Abstract
Primitive erythroblasts (EryPs) are the first hematopoietic cell type to form during mammalian embryogenesis and emerge within the blood islands of the yolk sac. Large, nucleated EryPs begin to circulate around midgestation, when connections between yolk sac and embryonic vasculature mature. Two to 3 days later, small cells of the definitive erythroid lineage (EryD) begin to differentiate within the fetal liver and rapidly outnumber EryPs in the circulation. The development and maturation of EryPs remain poorly defined. Our analysis of embryonic blood at different stages reveals a stepwise developmental progression within the EryP lineage from E9.5 to E12.5. Thereafter, EryDs are also present in the bloodstream, and the 2 lineages are not easily distinguished. We have generated a transgenic mouse line in which the human ϵ-globin gene promoter drives expression of green fluorescent protein exclusively within the EryP lineage. Here, we have used this line to characterize changes in cell morphology and surface-marker expression as EryPs mature and to track EryP numbers and enucleation throughout gestation. This study identifies previously unrecognized synchronous developmental stages leading to the maturation of EryPs in the mouse embryo. Unexpectedly, we find that EryPs are a stable cell population that persists through the end of gestation.
Introduction
Erythropoiesis initiates in the mammalian embryo with the emergence of primitive, nucleated erythroblasts (EryPs) in the yolk sac.1,2 EryPs constitute the predominant circulating blood cell until a second wave of definitive, enucleated erythrocytes (EryDs) arises within the fetal liver and then becomes the major erythroid-cell type in the fetal blood. Cells of the 2 erythroid lineages differ in size (EryPs are larger than EryDs) and express distinct sets of α- and β-like globin genes (embryonic/fetal in EryP, adult in EryD).1-5 It had long been accepted that a key distinguishing feature of primitive and definitive erythroid cells was the presence or absence of a nucleus: circulating EryPs retain their nuclei, whereas EryDs enucleate in the fetal liver or adult bone marrow, prior to entering the bloodstream.1 Recently, however, it was discovered that primitive erythroblasts also enucleate, beginning around E12.5 in the mouse embryo and continuing throughout gestation.6
The developmental origins of EryPs are poorly defined. Yolk sac and fetal liver erythroid cells are derived from distinct populations of mesoderm during gastrulation.7 In the yolk sac, erythroid and endothelial cells arise from mesodermal clusters to form morphologically identifiable “blood islands”8 late in gastrulation (around E7.5).5,9-12 These lineages were proposed to arise from a common progenitor, the hemangioblast, based on their close temporal and spatial association in the blood islands and on their shared expression of a number of genes.13-15 Some of these genes are required for normal development of both hematopoietic and endothelial cells.16-19 Formation of EryPs has been detected in the absence of endothelial cells in the more proximal yolk sac using confocal imaging.12,20 This finding suggests that hemangioblasts may not be the sole source of erythroid cells at this stage and/or that local signals control cell fate decisions in the hemangioblast. Cells with the properties of the hemangioblast were first identified in the embryonic stem cell system21-23 and were later shown to be present in gastrulating mouse embryos.24 Commitment of mesodermal progenitors to the hematopoietic and endothelial lineages begins prior to or shortly after these cells exit the primitive streak.24 In the mouse, erythroid progenitors (EryP colony-forming cells, or EryP-CFCs, identified in vitro) are found in the yolk sac between E7.25 and E9.0 but not at later stages in either the yolk sac or embryo proper.1 Once circulation has begun (around E9.5 in the mouse), EryPs are free to move into the embryo proper.25 We have begun to characterize EryP maturation during mouse embryogenesis, with a focus on circulating cells. Analysis of embryonic blood at different stages revealed a stepwise developmental progression within the primitive erythroid lineage: loss of nucleoli (E9.5-E10.5), decrease in cell diameter and cross-sectional area (E10.5-E11.5), progressive nuclear condensation (E10.5 onward), and enucleation (E12.5 onward). Thereafter, EryDs are also present in the bloodstream. A major obstacle to the study of primitive erythroid development is the current inability to easily distinguish EryPs from EryDs and to cleanly separate these populations, particularly given that enucleation can no longer be considered an exclusively definitive erythroid characteristic. No EryP-specific surface antigens have been identified that would permit the detection and isolation of cells of this lineage. We have exploited the restricted expression of embryonic globin genes to generate primitive erythroid lineage-specific transgenic mouse models, using upstream regulatory elements of the human embryonic β-like globin gene, ϵ26 to drive β-galactosidase27 and green fluorescent protein (GFP)28 reporters. These transgenic mouse lines have been useful in demonstrating a function for visceral endoderm signals in hematopoietic and endothelial development.27,28 The human ϵ-globin::KGFP transgenic line has permitted dynamic in vivo imaging of the developing yolk sac and circulatory system29 and quantitative analysis of hemodynamic changes during early embryogenesis.30 Here, we have used this line to track quantitatively the differentiation and maturation of circulating EryPs during embryogenesis. The maturation of primitive erythroblasts was reflected in their dynamic expression of Ter119 and CD71. Up-regulation of a number of nonerythroid-specific cell-surface markers, including integrins and other adhesion molecules, was apparent beginning around E12.5, reflecting a previously unrecognized EryP maturation process. Interestingly, enucleation of EryPs is accompanied by changes in surface antigen expression. Thus, the phenotype of primitive reticulocytes is distinct from that of their nucleated precursors. In contrast with the prevailing view that EryPs are present only transiently in the embryo,1,2 we find that they comprise a stable cell population that persists through the end of gestation. The human ϵ-globin::KGFP transgenic mouse model provides a novel system for defining, at the cellular and molecular level, the mechanisms involved in development of the primitive erythroid lineage. Our findings clearly indicate that primitive and definitive erythropoiesis have much in common.
Materials and methods
Generation of ϵ-globin::KGFP transgenic mice
The transgene31 included a BamHI/XhoI fragment from pUCϵX32 containing a human ϵ-globin minimal promoter26 ; jellyfish KGFP sequences amplified using the polymerase chain reaction (PCR) to include HindIII and XbaI sites at the 5′ and 3′ termini, respectively; an ϵ-globin gene BamHI fragment from pUCϵX32 containing splice and polyadenylation signals; and a 2.5-kb (kilobase) fragment containing a truncated human μLCR33 in which an internal HindIII site was destroyed by blunt ligation.26 Fragments were subcloned into pSP73 (Promega, Madison, WI).31 For microinjection, plasmid DNAs were digested with KpnI and NotI, and the eukaryotic portions were purified.34 Embryo injections and implantation into foster mothers were performed at the Mount Sinai Mouse Genetics Shared Resource Facility. Transgenic males (hemizygotes or homozygotes maintained on an ICR outbred background) were crossed with wild-type ICR females (Taconic, Germantown, NY). The morning of detection of the vaginal plug was taken as day 0.5 of gestation.
Embryo dissection and collection of embryonic blood
Timed pregnant females were killed by asphyxiation with CO2 according to institutional guidelines. Uteri were removed and washed with phosphate-buffered saline (PBS). Embryos were dissected free of decidual tissue in fluorescence-activated cell sorting (FACS) buffer (PBS + 5% fetal bovine serum, FBS; Hyclone, Logan, UT) and washed to remove contaminating maternal blood. After removal of the placenta, embryos were rinsed in PBS, transferred to dishes containing FACS buffer plus heparin (12.5 μg/mL; Sigma Aldrich, St Louis, MO), and exsanguinated by severing the vitelline and umbilical vessels and decapitating, then allowing the blood to drain into one well of a 12- or 24-well plate containing 1 mL FACS buffer plus heparin. Neonatal and postnatal pups were decapitated following CO2 anesthesia, and blood was collected by draining into 1 mL FACS buffer plus heparin.
Cytologic analyses
Embryonic blood or sorted cells were centrifuged onto glass slides for 3 minutes at 400g (Cytospin3 cytocentrifuge; Shandon, Pittsburgh, PA), air dried, and stained with Giemsa (Sigma). Samples were then fixed in 4% paraformaldehyde in PBS (Sigma) for 10 minutes at room temperature, blocked for 60 minutes in 5% normal mouse serum (NMS), and incubated with primary antibodies for 60 minutes at room temperature. To prepare NMS, blood was drained from a decapitated adult animal into PBS plus heparin and centrifuged (Beckman model GP; Beckman Coulter, Fullerton, CA) at 3300g for 15 minutes at room temperature, and the supernatant was harvested. Both primary rabbit anti-GFP (Molecular Probes, Eugene, OR) and secondary anti–rabbit IgG-FITC (Santa Cruz Biotechnologies, Santa Cruz, CA) were used at a dilution of 1:500 in blocking buffer (0.1% [wt/vol] Carnation instant nonfat dry milk [Nestle, Glendale, CA], 0.05% [vol/vol] Tween-20 [Sigma] in PBS). After 3 washes in PBS-T (PBS containing 0.05% [vol/vol] Tween-20), slides were mounted.35
Flow cytometry
Cell-surface antigen expression was examined by multicolor FACS using the antibodies shown in Table 1 Cells were incubated with primary antibodies (fluorochrome-conjugated or biotinylated) on ice for 15 to 20 minutes, washed with FACS buffer, collected by centrifugation, and, as required by the experiment, resuspended in streptavidin-APC (BD Pharmingen, San Diego, CA). Labeled cells were washed and resuspended in FACS buffer containing propidium iodide to exclude dead cells from analysis. Analytical FACS was performed using a FACSCalibur instrument (Becton Dickinson, San Jose, CA) in the Mount Sinai Flow Cytometry Shared Research Facility. Data were analyzed using the FlowJo software package (TreeStar, Ashland, OR). For quantitation of EryPs, data from at least 5 but generally 8 to 12 individual embryos per litter were obtained for each gestational age; data were collected from at least 3 independent pregnancies. Cell sorting was performed using a MoFlo instrument (DAKO Cytomation, Carpinteria, CA).
Antibodies used in this study
| Antigen-conjugate . | Clone . | Source* . |
|---|---|---|
| CD3E-PE | 145-2C11 | eBiosciences |
| CD4-PE | L3T4 | BD Pharmingen |
| CD8-PE | 53-6.7 | BD Pharmingen |
| CD11b (Mac-1)–PE | M1/70 | eBiosciences |
| CD19-PE | MB 19-1 | eBiosciences |
| CD24-bio | M1/69 | BD Pharmingen |
| CD25-APC | PC61 | BD Pharmingen |
| CD29 | 9EG7 | BD Pharmingen |
| CD31-PE | MEC 13.3 | BD Pharmingen |
| CD34-PE | RAM34 | BD Pharmingen |
| CD41-PE | MWReg30 | BD Pharmingen |
| CD44-APC | 1M7 | BD Pharmingen |
| CD45-bio | 30-F11 | eBiosciences |
| CD49d (α4 integrin)–PE | R1.2 | BD Pharmingen |
| CD49f (α6 integrin)–PE | GoH3 | BD Pharmingen |
| CD71-PE | R17217 | eBiosciences |
| CD105-bio | MJ7/18 | eBiosciences |
| CD106 (VCAM-1)-bio | 429 | eBiosciences |
| CD117 (c-kit)-APC | 2B8 | eBiosciences |
| CD133-PE | 13A4 | eBiosciences |
| CD140a (PDGFRα)–bio | APA5 | eBiosciences |
| CD140b (PDGFRβ)–bio | APB5 | eBiosciences |
| CD150-PE | TC15-12F12.2 | Biolegend |
| Sca-1-PE | E13-161.7 | BD Pharmingen |
| Gr-1-PE | RB6-8C5 | BD Pharmingen |
| Flk1-bio | AVAS12a1 | eBiosciences |
| Flk2-bio | A2F10 | eBiosciences |
| Flt4-bio | AFL4 | eBiosciences |
| F4/80-bio | BM8 | eBiosciences |
| CXCR4-bio | 2B11 | BD Pharmingen |
| α4β7 integrin-bio | DATK32 | BD Pharmingen |
| Ter119-APC | Ter119 | eBiosciences |
| Antigen-conjugate . | Clone . | Source* . |
|---|---|---|
| CD3E-PE | 145-2C11 | eBiosciences |
| CD4-PE | L3T4 | BD Pharmingen |
| CD8-PE | 53-6.7 | BD Pharmingen |
| CD11b (Mac-1)–PE | M1/70 | eBiosciences |
| CD19-PE | MB 19-1 | eBiosciences |
| CD24-bio | M1/69 | BD Pharmingen |
| CD25-APC | PC61 | BD Pharmingen |
| CD29 | 9EG7 | BD Pharmingen |
| CD31-PE | MEC 13.3 | BD Pharmingen |
| CD34-PE | RAM34 | BD Pharmingen |
| CD41-PE | MWReg30 | BD Pharmingen |
| CD44-APC | 1M7 | BD Pharmingen |
| CD45-bio | 30-F11 | eBiosciences |
| CD49d (α4 integrin)–PE | R1.2 | BD Pharmingen |
| CD49f (α6 integrin)–PE | GoH3 | BD Pharmingen |
| CD71-PE | R17217 | eBiosciences |
| CD105-bio | MJ7/18 | eBiosciences |
| CD106 (VCAM-1)-bio | 429 | eBiosciences |
| CD117 (c-kit)-APC | 2B8 | eBiosciences |
| CD133-PE | 13A4 | eBiosciences |
| CD140a (PDGFRα)–bio | APA5 | eBiosciences |
| CD140b (PDGFRβ)–bio | APB5 | eBiosciences |
| CD150-PE | TC15-12F12.2 | Biolegend |
| Sca-1-PE | E13-161.7 | BD Pharmingen |
| Gr-1-PE | RB6-8C5 | BD Pharmingen |
| Flk1-bio | AVAS12a1 | eBiosciences |
| Flk2-bio | A2F10 | eBiosciences |
| Flt4-bio | AFL4 | eBiosciences |
| F4/80-bio | BM8 | eBiosciences |
| CXCR4-bio | 2B11 | BD Pharmingen |
| α4β7 integrin-bio | DATK32 | BD Pharmingen |
| Ter119-APC | Ter119 | eBiosciences |
PE indicates phycoerythrin; bio, biotin; APC, allophycocyanin.
All companies are in San Diego, CA.
Semiquantitative reverse transcription–polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from FACS-sorted cells using Trizol (Invitrogen, Carlsbad, CA) and analyzed for expression of the indicated mouse genes using radiolabeled semiquantitative RT-PCR. Primers and reaction conditions for detection of endogenous mouse ϵY-globin and β-actin have been described.27 Primers for βh1- and βmaj-globin (243 and 442 bp [base pairs] products, respectively; annealed at 55°C) were βh1 (forward), 5′-CCTGATTGTTTACCCATGGAC-3′; βh1 (reverse), 5′-CAATCACCAACATGTTGCCCAG-3′; βmaj (forward), 5′-GGTGCACCTGACTGATG-3′; βmaj (reverse), 5′-AGTGGTACTTGTGAGCC -3′. Amplified products were electrophoretically resolved in acrylamide gels and visualized by autoradiography.
Assessment of enucleation
The nucleation status of circulating EryPs was assessed by FACS using the dye DRAQ5 (Alexis Biochemicals, Lausanne, Switzerland). Freshly harvested cells were loaded with DRAQ5 (5 μM in FACS buffer) for 5 minutes at 37°C, washed, and analyzed directly by FACS. In some cases, DRAQ5 loading was followed by staining with PE-conjugated antibodies.
Imaging
Embryos were imaged on a Zeiss (Jena, Germany) Lumar.V12 stereomicroscope equipped with epifluorescence illumination, a NeoLumar S 1.5 × FwD 30-mm objective, and appropriate filter sets (Chroma Technology, Rockingham, VT). Digital images were acquired using a Zeiss AxioCam MRM camera and Axiovision software. Fluorescently labeled cells were visualized using a Zeiss Axioplan-2 microscope with 63×/NA1.25 Plan NEOFLUAR or 100×/NA1.4 APOCHROMAT (adjustable iris) oil-immersion objectives. Giemsa-stained cytospin preparations were imaged using an AxioCam MRC camera mounted on a Zeiss Axiovert 25 microscope (LD A-Plan 32 × /NA0.40 objective lens). Images were postprocessed using Adobe (San Diego, CA) Photoshop software.
Results
Identification of developmental landmarks within the circulating primitive erythroid population of the mouse embryo
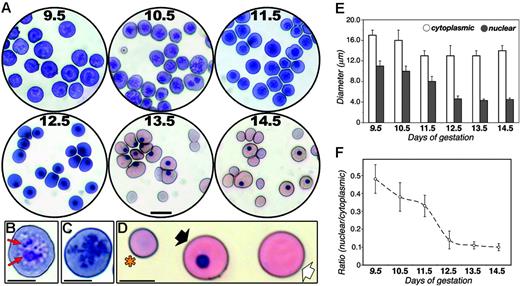
Despite more than a century of investigations on the biology of primitive erythropoiesis in the mammalian embryo, the maturation of EryPs during development remains poorly understood. The circulatory system of the mouse embryo is not fully functional until around E10.25 Therefore, we chose E9.5 as our starting point to examine EryP maturation. Cytospin preparations of peripheral embryonic blood isolated from E9.5 to E14.5 were stained with Giemsa (Figure 1). EryP maturation was assessed using the following cytologic characteristics: Giemsa dye reactivity, cell diameter and cross-sectional area, and nuclear diameter and cross-sectional area. From E9.5 to E12.5, the embryonic blood was dominated by large basophilic proerythroblasts36 (Figure 1A). A striking change in Giemsa reactivity was observed during the 24-hour period from E12.5 to E13.5, when the predominant cell type became the orthochromatophilic erythroblast (Figure 1A), presumably because of an increase in hemoglobin synthesis.37 Clear cytologic changes were evident as the primitive erythroblasts matured. Nucleoli were abundant in EryPs at E9.5 but were virtually undetectable by E10.5 (Figure 1B-C). From E13.5 onward, the peripheral blood contained a mixture of large nucleated cells, large cells lacking nuclei, and small enucleated (presumptive definitive) reticulocytes (Figure 1D).
Cytologic changes during primitive erythroid maturation. (A) Giemsa-stained cytospin preparations of blood from wild-type embryos at E9.5 to E14.5. Scale bar, 20 μm. Circulating blood cells from E9.5 (B) and E10.5 (C) embryos, showing loss of nucleoli (red arrows in B) within the intervening 24-hour period. Scale bar, 10 μm. (D) Enucleated definitive (*), larger nucleated primitive (black arrow) and enucleated primitive (white arrow) erythroid cells in circulation at E14.5. Scale bar, 10 μm. (E) Diameters of circulating E9.5 to E14.5 embryonic blood cells (□) and their nuclei (⊡), measured on cytospin preparations using the Axiovision program. (F) Ratio of mean cross-sectional area of nuclei and cytoplasms of circulating embryonic blood cells. A dramatic decrease in nuclear diameter and cross-sectional area was observed, coincident with nuclear condensation (compare with panel A). Data in panels E and F are expressed as mean ± SD.
Cytologic changes during primitive erythroid maturation. (A) Giemsa-stained cytospin preparations of blood from wild-type embryos at E9.5 to E14.5. Scale bar, 20 μm. Circulating blood cells from E9.5 (B) and E10.5 (C) embryos, showing loss of nucleoli (red arrows in B) within the intervening 24-hour period. Scale bar, 10 μm. (D) Enucleated definitive (*), larger nucleated primitive (black arrow) and enucleated primitive (white arrow) erythroid cells in circulation at E14.5. Scale bar, 10 μm. (E) Diameters of circulating E9.5 to E14.5 embryonic blood cells (□) and their nuclei (⊡), measured on cytospin preparations using the Axiovision program. (F) Ratio of mean cross-sectional area of nuclei and cytoplasms of circulating embryonic blood cells. A dramatic decrease in nuclear diameter and cross-sectional area was observed, coincident with nuclear condensation (compare with panel A). Data in panels E and F are expressed as mean ± SD.
To quantify changes in cell and nuclear size were observed as the EryPs matured, diameters and cross-sectional areas were measured. The diameter of cells in the peripheral blood decreased during embryonic development, from 16 to 17 μm at E9.5 to 13 to 14 μm from E11.5 to E14.5 (Figure 1E). Simultaneously, nuclear diameter shrank by nearly 60%. Even more strikingly, during this 5-day period, the cross-sectional areas of the cells and their nuclei decreased by greater than 90% (Figure 1F). Such drastic reduction in nuclear size is typical of nuclear condensation in maturing EryDs.38
Human ϵ-globin::KGFP transgenic mouse line as a model for investigating primitive erythroid development
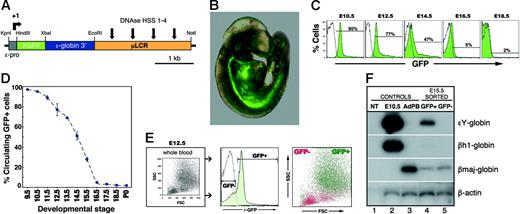
To prospectively identify and isolate EryPs and to characterize their maturational stages further, we used a transgenic mouse system in which EryPs are specifically labeled with GFP. This strategy allowed us to distinguish between the primitive and definitive erythroid lineages. A human ϵ-globin gene promoter and μLCR26 were used to target GFP expression to the primitive erythroid lineage (ϵ-globin::KGFP transgenic mouse line; Figure 2A)28 Displayed in Figure 2B is an embryo at E9.5, when the heart is beating and interconnections between the embryonic and yolk sac circulations are beginning to form.25 GFP+ cells were observed in both the yolk sac and embryo proper at this stage of development (Figure 2B; data not shown). GFP+ cells in the circulation were quantified using flow cytometry. GFP+ cells constituted 95% of peripheral-blood cells at E10.5 (Figure 2C). From E12.5 onward, the fraction of circulating GFP+ blood cells dropped rapidly, such that by E16.5, only 5% of the cells were GFP+ (Figure 2D; Table 2) Even in postnatal animals, a small number of GFP+ cells could be detected in the circulation (Table 2). The forward and side scatter properties of the GFP+ and GFP− populations were also examined by FACS. As anticipated from the morphologic analysis (Figure 1), the GFP+ cells were large (high FSC and SSC), whereas the GFP− cells were smaller and less granular (low FSC and SSC) (Figure 2E).
Frequency of GFP+ cells in circulation throughout development
| Embryonic stage . | Circulating blood cells expressing GFP, % . |
|---|---|
| E9.5 | 97 ± 2 |
| E10.5 | 95 ± 1 |
| E11.5 | 89 ± 8 |
| E12.5 | 77 ± 6 |
| E13.5 | 69 ± 5 |
| E14.5 | 51 ± 9 |
| E15.5 | 28 ± 10 |
| E16.5 | 5 ± 2 |
| E17.5 | 3 ± 0.8 |
| E18.5 | 2 ± 0.8 |
| P0 | 2 ± 0.6 |
| P8 | 0.6 ± 0.2 |
| P21 | 0.5 ± 0.2 |
| Adult* | 0.4 |
| Embryonic stage . | Circulating blood cells expressing GFP, % . |
|---|---|
| E9.5 | 97 ± 2 |
| E10.5 | 95 ± 1 |
| E11.5 | 89 ± 8 |
| E12.5 | 77 ± 6 |
| E13.5 | 69 ± 5 |
| E14.5 | 51 ± 9 |
| E15.5 | 28 ± 10 |
| E16.5 | 5 ± 2 |
| E17.5 | 3 ± 0.8 |
| E18.5 | 2 ± 0.8 |
| P0 | 2 ± 0.6 |
| P8 | 0.6 ± 0.2 |
| P21 | 0.5 ± 0.2 |
| Adult* | 0.4 |
Individual embryos were exsanguinated at different stages of development and peripheral blood was examined for the presence of GFP+ (primitive erythroid) cells by FACS. Percentages are expressed as mean ± SEM per embryo.
Two-year-old mouse.
Human ϵ-globin::KGFP transgenic mouse line as a model system for monitoring primitive erythroid development. (A) Cartoon of the ϵ-globin::KGFP transgenic construct. (B) Photograph of E9.5 ϵ-globin::KGFP embryo. GFP+ cells are seen throughout the circulation. (C) Flow cytometric analysis of circulating blood from E10.5 to E18.5. White area indicates cells from nontransgenic littermate. (D) Graphic representation of data from panel C and Table 2, highlighting the rapid decrease in the fraction of circulating blood cells that contains EryPs between E13.5 and E16.5. (E) Forward scatter (FSC) and side scatter (SSC) profiles of GFP+ and GFP− populations. (F) RT-PCR analysis of ϵY-, βh1-, and βmaj-globin gene transcription in FACS-sorted GFP+ and GFP− cells from E15.5 embryos. NT indicates no template (-DNA); E10.5, peripheral blood from E10.5 embryo; AdPB, adult peripheral blood.
Human ϵ-globin::KGFP transgenic mouse line as a model system for monitoring primitive erythroid development. (A) Cartoon of the ϵ-globin::KGFP transgenic construct. (B) Photograph of E9.5 ϵ-globin::KGFP embryo. GFP+ cells are seen throughout the circulation. (C) Flow cytometric analysis of circulating blood from E10.5 to E18.5. White area indicates cells from nontransgenic littermate. (D) Graphic representation of data from panel C and Table 2, highlighting the rapid decrease in the fraction of circulating blood cells that contains EryPs between E13.5 and E16.5. (E) Forward scatter (FSC) and side scatter (SSC) profiles of GFP+ and GFP− populations. (F) RT-PCR analysis of ϵY-, βh1-, and βmaj-globin gene transcription in FACS-sorted GFP+ and GFP− cells from E15.5 embryos. NT indicates no template (-DNA); E10.5, peripheral blood from E10.5 embryo; AdPB, adult peripheral blood.
To confirm the specificity of GFP expression in the primitive erythroid lineage, E15.5 GFP+ and GFP− cells were sorted by flow cytometry, and total cellular RNA was isolated and analyzed by semiquantitative RT-PCR. As shown in Figure 2F, expression of the endogenous mouse embryonic β-like globin gene ϵY was detected exclusively in the GFP+ population. Expression of βh1-globin was barely detectable and the adult βmaj-globin gene was up-regulated in GFP+ cells by this stage of development in comparison with E10.5 blood cells, both as expected.5 Thus, “maturational” globin gene switching5 is seen in the GFP+ cells. These findings establish the ϵ-globin::KGFP transgenic mouse line as a valuable tool for investigating the differentiation of EryPs. Hereafter, GFP+ cells are termed EryP/GFP+ cells.
Expression of surface antigens on circulating EryP/GFP+ cells during embryogenesis
The GFP expressed in primitive erythroid cells allowed us, for the first time, to perform multicolor FACS analyses of EryPs isolated at different times during embryogenesis. We examined the expression of surface antigens on EryP/GFP+ cells to identify differentiation landmarks and to make comparisons with cells of the definitive erythroid lineage. At E9.5, EryP/GFP+ cells expressed the erythroid-specific glycoprotein Ter119 and the transferrin receptor CD71 (Table 3), which together have been used as markers of erythroid maturation in the fetal liver.39,40 CD24,41 CD55,42,43 and CD147,44 which have been detected on definitive erythroid cells, were also expressed strongly on circulating EryP/GFP+ cells from E9.5 onward (Table 3; data not shown). Surprisingly, expression of several adhesion molecules was up-regulated on a subset of circulating EryP/GFP+ cells. CD44, the hyaluronic acid receptor,45 and α4, α5, and α6 integrins46 were all detected on EryP beginning around E12.5, reflecting a previously unappreciated EryP maturation process.
Expression of surface antigens on EryP/GFP+ cells during embryogenesis
| Category and antigens . | 9.5 dpc . | 12.5 dpc . | 14.5 dpc . |
|---|---|---|---|
| Erythroid | |||
| Ter119 | +++ | +++ | +++ |
| CD71 | +++ | +++ | ++ |
| Hematopoietic | |||
| CD3 | − | − | − |
| CD11b (Mac1) | − | − | − |
| CD19 | − | − | − |
| CD24 | +++ | +++ | +++ |
| CD31 (PECAM1) | − | − | − |
| CD34 | − | − | − |
| CD41 | − | − | − |
| CD45 | − | − | − |
| CD48 | − | − | − |
| CD55 (DAF) | +++ | +++ | +++ |
| CD105 (Endoglin) | − | − | − |
| CD117 (c-Kit) | − | − | − |
| CD147 (Basigin) | +++ | +++ | +++ |
| CD150 | − | − | − |
| Flk1 | − | − | − |
| Gr1 | − | − | − |
| Sca-1 | − | − | − |
| Adhesion molecules | |||
| CD49d (α4 integrin) | − | + | ++ |
| LPAM1 (α4β7 integrin) | − | − | − |
| CD49e (α5 integrin) | ND | + | ++ |
| CD49f (α6 integrin) | − | + | + |
| CD44 | − | + | ++ |
| MECA32 | − | − | − |
| Growth factor receptors | |||
| CD140a (PDGFRα) | − | − | − |
| CD140b (PDGFRβ) | − | − | − |
| CXCR4 | − | − | − |
| Flt4 | − | − | − |
| Category and antigens . | 9.5 dpc . | 12.5 dpc . | 14.5 dpc . |
|---|---|---|---|
| Erythroid | |||
| Ter119 | +++ | +++ | +++ |
| CD71 | +++ | +++ | ++ |
| Hematopoietic | |||
| CD3 | − | − | − |
| CD11b (Mac1) | − | − | − |
| CD19 | − | − | − |
| CD24 | +++ | +++ | +++ |
| CD31 (PECAM1) | − | − | − |
| CD34 | − | − | − |
| CD41 | − | − | − |
| CD45 | − | − | − |
| CD48 | − | − | − |
| CD55 (DAF) | +++ | +++ | +++ |
| CD105 (Endoglin) | − | − | − |
| CD117 (c-Kit) | − | − | − |
| CD147 (Basigin) | +++ | +++ | +++ |
| CD150 | − | − | − |
| Flk1 | − | − | − |
| Gr1 | − | − | − |
| Sca-1 | − | − | − |
| Adhesion molecules | |||
| CD49d (α4 integrin) | − | + | ++ |
| LPAM1 (α4β7 integrin) | − | − | − |
| CD49e (α5 integrin) | ND | + | ++ |
| CD49f (α6 integrin) | − | + | + |
| CD44 | − | + | ++ |
| MECA32 | − | − | − |
| Growth factor receptors | |||
| CD140a (PDGFRα) | − | − | − |
| CD140b (PDGFRβ) | − | − | − |
| CXCR4 | − | − | − |
| Flt4 | − | − | − |
Circulating GFP+ cells were assessed for surfce antigen expression by flow cytometry. Results represent data from at least 3 independent experiments.
— indicates antigen not detected on GFP+ cells; +, < 20% of GFP+ cells expressed antigen; ++, 20%-80% of GFP+ cells expressed antigen; +++, > 80 of GFP+ expressed antigen; ND, not determined; dpc, days after coitus.
Expression of a variety of lymphoid, myeloid, and endothelial markers was not detected in circulating EryP/GFP+ cells (Table 3). EryPs also lacked expression of the hematopoietic/vascular marker CD31/PECAM-1 (which has been identified on erythroid progenitors in the adult bone marrow47 ), c-kit (which has been detected on EryD progenitors in the fetal liver and bone marrow47,48 ), and other hematopoietic stem/progenitor-cell markers (Flk2, CXCR4, CD150; Table 3). Both Flk1 and CD41 have been detected on the surface of EryP progenitors in postgastrulation stage embryos but not on circulating primitive erythroblasts.20 Although expression of PDGF receptor α and β mRNA has been reported in circulating nucleated fetal erythroid progenitors using in situ hybridization,49 the corresponding receptor proteins were not detected on EryP/GFP+ cells (Table 3).
Maturation of primitive erythroblasts is reflected in their expression of Ter119 and CD71
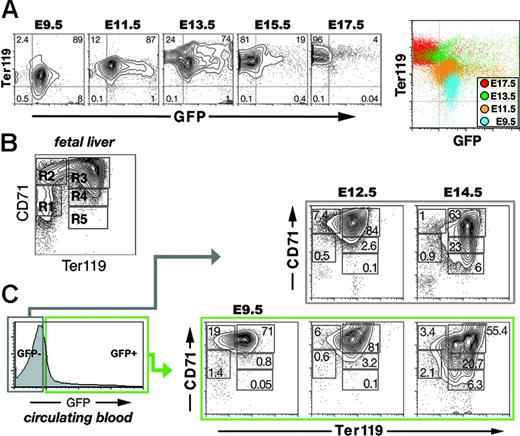
Ter119 associates with glycophorin A on the surface of maturing adult erythroid cells.39 A subset of unfractionated E10 yolk sac cells has been reported to express Ter119.39 Whether circulating EryPs express Ter119 is not known. Therefore, we harvested EryP/GFP+ cells from embryos at different stages and stained for Ter119 (Figure 3A). At E9.5, nearly 90% of EryP/GFP+ cells in the peripheral blood showed low levels of Ter119 staining. The intensity of Ter119 fluorescence increased over the next 4 days. By E13.5, EryP/GFP+ cells could be roughly divided into Ter119med and Ter119high populations. The GFP-negative population observed at this stage expressed Ter119 at high levels and presumably contains EryD.39 From E15.5 to 17.5, essentially all EryP/GFP+ cells expressed Ter119 at high levels (Figure 3A). The changes in Ter119 expression during embryogenesis are displayed in the color-coded composite density plot in Figure 3A (right).
Maturation of primitive erythroblasts is reflected in their expression of Ter119 and CD71. (A) Increase in Ter119 expression on the surface of live GFP+ cells from the circulation of E9.5 to E17.5 embryos. These changes are displayed in the color-coded composite density plot on the right. Axes indicate relative logarithmic fluorescence units for Ter119-PE (y-axis) and GFP (x-axis). (B) Density plot of a typical CD71 and Ter119 staining pattern on viable cells dispersed from wild-type whole E14.5 fetal liver. Axes indicate relative logarithmic fluorescence units for Ter119-APC (x-axis) and CD71-PE (y-axis). Regions R1 to R5 are defined by their characteristic CD71 and Ter119 staining pattern of their cells. The cells in each region can be classified by morphology as follows: primitive progenitor cells and proerythroblasts (R1), proerythroblasts and early basophilic erythroblasts (R2), early and late basophilic erythroblasts (R3), chromatophilic and orthochromatophilic erythroblasts (R4), and late orthochromatophilic erythroblasts and reticulocytes (R5).50 (C) Density plots of FACS-sorted GFP+ cells from E.9.5 and GFP− cells from E9.5, E12.5, and E14.5 embryos. As observed for unfractionated fetal liver cells (B), the circulating cells could be divided into 5 populations (R1 to R5). The greatest numbers of immature cells (R1 and R2) were found at E9.5. By E14.5, significantly larger numbers of mature EryP/GFP+ and EryD/GFP− cells (R4 and R5) were seen. Note that, although most EryPs were strongly GFP+, there was some heterogeneity in the level of expression of the transgene and this accounts for the appearance of a tail in the plot on the left.
Maturation of primitive erythroblasts is reflected in their expression of Ter119 and CD71. (A) Increase in Ter119 expression on the surface of live GFP+ cells from the circulation of E9.5 to E17.5 embryos. These changes are displayed in the color-coded composite density plot on the right. Axes indicate relative logarithmic fluorescence units for Ter119-PE (y-axis) and GFP (x-axis). (B) Density plot of a typical CD71 and Ter119 staining pattern on viable cells dispersed from wild-type whole E14.5 fetal liver. Axes indicate relative logarithmic fluorescence units for Ter119-APC (x-axis) and CD71-PE (y-axis). Regions R1 to R5 are defined by their characteristic CD71 and Ter119 staining pattern of their cells. The cells in each region can be classified by morphology as follows: primitive progenitor cells and proerythroblasts (R1), proerythroblasts and early basophilic erythroblasts (R2), early and late basophilic erythroblasts (R3), chromatophilic and orthochromatophilic erythroblasts (R4), and late orthochromatophilic erythroblasts and reticulocytes (R5).50 (C) Density plots of FACS-sorted GFP+ cells from E.9.5 and GFP− cells from E9.5, E12.5, and E14.5 embryos. As observed for unfractionated fetal liver cells (B), the circulating cells could be divided into 5 populations (R1 to R5). The greatest numbers of immature cells (R1 and R2) were found at E9.5. By E14.5, significantly larger numbers of mature EryP/GFP+ and EryD/GFP− cells (R4 and R5) were seen. Note that, although most EryPs were strongly GFP+, there was some heterogeneity in the level of expression of the transgene and this accounts for the appearance of a tail in the plot on the left.
A flow cytometric system has been used to identify progressive stages of erythroid maturation within the fetal liver based on expression of Ter119 and CD71.40,50 A density plot of Ter119 and CD71 staining of viable cells dispersed from a whole fetal liver (E14.5, wild-type) is shown in Figure 3B. Cells in regions R1 to R5 resemble erythroblasts at different developmental stages (R1 contains the least and R5 the most differentiated cells).50 Although the Ter119/CD71 antigen combination has been widely applied to erythroid development within the fetal liver and adult bone marrow,40,50-54 a comparable analysis of erythroid cells in the embryonic circulation has not been reported. Furthermore, the Ter119/CD71 classification of erythroid maturation does not account for the presence of primitive erythroblasts at midgestation. Therefore, we examined the expression of Ter119 and CD71 on GFP+ and GFP− cells from the blood of ϵ-globin::KGFP embryos.
At E9.5, prior to the appearance of EryD, circulating EryP/GFP+ cells could be divided into 5 subpopulations (R1 to R5) based on their expression of Ter119 and CD71 (Figure 3C). The R1 and R2 populations of definitive erythroid cells in the fetal liver are enriched in progenitors.50 EryP/GFP+ cells from the circulation were found in the R1 (Ter119negCD71med, 1.4%) and R2 (Ter119lowCD71high, 19%) populations. Most of the E9.5 EryP were found in the R3 (Ter119highCD71high) subpopulation (71%). At E12.5 and E14.5, when the bloodstream comprises a mixture of EryP and EryD, both circulating EryP/GFP+ and EryD/GFP− cells could be divided into these 5 subpopulations (Figure 3C). From E12.5 to E14.5, the R4 and R5 populations of EryP/GFP+ and EryD/GFP− cells became more abundant (eg, 3.2% and 0.1% for E12.5 EryP versus 20.7% and 6.3% for E14.5 EryP; Figure 3C), suggesting that maturation of the 2 erythroid lineages may be similar. The R3 population of EryP/GFP+ but not EryD/GFP− cells contained 2 distinct subsets at E14.5: Ter119medCD71high and Ter119highCD71high (discussed in “Enucleation of EryP is accompanied by changes in surface antigen expression”). Together, these findings strongly suggest that the embryonic circulation contains EryP at different stages of maturation.
Expression of definitive erythroid surface antigens on circulating EryP/GFP+ cells
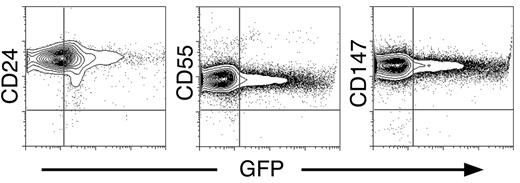
To characterize further the development of circulating EryP, we stained for 3 additional surface antigens that have been detected on definitive erythroid cells. The glycoproteins CD24,55 CD55,42 and CD147/Basigin44 were strongly expressed on all circulating EryP/GFP+ cells at the developmental stages examined (Table 3; Figure 4). The GFP− cells (presumed EryD) also expressed CD24, CD55, and CD147, as expected, at levels comparable to those measured for EryP/GFP+ cells.
Expression of definitive erythroid surface antigens on circulating primitive erythroid cells. Circulating E14.5 EryP/GFP+ cells showed strong surface expression of CD24, CD55, and CD147 by flow cytometry.
Expression of definitive erythroid surface antigens on circulating primitive erythroid cells. Circulating E14.5 EryP/GFP+ cells showed strong surface expression of CD24, CD55, and CD147 by flow cytometry.
Expression of adhesion molecules during EryP maturation
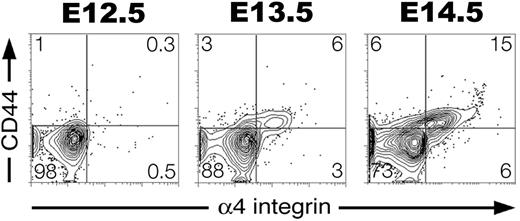
We next asked whether EryP, like definitive erythroblasts,56,57 show regulated expression of adhesion molecules during their maturation. No expression of α4, α5, and α6 integrins was detected on EryP/GFP+ cells at E9.5-10.5 (Table 3; data not shown). However, by E12.5 to E14.5, all 3 integrins were expressed on a subset of circulating EryP/GFP+ cells (Table 3). By E14.5, greater than 20% of EryP/GFP+ cells expressed α4 integrin (Figure 5). Thus, α4 integrin is activated after E9.5, increases over the next few days, and then decreases after enucleation (see “Enucleation of EryP is accompanied by changes in surface antigen expression”). α4 integrin is expressed on maturing definitive erythroblasts and is required for their normal development.58 The hyaluronate receptor CD44,59 also expressed on developing EryDs,60 was up-regulated on EryPs from E12.5 to E14.5 (Table 3; Figure 5). By E14.5, an α4 integrin+CD44+ double-positive cell population was present in the circulation (Figure 5).
Developmental regulation of adhesion molecule expression on maturing primitive erythroid cells. EryP/GFP+ cells were gated and assessed for expression of CD44 and α4 integrin from E12.5 to E14.5. A subset of EryP/GFP+ cells up-regulated expression of both CD44 and α4 integrin adhesion molecules.
Developmental regulation of adhesion molecule expression on maturing primitive erythroid cells. EryP/GFP+ cells were gated and assessed for expression of CD44 and α4 integrin from E12.5 to E14.5. A subset of EryP/GFP+ cells up-regulated expression of both CD44 and α4 integrin adhesion molecules.
Enucleation of EryP is accompanied by changes in surface antigen expression
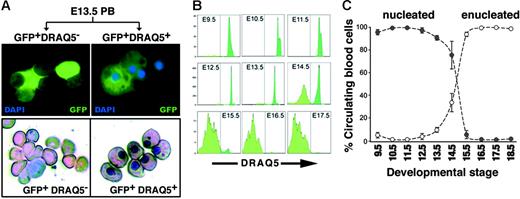
To distinguish between nucleated and enucleated EryPs in circulating blood, we took advantage of the cell-permeable DNA-binding fluorescent dye DRAQ5.61,62 Peripheral blood from E13.5 embryos was stained with DRAQ5 and sorted by FACS. DRAQ5high (nucleated) and DRAQ5neg (enucleated) cells could be clearly distinguished. Giemsa-stained cytospin preparations of the sorted cells confirmed that the DRAQhigh cells contained nuclei, whereas the DRAQ5neg cells did not (Figure 6A). The nucleation status of circulating EryP/GFP+ cells was followed from E9.5 to E18.5 (Figure 6B-C). Early in development of the circulation (E9.5), all EryP/GFP+ cells were DRAQ5+. Between E13.5 and E14.5, a precipitous (50%) reduction in DRAQ5+ cell number was observed (Figure 6B-C). The rapid loss of DRAQ5 signal continued until, by E18.5, all GFP+ cells were DRAQ5neg However, the total number of Eryp/GFP+ cells (DRAQ5high + DRAQ5neg) remained approximately the same from E12.5 to the end of gestation (Figure 6C), indicating that EryP are a stable population and that enucleation does not foreshadow their death. Thus, the combination of DRAQ5 and GFP permitted, for the first time, a quantitative analysis of enucleation by primitive erythroid cells.
Monitoring enucleation of the primitive erythroid population using the cell-permeable DNA-binding dye DRAQ5. (A) Blood cells were harvested from the circulation of GFP+ E13.5 embryos, incubated with DRAQ5, and sorted according to expression of GFP and uptake of DRAQ5. DRAQ5 uptake was a clear indicator of the presence of a nucleus in each cell, as revealed by Giemsa staining (bottom). No fluorescent signal was detected in the GFP− cell populations. (B) DRAQ5 profiles of GFP+ cells harvested from E9.5 (when all EryP/GFP+ cells were DRAQ5high) to E18.5 (when almost all EryP/GFP+ cells were DRAQ5neg). (C) Decline in numbers of nucleated EryP (•) versus the increase in numbers of enucleated EryP (○) in the circulation during embryogenesis, expressed as mean ± SEM. The total numbers of EryP remained stable throughout gestation.
Monitoring enucleation of the primitive erythroid population using the cell-permeable DNA-binding dye DRAQ5. (A) Blood cells were harvested from the circulation of GFP+ E13.5 embryos, incubated with DRAQ5, and sorted according to expression of GFP and uptake of DRAQ5. DRAQ5 uptake was a clear indicator of the presence of a nucleus in each cell, as revealed by Giemsa staining (bottom). No fluorescent signal was detected in the GFP− cell populations. (B) DRAQ5 profiles of GFP+ cells harvested from E9.5 (when all EryP/GFP+ cells were DRAQ5high) to E18.5 (when almost all EryP/GFP+ cells were DRAQ5neg). (C) Decline in numbers of nucleated EryP (•) versus the increase in numbers of enucleated EryP (○) in the circulation during embryogenesis, expressed as mean ± SEM. The total numbers of EryP remained stable throughout gestation.
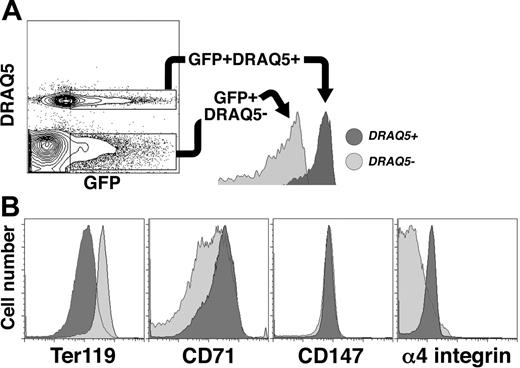
The process of erythroblast enucleation is accompanied by a profound reorganization of cellular structure.63 Studies of definitive erythroid progenitors have shown that cytoskeletal components such as actin and spectrin64-66 are partitioned into reticulocytes after the vimentin-based microfilaments are degraded, allowing the nucleus to move freely about the cell.64 The expelled nuclei are encapsulated by a thin membrane into which cell-surface molecules such as glycophorin A and phosphatidylserine are selectively partitioned.64,67,68 To determine whether sorting of surface antigens occurs during primitive erythroid maturation, we examined the expression of Ter119, CD71, CD147, and α4 integrin in nucleated (DRAQ5high) and enucleated (DRAQ5neg) EryP/GFP+ cells from embryos at E14.5, a stage when both populations are present in the circulation in roughly equal numbers (Figure 6C). Nucleated and enucleated EryP/GFP+ cells could be clearly distinguished by FACS (Figure 7A). Ter119 expression was higher in enucleated than in nucleated EryP/GFP+ cells. This difference in Ter119 expression is interesting, given our earlier observation that expression of Ter119 increases on circulating EryPs during embryogenesis (Figure 3). Ter119 is partitioned to the membrane of reticulocytes during the enucleation of adult mouse bone marrow–derived erythroblasts.67 Our findings suggest that partitioning of Ter119 occurs during enucleation of EryPs as well. A reciprocal pattern of expression was observed for CD71 and α4 integrin, with high levels on nucleated and greatly reduced levels on the cells that had extruded their nuclei (young primitive reticulocytes, Figure 7B). In contrast, no differences in expression of CD147 were detected in nucleated versus enucleated EryP (Figure 7B). Therefore, the reorganization of antigens on the primitive erythroblast membrane is a selective process.
Redistribution of surface antigens following enucleation of primitive erythroid cells. (A) Flow cytometric identification of nucleated and enucleated EryP based on DRAQ5 uptake. GFP+DRAQ5high (nucleated) cells, dark gray area; GFP+DRAQ5neg (enucleated) cells, light gray area. (B) GFP+ cells were gated according to DRAQ5 uptake (A) and assessed for surface antigen expression. Ter119 levels were higher on enucleated reticulocytes (dark gray area) than on nucleated primitive erythroblasts (light gray area). Expression of CD71 and α4 integrin were lower on enucleated than on nucleated EryP/GFP+ cells. No differences in expression of CD147 were seen.
Redistribution of surface antigens following enucleation of primitive erythroid cells. (A) Flow cytometric identification of nucleated and enucleated EryP based on DRAQ5 uptake. GFP+DRAQ5high (nucleated) cells, dark gray area; GFP+DRAQ5neg (enucleated) cells, light gray area. (B) GFP+ cells were gated according to DRAQ5 uptake (A) and assessed for surface antigen expression. Ter119 levels were higher on enucleated reticulocytes (dark gray area) than on nucleated primitive erythroblasts (light gray area). Expression of CD71 and α4 integrin were lower on enucleated than on nucleated EryP/GFP+ cells. No differences in expression of CD147 were seen.
Discussion
A transgenic mouse model for tagging, tracking, and purifying cells of the primitive erythroid lineage
The primitive erythroid lineage is the first to differentiate during mammalian embryogenesis. Despite many decades of study, the developmental biology of these cells is still very poorly understood. In the mouse, more than 6 million EryPs are found in the circulation by E14.5.6 At this stage, active production of definitive erythroid cells from hematopoietic stem cells is in progress in the fetal liver. A major technical hurdle in the analysis of primitive erythropoiesis has been the lack of a method for the rapid and quantifiable segregation of EryPs from EryDs. Here, we use a human ϵ-globin::KGFP transgenic mouse line to tag and track primitive erythroid cells in circulation stage embryos. By a variety of criteria, we conclude that these mice provide a novel model system for studying primitive erythropoiesis: GFP expression in the blood islands of the yolk sac28-30,69 and in virtually all blood cells in the circulation of the preliver embryo (this work); GFP expression in EryP colonies formed in vitro after secondary plating of cells from explanted embryos28 ; rapid decrease in the numbers of circulating GFP+ cells around E14.5, when active definitive erythropoiesis is under way (this study); and the size distribution, cell morphology, pattern of Giemsa staining, and expression of mouse ϵY-globin mRNA in GFP+ cells (this study).
The exclusive expression of the human ϵ-globin::KGFP transgene in EryPs made it possible to distinguish, for the first time, the maturational stages of primitive erythroid cells from those of their definitive counterparts. In this report, we demonstrate that circulating EryPs in the mouse embryo undergo a stepwise developmental progression from proerythroblast to orthochromatic erythroblast to reticulocyte, characterized by loss of nucleoli (E9.5-10.5), decreased cell diameter and cross-sectional area (E10.5-11.5), nuclear condensation (E10.5 onward), changes in expression of cell-surface molecules (E12.5-14.5), and enucleation (E12.5 onward). Thus, the maturation of primitive erythroblasts is developmentally synchronized. The final product of the enucleation process6 is a primitive reticulocyte. Unexpectedly,1,2 we find that EryPs are not a transient population in the embryo; rather, their numbers remain stable through the end of gestation (Figure 6C). The fate of these cells after birth awaits further investigation. Maturing EryPs express some of the same surface antigens as do EryDs. Interestingly, the cell-surface phenotype of enucleated EryPs is distinct from that of their nucleated precursors. We conclude that EryPs undergo a maturation process analogous, at least in part, to that of EryDs.
Immature EryPs in the embryonic circulation
It is of particular interest that at E9.5, prior to the appearance of EryDs, circulating EryP/GFP+ cells were found in the R1 and R2 populations. Within 3 days, these populations had been largely depleted, falling from 1.4% and 19%, respectively, at E9.5 to 0.6% and 6%, respectively, at E12.5. EryP progenitors, as defined by EryP-CFCs, were not detected at this stage of development.1 The R2 population of EryPs identified here might contain high proliferative potential colony-forming cells (HPP-CFCs). In an earlier study, embryonic HPP-CFCs were reported to give rise to definitive erythroid and macrophage (but not to primitive erythroid) progenitors on secondary replating.70 However, a clonogenic analysis of FACS-purified R2 EryP/GFP+ cells might reveal a population of HPP-CFCs that had previously escaped detection. This possibility is currently under investigation.
Cell-surface phenotype of maturing primitive erythroblasts
One goal of this study was to define the surface phenotype of the primitive erythroid lineage. We found that EryPs expressed several surface antigens found on EryDs, including Ter119,39 CD71,39 CD24,41 CD55,42 and CD147.44 Simultaneous analysis of Ter119 and CD71 by flow cytometry revealed a developmental progression for circulating EryPs reminiscent of that found for EryDs.50 Therefore, maturation of primitive erythroblasts involves stepwise changes in surface antigen expression, reflecting the formation of discrete, increasingly more mature populations of cells, followed by their terminal enucleation to reticulocytes.
Redistribution of surface antigens prior to EryP enucleation
The use of multicolor flow cytometry in combination with a lineage-specific GFP reporter, a vital dye (DRAQ5), and selective surface antigen labeling allowed us to determine that the R3 subset of EryPs at E14.5 contained nucleated and enucleated cells, respectively, both of which are found in the circulation. In contrast, the R3 subset of circulating EryD/GFP− cells comprised a uniform population (Ter119highCD71high). EryDs do not enter the circulation until after their enucleation in the fetal liver. Glycophorin A, with which Ter119 interacts,39 has been shown to partition selectively to the plasma membrane of definitive reticulocytes during erythroblast enucleation.67 In contrast with the increase in Ter119 on newly formed EryP reticulocytes, a reciprocal decrease in expression of CD71 and α4 integrin was detected, suggesting preferential redistribution of these antigens from the nascent EryP reticulocyte to the plasma membrane surrounding the expelled nucleus. Almost nothing is known about the structure and behavior of the nucleus in developing EryPs. The ability to distinguish and purify primitive erythroid cells at different stages of maturation will permit investigations of the mechanisms underlying EryP nuclear condenstation and extrusion.
The up-regulation of the adhesion molecules α4 integrin and CD44 on maturing EryP in the circulation is particularly intriguing. Both antigens are found on definitive human erythroblasts in the bone marrow.46,57 α4 Integrins are essential for the efficient differentiation and proliferation of erythroid progenitors in vitro and in vivo.58 CD44 antigens are a family of highly glycosylated proteins encoded by a single gene and are thought to play an important role in the adhesion, migration, and development of hematopoietic progenitor cells.71 Effective cell adhesion requires the coordinated action of integrins and cell-surface proteoglycans such as CD44. Interestingly, an α4 integrin+CD44+ double-positive cell population was present in the circulation by E14.5. CD147, which interacts with integrins on the surface of myeloid cells,72 was also expressed at high levels on EryPs. These observations suggest that a subset of EryPs may home to one or more anatomical sites (eg, the placenta, for oxygenation, or the fetal liver) within the embryo.
Conclusion
The power of the ϵ-globin::KGFP transgenic model for future studies of normal primitive erythroid development will perhaps become most evident when these mice are crossed onto mutant backgrounds. With the novel strategy of primitive erythroid-specific transgene expression, combined with selective cell-surface labeling to facilitate single-step purification of EryPs at different stages during their maturation, it will now be possible to identify critical genes and analyze the molecular mechanisms that control the development of this lineage.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: S.T.F., J.I., and M.H.B. designed the experiments and prepared the figures; S.T.F. and M.H.B. wrote the paper; and S.T.F. and J.I. performed the experiments.
S.T.F. and J.I. contributed equally to this work.
Acknowledgments
We thank Alexandra Snyder for assistance during the early phases of this work and Drs Anna-Katerina Hadjantonakis and Michael A. Dyer for helpful discussions.
This work was supported by grants from the National Institutes of Health (grants RO1 HL62248, DK52191, and EB02209) (M.H.B.), from the Cooley's Anemia Foundation (J.I.), and to the Mount Sinai Mouse Genetics Shared Resource Facility (R24 CA88302).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal