as more than 1000 copies of EBV DNA per milliliter of whole blood. EBV PTLD was defined as biopsy- or autopsyproven posttransplantation lymphoma, or viremia along with computerized tomography nodal or soft-tissue abnormalities consistent with PTLD. Patients treated with rituximab received 375 mg/m2 weekly for 4 weeks.
Statistical considerations
The cumulative incidence of EBV-related complications was estimated by treating deaths from other causes as competing risks.31 Survival after documented EBV viremia or PTLD was estimated by the Kaplan-Meier method. Comparison of incidence between subgroups was done using the log-rank test.32 Cox regression analysis was performed to test the independent effect of factors on EBV-related complications.33 Events were analyzed as of October 2005. Statistical comparison of continuous factors was performed by the Wilcoxon 2-sample test or the Kruskal-Wallis test. Differences in categoric factors were tested across subgroups by the use of the chi-square test or Fisher exact test.34
Results
EBV-related events
Fifteen of 335 patients developed EBV-related complications at a median of 133 days (range, 52-407 days) after UCBT. A summary of the 15 cases of EBV-related complications is detailed in Table 1. Four patients had viremia and 11 had PTLD involving bone marrow, lymph nodes, tonsil, liver, skin, stomach, or lung. Among 11 patients who developed EBV PTLD, 5 survived 113 to 1668 days after UCBT. Five of 9 patients treated with rituximab responded to therapy and survived. At 1 year, 45% (95% confidence interval [CI], 15%-75%) survived following the diagnosis of EBV-related complication. The overall incidence of EBV viremia and EBV-associated PTLD was 4.5%, similar to that previously reported.22 The incidence of EBV viremia and EBV-associated PTLD was 3.3% and 7.4% in recipients of MA and NMA preparative therapy, respectively. Demographic characteristics of the 2 groups are summarized in Table 2. In the univariate analysis, age, sex, CMV serostatus, number of UCB units composing the graft, prior autologous transplantation, and disease group (malignant or nonmalignant) were not significantly associated with the incidence of EBV-related complications. An increased risk, however, was observed with HLA mismatch (P = .03). In contrast to our prior report where all patients had received ATG as part of an MA conditioning, a higher incidence of EBV-associated complications was found in patients treated with ATG (14/204 [7%]) compared with those without ATG (1/131 [0.8%]; P = .02). As shown in Table 3, patients who received ATG as part of the preparative regimen had a significantly lower incidence of acute GVHD, but the incidence of EBV-related complications was similar between patients who did and did not develop grades II-IV acute GVHD (7/149 [4.6%] vs 8/186 [4.3%]; P = .86). Table 3 summarizes outcomes by preparative regimen and administration of ATG as part of the preparative regimen.
Characteristics of patients who developed EBV-related complications
Pt no. . | Diagnosis . | Preparative regimen . | Age, y . | CMV status . | Time to EBV event, d . | EBV event . | Method of diagnosis . | Treatment . | GVHD prophylaxis regimen . | Time to acute GVHD grade II-IV, d . | Outcome/cause of death . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MDS | NMA with ATG | 58 | + | 247 | PTLD | Colon biopsy | Rituximab | CsA/MMF | 50 | Alive |
| 2 | AML | NMA with ATG | 60 | – | 133 | PTLD | Tonsil biopsy | Rituximab | CsA/MMF | 35 | Alive |
| 3 | SAA | NMA with ATG | 35 | – | 122 | PTLD | Multiple biopsies* | Rituximab | CsA/MMF | None | Dead/PTLD |
| 4 | CML | NMA with ATG | 48 | + | 185 | PTLD | Multiple biopsies† | Rituximab | CsA/MMF | 24 | Dead/PTLD |
| 5 | CLL | NMA with ATG | 50 | – | 54 | PTLD | PCR | Rituximab | CsA/MMF | None | Alive |
| 6 | SAA | NMA with ATG | 18 | + | 117 | Viremia | PCR | None | CsA/MMF | None | Alive |
| 7 | AML | NMA | 49 | – | 603 | PTLD | Autopsy | None | CsA/MMF | 30 | Dead/hemophagocytic syndrome |
| 8 | CML | MA with ATG | 49 | – | 133 | PTLD | LND biopsy | Rituximab vincristine | CsA/M-pred | None | Dead/PTLD |
| 9 | MDS | MA with ATG | 49 | – | 131 | PTLD | Stomach biopsy | Rituximab | CsA/M-pred | 72 | Alive |
| 10 | AML | MA with ATG | 7 | – | 407 | PTLD | Autopsy | None | CsA/M-pred | 38 | Dead/alveolar hemorrhage and PTLD |
| 11 | ALL | MA with ATG | 14 | + | 112 | Viremia | PCR | None | CsA/M-pred | 16 | Dead/MOF and sepsis |
| 12 | CML | MA with ATG | 17 | – | 52 | Viremia | PCR | None | CsA/M-pred | 36 | Alive |
| 13 | ALD | MA with ATG | 8 | – | 93 | PTLD | PCR CT scan | Rituximab | CsA/M-pred | None | Alive |
| 14 | OP | MA with ATG | 4 | – | 61 | Viremia | PCR | None | CsA/MMF | 15 | Dead/GVHD |
| 15 | MLS | MA with ATG | 7 | – | 92 | PTLD | Liver biopsy | Rituximab methypred | CsA/M-pred | None | Dead/PTLD |
Pt no. . | Diagnosis . | Preparative regimen . | Age, y . | CMV status . | Time to EBV event, d . | EBV event . | Method of diagnosis . | Treatment . | GVHD prophylaxis regimen . | Time to acute GVHD grade II-IV, d . | Outcome/cause of death . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MDS | NMA with ATG | 58 | + | 247 | PTLD | Colon biopsy | Rituximab | CsA/MMF | 50 | Alive |
| 2 | AML | NMA with ATG | 60 | – | 133 | PTLD | Tonsil biopsy | Rituximab | CsA/MMF | 35 | Alive |
| 3 | SAA | NMA with ATG | 35 | – | 122 | PTLD | Multiple biopsies* | Rituximab | CsA/MMF | None | Dead/PTLD |
| 4 | CML | NMA with ATG | 48 | + | 185 | PTLD | Multiple biopsies† | Rituximab | CsA/MMF | 24 | Dead/PTLD |
| 5 | CLL | NMA with ATG | 50 | – | 54 | PTLD | PCR | Rituximab | CsA/MMF | None | Alive |
| 6 | SAA | NMA with ATG | 18 | + | 117 | Viremia | PCR | None | CsA/MMF | None | Alive |
| 7 | AML | NMA | 49 | – | 603 | PTLD | Autopsy | None | CsA/MMF | 30 | Dead/hemophagocytic syndrome |
| 8 | CML | MA with ATG | 49 | – | 133 | PTLD | LND biopsy | Rituximab vincristine | CsA/M-pred | None | Dead/PTLD |
| 9 | MDS | MA with ATG | 49 | – | 131 | PTLD | Stomach biopsy | Rituximab | CsA/M-pred | 72 | Alive |
| 10 | AML | MA with ATG | 7 | – | 407 | PTLD | Autopsy | None | CsA/M-pred | 38 | Dead/alveolar hemorrhage and PTLD |
| 11 | ALL | MA with ATG | 14 | + | 112 | Viremia | PCR | None | CsA/M-pred | 16 | Dead/MOF and sepsis |
| 12 | CML | MA with ATG | 17 | – | 52 | Viremia | PCR | None | CsA/M-pred | 36 | Alive |
| 13 | ALD | MA with ATG | 8 | – | 93 | PTLD | PCR CT scan | Rituximab | CsA/M-pred | None | Alive |
| 14 | OP | MA with ATG | 4 | – | 61 | Viremia | PCR | None | CsA/MMF | 15 | Dead/GVHD |
| 15 | MLS | MA with ATG | 7 | – | 92 | PTLD | Liver biopsy | Rituximab methypred | CsA/M-pred | None | Dead/PTLD |
Pt indicates patient; CMV indicates cytomegalovirus; GVHD, graft-versus-host disease; MDS, myelodysplastic syndrome; NMA, nonmyeloablative; ATG, antithymocyte globulin; PTLD, posttransplantation lymphoproliferative disorder; CsA, cyclosporine A; AML, acute myeloid leukemia; SAA, severe aplastic anemia; CML, chronic myelogenous leukemia; CLL, chronic lymphocytic leukemia; PCR, polymerase chain reaction; MA, myeloablative; LND, lymph node; M-pred, Methylprednisolone; MOF, multiple organ failure; ALD, adrenoleukodystrophy; CT scan, computerized tomography; OP, osteopetrosis; and MLS, Maroteaux-Lamy syndrome
Liver, marrow, and skin biopsies
Liver, tonsil, and lung biopsies
Demographic characteristics of all patients by intensity of the preparative regimen
Characteristic . | Myeloablative . | Nonmyeloablative . | P . |
|---|---|---|---|
| No. of patients | 240 | 95 | |
| Median age, y (range) | 8 (0.2-53) | 50 (18-69) | < .01 |
| Median weight, kg (range) | 30 (4-120) | 78 (50-134) | < .01 |
| CMV-positive recipients, no. (%) | 108 (45) | 47 (49) | .46 |
| HLA match, no. (%) | .02 | ||
| 6 of 6 | 28 (12) | 6 (6) | |
| 5 of 6 | 109 (45) | 29 (31) | |
| 3-4 of 6 | 103 (43) | 60 (63) | |
| No. treated with ATG (%) | 174 (73) | 30 (32) | < .01 |
| No. of UCB recipients (%) | < .01 | ||
| Single unit | 192 (80) | 17 (18) | |
| Double unit | 48 (20) | 78 (82) | |
| Median infused nucleated cell dose, × 107/kg (range) | 4.1 (0.7-28.1) | 3.6 (1.1-6.8) | < .01 |
| Median infused CD34+ cell dose × 105/kg (range) | 4.3 (0.4-96.7) | 4.5 (0.7-18.8) | .57 |
| No. with malignant diagnosis (%) | 157 (65) | 93 (98) | .09 |
| Median time to follow-up, y (range) | 1.5 (0.-9.2) | 1.2 (0.3-3.5) | .02 |
Characteristic . | Myeloablative . | Nonmyeloablative . | P . |
|---|---|---|---|
| No. of patients | 240 | 95 | |
| Median age, y (range) | 8 (0.2-53) | 50 (18-69) | < .01 |
| Median weight, kg (range) | 30 (4-120) | 78 (50-134) | < .01 |
| CMV-positive recipients, no. (%) | 108 (45) | 47 (49) | .46 |
| HLA match, no. (%) | .02 | ||
| 6 of 6 | 28 (12) | 6 (6) | |
| 5 of 6 | 109 (45) | 29 (31) | |
| 3-4 of 6 | 103 (43) | 60 (63) | |
| No. treated with ATG (%) | 174 (73) | 30 (32) | < .01 |
| No. of UCB recipients (%) | < .01 | ||
| Single unit | 192 (80) | 17 (18) | |
| Double unit | 48 (20) | 78 (82) | |
| Median infused nucleated cell dose, × 107/kg (range) | 4.1 (0.7-28.1) | 3.6 (1.1-6.8) | < .01 |
| Median infused CD34+ cell dose × 105/kg (range) | 4.3 (0.4-96.7) | 4.5 (0.7-18.8) | .57 |
| No. with malignant diagnosis (%) | 157 (65) | 93 (98) | .09 |
| Median time to follow-up, y (range) | 1.5 (0.-9.2) | 1.2 (0.3-3.5) | .02 |
CMV indicates cytomegalovirus; HLA, human major histocompatibility complex; ATG, antithymocyte globulin; and UCB, umbilical cord blood
Outcomes of the 335 umbilical cord blood transplant recipients by preparative regimen and administration of ATG
. | Myeloablative . | . | . | Nonmyeloablative . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome . | Without ATG (95% CI) . | With ATG (95% CI) . | P . | Without ATG (95% CI) . | With ATG (95% CI) . | P . | ||||
| No. of patients | 66 | 174 | 65 | 30 | ||||||
| Grade II-IV acute GVHD, % | 58 (45-71) | 34 (27-41) | <.01 | 63 (49-77) | 37 (19-55) | .04 | ||||
| Extensive chronic GVHD at 1 y, % | 20 (10-30) | 8 (4-12) | <.01 | 28 (16-40) | 21 (5-37) | .92 | ||||
| Primary neutrophil engraftment, % | 97 (93-100) | 92 (88-96) | .17* | 92 (86-98) | 94 (84-100) | .50* | ||||
| Survival at 2 y, % | 60 (56-76) | 52 (44-60) | .58 | 46 (32-60) | 45 (25-65) | .14 | ||||
. | Myeloablative . | . | . | Nonmyeloablative . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome . | Without ATG (95% CI) . | With ATG (95% CI) . | P . | Without ATG (95% CI) . | With ATG (95% CI) . | P . | ||||
| No. of patients | 66 | 174 | 65 | 30 | ||||||
| Grade II-IV acute GVHD, % | 58 (45-71) | 34 (27-41) | <.01 | 63 (49-77) | 37 (19-55) | .04 | ||||
| Extensive chronic GVHD at 1 y, % | 20 (10-30) | 8 (4-12) | <.01 | 28 (16-40) | 21 (5-37) | .92 | ||||
| Primary neutrophil engraftment, % | 97 (93-100) | 92 (88-96) | .17* | 92 (86-98) | 94 (84-100) | .50* | ||||
| Survival at 2 y, % | 60 (56-76) | 52 (44-60) | .58 | 46 (32-60) | 45 (25-65) | .14 | ||||
ATG indicates antithymocyte globulin; CI, confidence interval; and GVHD, graft-versus-host disease
Comparison of proportions at day 42 after UCB transplantation
EBV-related events after an MA therapy
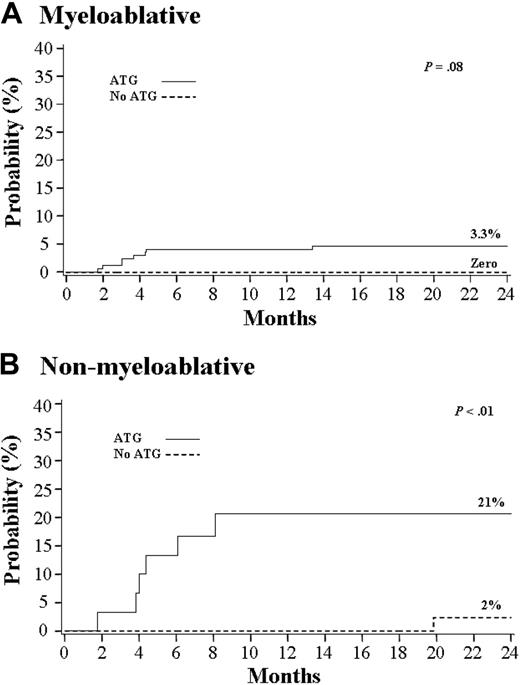
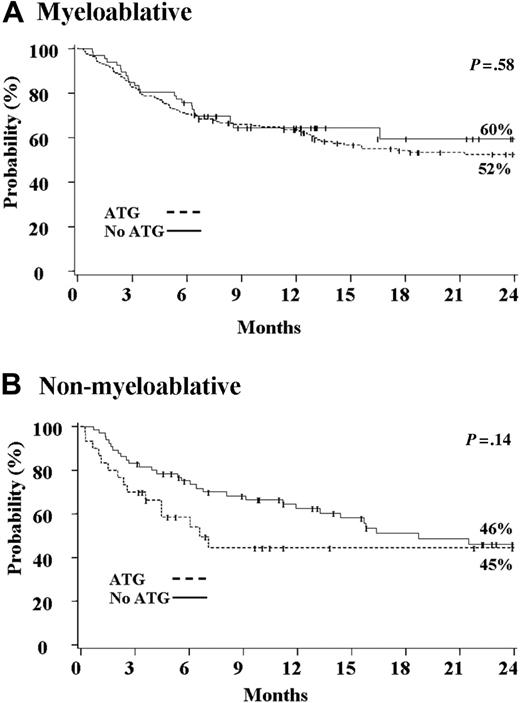
Among 240 patients who received an MA preparative regimen, the incidence of EBV viremia or PTLD was 3.3% (95% CI, 1.0%-5.6%). However, all 8 cases were observed among the 174 patients who received ATG as part of the preparative regimen and in none of 166 who did not. This difference, however, was not statistically significant (P = .08; Figure 1A).As it is shown in Table 2, patients who received an MA preparative regimen with ATG were more likely to be younger, weigh less, be CMV seronegative, and be a recipient of a single UCB unit. The median time to the development of EBV-related complications was 102 days (range, 52-407 days). Patients who received ATG were less likely to develop grades II-IV acute GVHD or extensive chronic GVHD (Table 3). There was no significant difference in the proportion with primary neutrophil engraftment and survival between patients who did or did not receive ATG as part of the preparative regimen (Table 3; Figure 2A).
EBV-related events after an NMA therapy
Among 95 patients who received an NMA preparative regimen, the incidence of EBV viremia or PTLD was 7% (95% CI, 2%-14%). However, there was a significantly higher risk among patients who received ATG (21% vs 2%, P < .01; Figure 1B). Among 30 patients who received ATG, 5 developed EBV PTLD and 1 developed EBV viremia. Patients who received an NMA preparative regimen with ATG were more likely to be older, male, weigh more, and be a recipient of 2 UCB units (Table 2). Among patients who received an NMA preparative regimen, the median time to development of EBV-related complications was 133 days (range, 54-603 days), with EBV PTLD occurring at a median of 133 days (range, 54-247 days). Patients who received ATG were less likely to develop grades II-IV acute GVHD (Table 3). There was no significant difference in the proportion with primary neutrophil engraftment, extensive chronic GVHD, and survival between patients who did or did not receive ATG as part of the preparative regimen (Table 3; Figure 2B).
As shown in Table 4, for Cox multivariate regression analysis the only independent predictor of an increased risk of EBV-related complications was receiving an NMA preparative regimen with ATG (relative risk [RR], 15.4; 95% CI, 2-116; P < .01). Neither prior CMV serostatus, HLA match, nor GVHD prophylaxis were predictors of EBV-related complications.
Cox regression on EBV-related complications
Factor . | Relative risk (95% CI) . | P . |
|---|---|---|
| Conditioning | ||
| Myeloablative* | 1.0 | |
| Nonmyeloablative, without ATG | 0.7 (0.1-6.5) | .51 |
| Nonmyeloablative, with ATG | 15.4 (2.0-116.1) | <.01 |
| CMV serostatus | ||
| Positive* | 1.0 | |
| Negative | 3.0 (0.9-9.7) | .07 |
| HLA, engrafted in doubles | ||
| 6 of 6 | 1.0 | |
| 5 of 6 | 0.2 (0.1-1.5) | .12 |
| 3-4 of 6 | 0.9 (0.2-4.7) | .94 |
| No. of donors | ||
| 1 | 1.0 | |
| 2 | 0.4 (0.1-2.4) | .29 |
Factor . | Relative risk (95% CI) . | P . |
|---|---|---|
| Conditioning | ||
| Myeloablative* | 1.0 | |
| Nonmyeloablative, without ATG | 0.7 (0.1-6.5) | .51 |
| Nonmyeloablative, with ATG | 15.4 (2.0-116.1) | <.01 |
| CMV serostatus | ||
| Positive* | 1.0 | |
| Negative | 3.0 (0.9-9.7) | .07 |
| HLA, engrafted in doubles | ||
| 6 of 6 | 1.0 | |
| 5 of 6 | 0.2 (0.1-1.5) | .12 |
| 3-4 of 6 | 0.9 (0.2-4.7) | .94 |
| No. of donors | ||
| 1 | 1.0 | |
| 2 | 0.4 (0.1-2.4) | .29 |
Factors included in the model and tested for proportional hazards were conditioning regimen, CMV serostatus, age, weight, graft-versus-host disease prophylaxis, cell dose, diagnosis, number of UCB donor units, HLA match, prior autologous transplant, and sex
CI indicates confidence interval; ATG, antithymocyte globulin; and CMV, cytomegalovirus
Discussion
An unexpectedly high incidence of EBV-related complications has recently been observed for patients undergoing UCBT with an NMA preparative regimen including ATG. As transplant centers increasingly use an NMA preparative regimen in the context of UCBT, there needs to be an awareness of this new risk. At our center, the magnitude of the risk necessitated patient notification in those previously treated and alteration of the consent for those receiving ATG. Further, these observations demand closer monitoring in recipients of UCB and ATG for evidence of EBV reactivation after transplantation and the consideration of preemptive anti-CD20 therapy.
Cumulative incidence of Epstein-Barr virus-related complications.
Complications (A) after myeloablative (n = 240) and (B) after nonmyeloablative (n = 95) umbilical cord blood transplantation.
Cumulative incidence of Epstein-Barr virus-related complications.
Complications (A) after myeloablative (n = 240) and (B) after nonmyeloablative (n = 95) umbilical cord blood transplantation.
In this study, we found a cumulative incidence of EBV-related complications of 4.5% and EBV PTLD of 3%. This rate is similar to the 2% incidence of EBV PTLD in the combined datasets of the University of Minnesota and Duke University Medical Center previously reported.22 Furthermore, this result compares favorably with the incidence of EBV PTLD observed after transplantations from other allogeneic stem cell sources, which ranges from less than 1% to 29%, depending upon the use of T-cell depletion, posttransplantation immune suppression, and HLA match between the donor and recipient.13-20,35,36 Higher risks have been associated with unrelated donor, T-cell depletion (TCD), HLA mismatch, and administration of ATG.11-18,20,23 A recent report showed a significantly higher incidence of EBV-related complications after an NMA preparative regimen.23 In contrast to our series, this study included only children, 41 of 65 patients had primary immunodeficiency, and grafts were from matched and mismatched related and unrelated donors.23
Until the mid 1990s, quantitative measurements of EBV load were not determined routinely. This may account for the lower incidence of viremia reported in earlier series. More recently, patients with persistent fever with or without associated adenopathy had EBV viral load measurements. Six of 15 patients who developed EBV-related complications were diagnosed by the EBV assay using real-time TaqMan PCR.30 Although this is a more sensitive technique, it is not likely to explain our findings as in our retrospective analysis, as EBV PCR was only obtained for those patients with clinical manifestations suspicious for EBV reactivation and patients were not being routinely monitored for EBV reactivation. This is also evidenced by the fact that 2 patients were found to have EBV PTLD on autopsy. After allogeneic HSCT, EBV viremia is a frequent event, with incidence ranging between 29% and 65%,4,6,8-11 and increased risk has been associated with unrelated donor, TCD, and administration of ATG.4,6,8,10,11,23 The effect of donor source on EBV viremia has not been reported.
Kaplan-Meier probability of overall survival. Overall survival (A) after myeloablative (n = 240) and (B) after nonmyeloablative (n = 95) umbilical cord blood transplantation.
Kaplan-Meier probability of overall survival. Overall survival (A) after myeloablative (n = 240) and (B) after nonmyeloablative (n = 95) umbilical cord blood transplantation.
Importantly, all but 1 case of EBV-related complications were diagnosed in patients who received ATG as part of their preparative regimen, particularly after an NMA preparative regimen. The 21% incidence found in the subgroup who received ATG with NMA conditioning far exceeds the expected overall low risk after UCBT,22 similar to what has recently been reported for children receiving adult derived HSCs with ATG or alemtuzumab as part of an NMA preparative regimen.23 Clave et al11 have shown that patients who have EBV-specific T cells at the onset of reactivation are more likely to control the viral reactivation without additional therapy. The absence of EBV-specific memory T cells in UCB grafts, more frequent use of HLA-mismatch grafts, and incorporation of ATG inducing in vivo TCD may all contribute to a higher risk of EBV-related complications in this subset of patients after UCBT.11 However, as we observed no increased risk of EBV complications after an MA preparative regimen, even with ATG, other factors such as patient age, diagnosis, prior therapy, and nucleated cell dose may modify the risk. Furthermore, it is possible that following NMA conditioning the number of residual recipient B cells may play a role. In multivariate analysis, the only independent predictor of an increased risk of EBV-related complications was an NMA preparative regimen with ATG. In our cohort, HLA mismatch itself, a risk factor for EBV PTLD for other HSC sources,13,16,20 does not appear to be a predictor for EBV-related complications.
Rituximab is an anti-CD20 humanized monoclonal antibody that has been shown to be active against malignant37-39 and nonmalignant40-45 B-cell diseases. Recent reports have shown its activity against EBV PTLD.8,9,11,23 Rituximab was administered in 9 of 15 patients who developed EBV-related complications, with 5 responding and all 5 alive and EBV disease free beyond 1 year. One of the rituximab responders had also received methylprednisolone. Among the 4 patients who failed rituximab, 3 received the drug alone and 1 received the drug in combination with vincristine.
Reduction of immune suppression, administration of anti-CD20 antibody, and donor lymphocyte infusions (DLIs) have been used for the treatment of EBV PTLD with variable success.5,7,9,46-48 One of the limitations of UCBT is the unavailability of donor lymphocytes for treatment of EBV PTLD or relapse. Alternatives for recipients of UCB would be (1) elimination of ATG from the preparative regimen, (2) addition of rituximab to eliminate B cells, or (3) incorporation of an agent that eliminates both B and T cells, such as alemtuzumab. Alemtuzumab has been associated with a lower risk of EBV complications than ATG.13,23 However, alemtuzumab has been associated with opportunistic infections, particularly viral reactivation including CMV,49-52 and loss of complete chimerism.53-55 However, in recipients of UCB, early diagnosis, reduction of immune suppression when possible, and use of rituximab are the principal options in those with EBV-related complications.
Alternatively, monitoring for EBV with therapeutic intervention only in those patients with increasing viral load may be a safer approach. More recently, studies in HSCT7-11,23,56 as well as solid organ transplantation56-60 suggest that EBV viral load monitoring may be worthwhile in high-risk populations. Some suggest that preemptive therapy is highly effective in controlling viral proliferation and avoiding progression into EBV PTLD.7-10,57 After HSCT, rituximab seems to be effective preemptive therapy once viremia is detected7-10 but of lesser efficacy once EBV PTLD is fully established.10 It is suggested that patients with reduction in viral load after a single dose of rituximab are likely to become complete responders, whereas a rising load is predictive of failure.7 Close viral monitoring during therapy may be valuable, particularly in high-risk populations.
Patients undergoing an NMA UCBT with ATG are at a uniquely higher risk for the development of EBV-related complications, in particular PTLD. Although reduction of immune suppression should always be considered, donor lymphocyte infusions are not available from the UCB donor. Recent data suggest that EBV viral load monitoring with preemptive rituximab treatment for those who develop viremia may halt the progression to PTLD. Therefore, patients who receive ATG as part of an NMA preparative regimen should have quantitative EBV monitoring between days 30 and 180 after UCBT. At our institution, patients who develop EBV viremia (> 1000 copies of EBV DNA per milliliter of whole blood) receive therapy with a single dose of rituximab. Patients with persistent viremia or evidence of PTLD receive more aggressive therapy with additional doses of rituximab with or without chemotherapy. Regardless, all potential recipients of UCB and ATG must be appropriately counseled on this potential risk.
Prepublished online as Blood First Edition Paper, June 27, 2006; DOI 10.1182/blood-2006-03-011791.
Supported in part by grants from the National Institutes of Health (grant NCI P01-CA65493) and Children's Cancer Research Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal