The studies of Robach and colleagues offer new insights into the interactions between iron and oxygen homeostasis and may have important implications for clinical conditions associated with hypoxia and altered erythropoiesis.
Oxygen homeostasis in hypoxia is maintained by stimulating erythropoiesis and promoting hemoglobin synthesis. This implies increased iron supply to the erythropoietic tissue that, in turn, may result in inadequate iron availability for other body compartments. The skeletal muscle represents about 40% of the body mass and contains 10% to 15% of body iron, mainly in the form of myoglobin. Anticipating a competition for iron between the erythropoietic and the skeletal muscle compartments under the increased demands of hypoxia, Robach and colleagues embarked on a study examining simultaneously, in blood and muscle, the effect of hypoxia on proteins of iron metabolism and hemoproteins. To achieve this, healthy young adults were studied at sea level and, again, at an altitude of 4559 m after a period of acclimatization and rest lasting 7 to 9 days. Measurements were performed on blood samples and suction biopsies of the vastus lateralis muscle. The methodological aspects of the study are commendable and even heroic. In order to perform the high-altitude studies, the volunteers, and presumably the scientific team, had to perform a major mountaineering exercise. Managing to derive so much information from skeletal muscle biopsies in healthy volunteers is a major technical achievement.
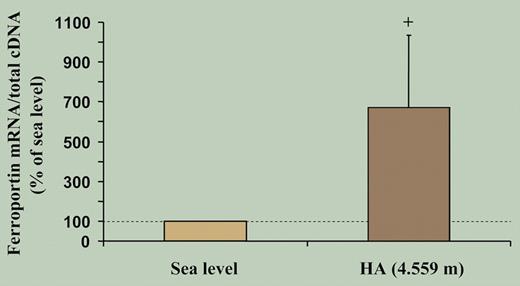
As expected, after 7 to 9 days of high-altitude hypoxia there was a marked increase in hemoglobin, decrease in serum ferritin and transferrin saturation, and increase in serum transferrin and transferrin receptors. However, contrary to expectations, the investigators found a decrease in muscular myoglobin expression and down-regulation of a number of iron-related proteins in skeletal muscle, including L-ferritin, transferrin receptor (TfR) expression, and total iron content. The simultaneous decrease in L-ferritin and TfR in hypoxia occurred despite increased HIF-1 mRNA levels. They also observed unchanged binding activity of iron regulatory proteins (IRPs). Hepcidin measurements were not included because at the time of designing the study the central role of hepcidin in iron homeostasis was not yet established. However, ferroportin mRNA, a key protein responsible for iron export directly regulated by hepcidin, showed a more than 6-fold increase (see the figure), implying increased export of muscle iron at high altitude. The authors conclude that the increased iron requirements of enhanced erythropoiesis under hypoxia result in increased muscle iron mobilization and down-modulation of myoglobin, implying altered muscle oxygen homeostasis.
High-altitude exposure is associated with an increase in skeletal muscle ferroportin mRNA levels. Measurements obtained from muscle biopsies under normoxic conditions at sea level and after exposure to high-altitude hypoxia for 7 to 9 days (HA). See the complete figure in the article beginning on page 4724.
High-altitude exposure is associated with an increase in skeletal muscle ferroportin mRNA levels. Measurements obtained from muscle biopsies under normoxic conditions at sea level and after exposure to high-altitude hypoxia for 7 to 9 days (HA). See the complete figure in the article beginning on page 4724.
A number of possible mechanisms may be invoked to explain the observed findings. First, the trauma of strenuous exercise may have resulted in rhabdomyolysis. This is unlikely because the tests were performed after a prolonged period of acclimatization. Second, hypoxia could result in muscle damage, but after the observed increase in hemoglobin concentration, blood oxygen-carrying capacity was shown to be normal. Consequently, the most likely mechanism was a physiologic enhancement of cellular iron release in response to accelerated erythropoiesis resulting in the suppression of hepcidin expression,1 allowing increased cellular iron export by ferroportin. The associated parallel decrease in both ferritin and TfR implies that these changes were not IRP regulated and were likely caused by the overexpression of ferroportin.2,3
The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal